Abstract
Neurocysticercosis, caused by the cestode Taenia solium, is the most common parasitic infection of the human central nervous system that leads to seizures. Taenia crassiceps cysticercosis in mice is an experimental model for Taenia solium cysticercosis. Similar to the human infection, live parasites cause little or no granulomatous inflammation. Dying parasites initiate a granulomatous reaction. The neuropeptide, Substance P (SP), stimulates Th1 cytokine production. In the current studies, we determined if absence of SP/SP receptor circuitry in the SP precursor, preprotachykinin knockout or SP-receptor, neurokinin (NK1) knockout mice, affected granuloma cytokine production. We hence compared the levels of Th1 cytokines, IL-2 and IFN-γ, and levels of Th2/immunoregulatory cytokines, IL-4 and IL-10, by ELISA in T. crassiceps-induced granulomas derived from infected C57BL/6 wild type (WT) versus SP-Precursor knockout and NK1 knockout mice. We found that mean levels of IL-2, IFN-γ, IL-4, and IL-10 in infected, WT-derived granulomas were significantly higher than those of granulomas derived from infected SP-Precursor knockout or the NK1 receptor knockout mice. Levels of Th2/immunoregulatory cytokines, IL-4 and IL-10, were higher in early stage granulomas (histologically-staged on basis of evidence of parasite remnants) versus late stage granulomas (no parasite-remnants) of both knockouts, whereas the reverse was noted in WT-derived granulomas. These studies established that the absence of an SP/SP receptor circuitry in the SP precursor knockout mice or NK1 receptor knockout led to an inhibited cytokine response.
Neurocysticercosis, caused by the cestode Taenia solium is the most common parasitic infection of the human central nervous system that leads to epileptic-like seizures. Neurocysticercosis was previously prevalent mostly in the developing countries such as India and China, and in countries of Africa and Latin America (Bharucha, 2003; deBittencourt et al., 1996; Jallon, 1997; Sanchez et al., 1999; Rajshekhar et al., 2003). It is now becoming an increasingly important cause of seizures in the United States due to immigration from Mexico, and Central and South America (Sorvillo et al., 1992; Ong et al., 2002).
Food contaminated with feces of a T. solium tapeworm carrier (containing eggs of the tapeworm) is one of the primary causes leading to neurocysticercosis (Carpio, 2002). Eggs hatch in the small intestine. Oncospheres that emerge, penetrate the gut wall, enter the blood stream, and migrate to various sites, including the brain, subcutaneous tissues, heart muscle, and eyes, where they mature into cysticerci. Viable parasites are capable of modulating and suppressing the host immune responses (White et al., 1997). Initially, viable cysticerci induce a minimal granulomatous inflammatory response. However, as the cysticerci die, they loose their ability to control the host’s inflammatory response. The cyst wall is then infiltrated and surrounded by host inflammatory granulomatous cells composed primarily of mononuclear macrophages. Inflammatory cells are also known to enter the fluid of the parasite. This inflammatory response is associated with production of type 1 cytokines such as interferon gamma (IFN-γ), Interleukin-2 (IL-2), and Interleukin-12 (IL-12) (Restrepo et al., 1998). As the host response progresses, fibrosis permeates the cysticercus with collapse of the cyst cavity. Eventually, the parasite is replaced totally by fibrotic tissue, which may ultimately calcify.
Taenia crassiceps cysticercosis in mice is an experimental model for Taenia solium cysticercosis (Kunz et al., 1989; Larralde et al., 1990; Villa and Kuhn, 1991; Sciutto et al.,1995; Villa and Kuhn, 1997). In this model, inoculation intraperitoneally with 10 small parasite cysts of T. crassiceps leads to loading within 3–6 mo the entire peritoneal cavity crassiceps cysts that have been propagated by asexual, exogenous budding of mature cysticerci. As in the human infection, live cysticerci of T. crassiceps induce little or no granulomatous inflammation, whereas dying parasites initiate a granulomatous reaction. The mediators responsible for modulating the granulomatous cytokine responses are not known.
Bioactive substances that can modify granulomatous cytokine responses include substance P (SP), which is a neuropeptide involved in pain transmission (Honore et al., 2000). Neurons, endothelial cells, and cells of the immune system synthesize SP; receptors for SP are distributed throughout the body on neurons, endothelial cells, lymphocytes, and macrophages (Weinstock et al., 1988; Cook et al., 1994; Ho et al., 1997; Maggi, 1997; Goode et al.,1998). SP plays an important role in immune-mediated inflammatory processes. Normal induction of the granulomatous inflammation in other parasitic infections, e.g., murine schistosomiasis, requires binding of SP to its specific high affinity receptor (Blum et al., 1999). Substance P can also bind to NK2 and NK3 receptors with very low affinity. Most importantly, SP stimulates the production of the Th1 cytokine, IFN-γ (Blum et al., 1998; Weinstock and Elliott, 1998).
We previously examined mRNA and protein expression of Th1 cytokines, IL-2 and IFN-γ, and Th2/immunoregulatory cytokines, IL-4 and IL-10, in granulomas from infected wild type BALB/c mice by in situ hybridization and immunohistochemistry (Robinson et al., 1997). Early stage granulomas (characterized on basis of parasite remnants) predominantly express Th1 cytokines, IL-2 and IFN-γ, whereas Th2/immunoregulatory cytokines, IL-4 and IL-10, are expressed in the later stages (no parasite remnants). We also detected SP mRNA and protein predominantly in early stage granulomas (Robinson et al., 2002). The role of SP in the regulation of cytokine production in murine cysticercosis was not addressed. Therefore, in the current studies, we determined if mice devoid of SP or its receptor generate granulomas in cysticercosis with altered capacity to make cytokines.
MATERIALS AND METHODS
In vivo animal studies
Murine cysticercosis model
Granulomas were removed from the peritoneal cavities of female mice that had been infected for 3 mo after intra-peritoneal inoculation with 10 cysts of the ORF strain of T. crassiceps. Three groups of mice were included in the experiments: (1) wild type C57BL/6 mice; (2) preprotachykinin or SP precursor knockout mice (bred 10 generations onto the C57BL/6 background); and (3) SP receptor, neurokinin (NK1 knockout mice bred 10 generations onto the C57BL/6 background). Five to 8 infected mice from each of the 3 groups were used for this study. Eleven to 18 granulomas/mouse group were used for the study. Granulomas associated with parasites were identified visually, removed from the peritoneal cavity, and divided into 2 portions; 1 portion was fixed in 4% paraformaldehyde to be utilized for histological staging, and the other was used for quantifying cytokine proteins by ELISA.
Granuloma staging
The portion of each granuloma that was fixed with 4% paraformaldehyde was paraffin-embedded and cut into 5-μm sections, which were then stained with Giemsa and examined microscopically. We separated the granulomas into early and late stages based on the histological appearance of the degenerating parasite according to previously described methods (Robinson et al., 1997). Briefly, granulomas were classified as follows. Early stage granulomas were divided into stages 1 and 2; the former exhibited areas of intact tegument of the dead parasites, but also possessed areas with infiltration with host cells. Stage 2 granulomas displayed no areas of normal tegument of the parasite, infiltration with lymphocytes, but exhibited intact parasite morphology of the dead parasite, including a cyst cavity. Late stage granulomas were divided into stages 3 and 4; stage 3 granulomas revealed complete infiltration with host mononuclear cells and no evidence of a parasite cyst cavity, but a suggestion of the underlying degenerating parasite morphology. Stage 4 granulomas revealed only host cells and debri without clearly identifiable parasite elements.
Sandwich ELISA for the detection of cytokines
We determined protein levels of the different cytokines in 15–18 different granulomas derived from T. crassiceps infected wild type mice, 10–13 granulomas derived from T. crassiceps infected, NK1 knockout mice, and 9–11 granulomas derived from T. crassiceps infected Substance P knockout mice. To quantify cytokine protein, a portion of each granuloma was homogenized in PBS, followed by centrifugation at 14,000 RPM. Supernatant was analyzed for IL-2, IL-4, IL-10, and IFN-γ protein by sandwich ELISA (R&D Systems, San Diego, California) as per manufacterer’s instruction. Results are expressed as pg/mg of total protein.
Statistical analysis
Statistical differences were determined using 1-way ANOVA, followed by pairwise comparison using Tukey’s or Dunn’s test as appropriate. Significance was set at P<0.05.
RESULTS
Relative levels of Th1 cytokines, IL-2, and IFN-γ in infected, WT-derived granulomas compared to granulomas derived from infected SP precursor knockout or NK1 receptor knockout mice
Interleukin-2
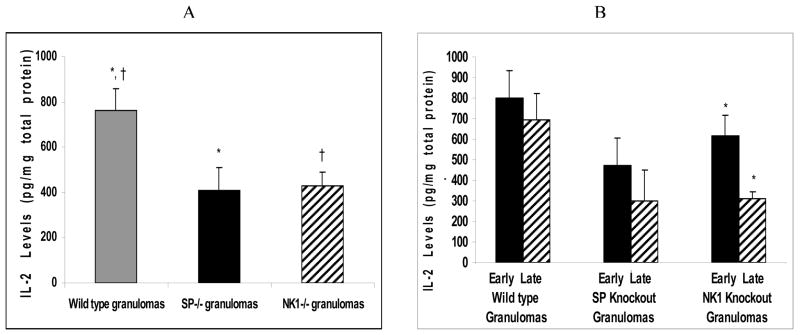
The mean IL-2 levels in the granulomas derived from infected wild type mice were significantly higher than those from granulomas derived from infected NK1R knockout mice (ANOVA; P<0.05) (Fig. 1A). Similarly, the mean IL-2 levels in the granulomas derived from infected wild type mice were significantly higher than those from granulomas derived from infected SP-Precursor knockout mice (ANOVA; P<0.05) (Fig. 1B).
Figure 1.
Quantitative levels of Th1 cytokine (IL-2) in (A) granulomas derived from Taenia crassiceps infected, wild type versus SP-Precursor knockout and NK1 receptor knockout mice. *P< 05, IL-2 levels in infected, SP-Precursor knockout-derived granulomas versus wild type-derived granulomas, †P< .05, IL-2 levels in infected, NK1 knockout-derived granulomas versus wild type-derived granulomas. (B) Early versus late granulomas derived from Taenia crassiceps infected, wild type versus SP-Precursor knockout and NK1 receptor knockout mice. *P< 05, IL-2 levels in infected, early stage versus late stage NK1 knockout derived granulomas.
Interferon-γ
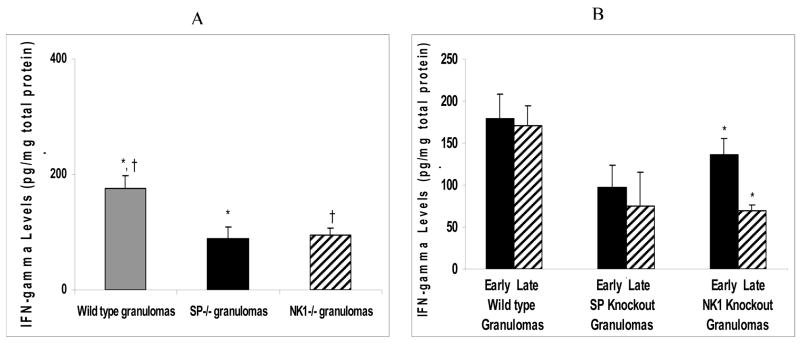
Similar to the Th1 cytokine IL-2, the IFN-γ levels in the granulomas derived from infected wild type mice were significantly higher than those of the granulomas derived from infected NK1R knockout or SP knockout mice (ANOVA; P<0.05) (Fig. 2A).
Figure 2.
Quantitative levels of Th1 cytokine (IFN-γ) in (A) granulomas derived from Taenia crassiceps infected, wild type versus SP-Precursor knockout and NK1 receptor knockout mice. *P< 05, IFN-γ levels in infected, SP-Precursor knockout-derived granulomas versus wild type-derived granulomas, †P< .05, IFN-γ levels in infected, NK1 knockout-derived granulomas versus wild type-derived granulomas. (B) Early versus late granulomas derived from Taenia crassiceps infected, wild type versus SP-Precursor knockout and NK1 receptor knockout mice. *P< 05, IFN-γ levels in infected, early stage versus late stage NK1 knockout-derived granulomas.
Relative levels of Th1 cytokines, IL-2 and IFN-γ derived from different stages of granuloma from infected, wildtype, SP Precursor knockout or NK1 receptor knockout mice
Interleukin-2
There was no major difference in the amount of IL-2 in early versus late stage granulomas derived from infected, wild type, or SP knockout mice (ANOVA; P>0.05) (Fig. 1B). However, significant differences were noted when comparing IL-2 levels in early versus late stage granulomas from infected NKR1 knockout mice (ANOVA; P<0.05) (Fig. 1B).
Interferon-γ
There was no major difference in the amount of IFN-γ in early versus late stage granulomas derived from infected, wild type or SP knockout mice (ANOVA; P>0.05) (Fig. 2B). In contrast, IFN-γ levels in the early granulomas derived from infected NK1R knockout mice was significantly higher than that of the late stage granulomas derived from infected NK1R knockout mice (ANOVA; P<0.05) (Fig. 2B).
Relative levels of Th2/immunoregulatory cytokines, IL-10 and IL-4 in infected, WT-derived granulomas compared to granulomas derived from infected SP knockout or NK1 receptor knockout mice
Interleukin-10
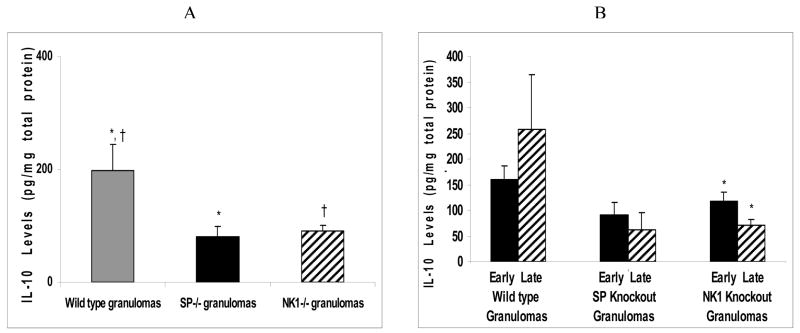
Similar to the Th1 cytokines, IL-10 levels in the infected, wild type derived granulomas was higher than that of the infected, NK1R knockout derived granulomas or SP-Precursor knockout derived granulomas (ANOVA; P<0.05) (Fig. 3A).
Figure 3.
Quantitative levels of IL-10 in (A) granulomas derived from Taenia crassiceps infected, wild type versus SP-Precursor knockout and NK1 receptor knockout mice. *P< 05, IL-10 levels in infected, SP-Precursor knockout-derived granulomas versus wild type-derived granulomas, †P< .05, IL-10 levels in infected, NK1 knockout-derived granulomas versus wild type-derived granulomas. (B) Early versus late granulomas derived from Taenia crassiceps infected, wild type versus SP-Precursor knockout and NK1 receptor knockout mice. *P< 05, IL-10 levels in infected, early stage versus late stage NK1 knockout-derived granulomas.
Interleukin-4
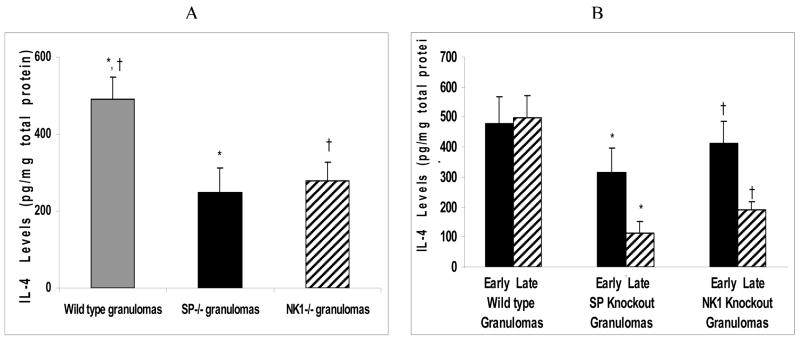
Similar to the Th1 cytokines and the regulatory cytokine IL-10, levels of the Th2 cytokine, IL-4 in the granulomas derived from infected wild type mice were higher than those of the granulomas derived from infected, NK1R knockout or the SP-Precursor knockout mice (ANOVA; P<0.05)(Fig. 4A).
Figure 4.
Quantitative levels of Th2 cytokine (IL-4) in (A) granulomas derived from Taenia crassiceps infected, wild type versus SP-Precursor knockout and NK1 receptor knockout mice. *P< 05, IL-4 levels in infected, SP-Precursor knockout-derived granulomas versus wild type-derived granulomas, †P< .05, IL-4 levels in infected, NK1 knockout-derived granulomas versus wild type-derived granulomas. (B) Early versus late granulomas derived from Taenia crassiceps infected, wild type versus SP-Precursor knockout and NK1 receptor knockout mice., *P< 05, IL-4 levels in infected, early stage versus late stage SP-Precursor knockout-derived granulomas, †P< .05, IL-4 levels in infected, early stage versus late stage NK1 knockout-derived granulomas.
Relative levels of Th2/immunoregulatory cytokines, IL-4 and IL-10, derived from different stages of granulomas from infected, wildtype, SP-Precursor knockout or NK1 receptor knockout mice
Interleukin-10
Although the IL-10 levels in the early granulomas derived from infected wild type mice were somewhat lower than the late stage granulomas derived from infected wild type mice, these differences were not statistically different (ANOVA; P>0.05) (Fig. 3B). In contrast to wild type mice, mean IL-10 levels in the early stage granulomas derived from infected NK1R knockout mice were higher than those of the late stage granulomas derived from infected NK1R knockout mice (ANOVA; P<0.05) (Fig. 3B). Also, similar to that of the NK1R knockout granulomas, it was noted that the mean IL-10 levels in the early stage granulomas derived from infected, SP-Precursor knockout mice were higher than that of the late stage SP-Precursor derived granulomas. However, the increase was not statistically significant (ANOVA; P>0.05) (Fig. 3B).
Interleukin 4
The mean IL-4 levels in the early granulomas derived from infected NK1R knockout mice was significantly higher than those of the late granulomas derived from infected NK1R knockout mice. (ANOVA; P<0.05) (Fig. 4B). Similarly, we noted that the mean IL-4 levels in the early stage granulomas derived from infected, SP-Precursor knockout mice were significantly higher than those of the late stage SP-Precursor derived granulomas (ANOVA; P<0.05) (Fig. 4B). The IL-4 levels in the early granulomas derived from infected, wild type mice were not significantly different from those of the late stage granulomas derived from infected wild type mice (ANOVA; P>0.05) (Fig. 4B).
DISCUSSION
In the current studies, we determined if the absence of SP and its specific receptor, NK1R, affects cytokine expression in peritoneal granulomas induced by murine T. crassiceps infection. We thus measured cytokine content of granulomas that developed in infected C57BL/6, wild type, SP-Precursor knockout, and NK1R knockout mice. We noted that the levels of Th1 cytokines, IL-2 and IFN-γ, and Th2/immunoregulatory cytokines, IL-4 and IL-10, in the wild type granulomas were significantly higher than those of the SP-Precursor and NK1R, knockout granulomas.
Substance P is known to stimulate the production of Th1 cytokines (Blum et al., 1998; Weinstock and Elliott, 1998). We had thus anticipated that if SP is an important factor in the stimulation of Th1 cytokines; in the absence of SP-SP receptor interaction, a blunted Th1 response should occur. As anticipated, we noted that in the granulomas derived from both infected SP-Precursor and the NK1R knockout mice, there was significantly lower Th1 cytokine levels as compared to granulomas derived from infected, wild type mice.
Since there are no studies implicating the role of SP-NK1R interaction in Th2/immunoregulatory cytokine production, our results demonstrating a blunted Th2/immunoregulatory cytokine production in the absence of the SP-NK1 receptor interaction network were unexpected. We do not know why the Th2/immunoregulatory cytokine response is blunted in the absence of the SP-NK1 receptor interaction pathway.
In the current studies, we also observed that the levels of Th2/immunoregulatory cytokines, IL-4 and IL-10, were higher in early stage versus late stage granulomas of both the knockouts. Although not significant, the Th2/immunoregulatory cytokine levels were higher in late stage versus early stage granulomas of the wild type mice. We do not know the reason why the pattern of Th2/immunoregulatory cytokine expression is different from that of the wild type infected mice.
The present studies show that the absence of SP precursor (preprotachykinin) and the specific substance P receptor, NK1R, affects cytokine expression in peritoneal granulomas induced by T. crassiceps. Preprotachykinin encodes for substance P and neurokinin A. Therefore, preprotachykinin knockout mice do not produce either substance P or neurokinin A. However, because NK1 receptor is specific for substance P and not for neurokinin A, and the current studies demonstrate that absence of SP precursor (preprotachykinin) and the specific substance P receptor, NK1R, affects cytokine expression in peritoneal granulomas induced by T. crassiceps, it is logical to deduce that most if not all of the effects on altered cytokine production is due to substance P and not by neurokinin A.
It has been demonstrated by Metwali et al. (2004) that peptide hormones such as hemokinin can bind and activate the NK1 receptor at sites of chronic inflammation (Metwali et al., 2004). Therefore, although the current studies suggest that SP may be an important mediator associated with cytokine production, there may be other peptide hormones like hemokinin that also bind and activate the NK1 receptor that may be associated with cytokine production. Therefore, it may be possible that in the NK1 receptor knockout mice, besides the obstruction of the SP mediated affects, there may be additional blockage via the effects of other possible mediators such as hemokinin.
These studies determined that the absence of an SP/SP receptor circuitry in the SP precursor knockout mice or NK1 receptor knockout led to an inhibited cytokine response.
Acknowledgments
These studies were supported by the National Institutes of Health, 1 R01 NS042604-01A2 to P. Robinson. Taenia crassiceps was provided by Dr. Raymond Kuhn, Wake Forest University, Winston-Salem, North Carolina. SP precursor knockout mice were provided by Dr. Julio Pérez Fontán, University of Texas Southwestern School of Medicine, Houston, Texas. NK1 knockout mice were provided by Dr. Weinstock, University of Iowa, Iowa City, Iowa.
LITERATURE CITED
- Bharucha NE. Epidemiology of epilepsy in India. Epilepsia. 2003;44(Suppl 1):9–11. doi: 10.1046/j.1528-1157.44.s.1.5.x. [DOI] [PubMed] [Google Scholar]
- Blum AM, Elliott DE, Metwali A, Li J, Qadir K, Weinstock JV. Substance P regulates somatostatin expression in inflammation. Journal of Immunology. 1998;161:6316–6322. [PubMed] [Google Scholar]
- Blum AM, Metwali A, Kim-Miller M, Li J, Qadir K, Elliott DE, Lu B, Fabry Z, Gerard N, Weinstock JV. The substance P receptor is necessary for a normal granulomatous response in murine schistosomiasis mansoni. Journal of Immunology. 1999;162:6080–6085. [PubMed] [Google Scholar]
- Carpio A. Neurocysticercosis: An update. Lancet Infectious Diseases. 2002;2:751–762. doi: 10.1016/s1473-3099(02)00454-1. [DOI] [PubMed] [Google Scholar]
- Cook GA, Elliott D, Metwali A, Blum AM, Sandor M, Lynch R, Weinstock JV. Molecular evidence that granuloma T lymphocytes in murine schistosomiasis mansoni express an authentic substance P (NK-1) receptor. Journal of Immunology. 1994;152:1830–1835. [PubMed] [Google Scholar]
- De Bittencourt PR, Adamolekum B, Bharucha N, Carpio A, Cossio OH, Danesi MA, Dumas M, Fernandes JG, Genton P, Manreza ML, et al. Epilepsy in the tropics: II. Clinicalpresentations, pathophysiology, immunologic diagnosis, economics, and therapy. Epilepsia. 1996;37:1128–1137. doi: 10.1111/j.1528-1157.1996.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Goode T, O’Connell J, Sternini C, Anton P, Wong H, O’Sullivan GC, Collins JK, Shanahan F. Substance P (neurokinin-1) receptor is a marker of human mucosal but not peripheral mononuclear cells: Molecular quantitation and localization. Journal of Immunology. 1998;161:2232–2240. [PubMed] [Google Scholar]
- Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. Journal of Immunology. 1997;159:5654–5660. [PubMed] [Google Scholar]
- Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Jallon P. Epilepsy in developing countries. Epilepsia. 1997;38:1143–1151. doi: 10.1111/j.1528-1157.1997.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Kunz J, Kalinna B, Watschke V, Geyer E. Taenia crassiceps metacestode vesicular fluid antigens shared with the Taenia solium larval stage and reactive with serum antibodies from patients with neurocysticercosis. Zentralblatt fur Bakteriologie. 1989;271:510–520. doi: 10.1016/s0934-8840(89)80113-6. [DOI] [PubMed] [Google Scholar]
- Larralde C, Montoya RM, Sotelo J, Hayunga J, Sciutto E, Palencia G, Padilla A, Govensky T, Diaz ML. Murine T. crassiceps antigens in immunodiagnosis of T. solium human neurocysticercosis, T. saginata bovine cysticercosis, and human E. granulosus hydatidosis. Bulletin de la Societe Francaise de Parasitology. 1990;8:S8B. [Google Scholar]
- Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regulatory Peptides. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- Metwali A, Blum AM, Elliott DE, Setiawan T, Weinstock JV. Cutting edge: Hemokinin has substance P-like function and expression in inflammation. Journal of Immunology. 2004;172:6528–6532. doi: 10.4049/jimmunol.172.11.6528. [DOI] [PubMed] [Google Scholar]
- Ong S, Talan DA, Moran GJ, Mower W, Newdow M, Tsang VC, Pinner RW. Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerging Infectious Diseases. 2002;8:608–613. doi: 10.3201/eid0806.010377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajshekhar V, Joshi DD, Doanh NQ, van De N, Xiaonong Z. Taenia solium taeniosis/cysticercosis in Asia: Epidemiology, impact and issues. Acta Tropica. 2003;87:53–60. doi: 10.1016/s0001-706x(03)00055-x. [DOI] [PubMed] [Google Scholar]
- Restrepo BI, Llaguno P, Sandoval MA, Enciso JA, Teale JM. Analysis of immune lesions in neurocysticercosis patients: Central nervous system response to helminth appears Th1-like instead of Th2. Journal of Neuroimmunology. 1998;89:64–72. doi: 10.1016/s0165-5728(98)00112-x. [DOI] [PubMed] [Google Scholar]
- Robinson P, Atmar RL, Lewis DE, White AC., Jr Granuloma cytokines in murine cysticercosis. Infection and Immunity. 1997;65:2925–2931. doi: 10.1128/iai.65.7.2925-2931.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P, White AC, Lewis DE, Thornby J, David E, Weinstock J. Sequential expression of the neuropeptides substance P and somatostatin in granulomas associated with murine cysticercosis. Infection and Immunity. 2002;70:4534–4538. doi: 10.1128/IAI.70.8.4534-4538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AL, Lindback J, Schantz PM, Sone M, Sakai H, Medina MT, Ljungstrom I. A population-based, case-control study of Taenia solium taeniasis and cysticercosis. Annales of Tropical Medical Parasitology. 1999;93:247–258. [PubMed] [Google Scholar]
- Sciutto E, Fragoso G, Baca M, De la Cruz V, Lemus L, Lamoyi E. Depressed T-cell proliferation associated with susceptibility to experimental Taenia crassiceps infection. Infection and Immunity. 1995;63:2277–2281. doi: 10.1128/iai.63.6.2277-2281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvillo FJ, Waterman SH, Richards FO, Schantz PM. Cysticercosis surveillance: Locally acquired and travel-related infections and detection of intestinal tapeworm carriers in Los Angeles County. American Journal of Tropical Medicine and Hygiene. 1992;47:365–371. doi: 10.4269/ajtmh.1992.47.365. [DOI] [PubMed] [Google Scholar]
- Villa O, Kuhn R. Antigenic and immunogenic analysis of Taenia crassiceps and Taenia solium using sera from their natural intermediate hosts. ASM Bulletin. 1991;39:99–103. [Google Scholar]
- Villa O, Kuhn R. Mice infected with the larvae of Taenia crassiceps exhibit a Th2 like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology. 1996;112:561–570. doi: 10.1017/s0031182000066142. [DOI] [PubMed] [Google Scholar]
- Weinstock JV, Blum A, Walder J, Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. Journal of Immunology. 1988;141:961–966. [PubMed] [Google Scholar]
- Weinstock JV, Elliott D. The substance P and somatostatin interferon-gamma immunoregulatory circuit. Annals of the New York Academy of Sciences. 1998;840:532–539. doi: 10.1111/j.1749-6632.1998.tb09592.x. [DOI] [PubMed] [Google Scholar]
- White AC, Jr, Robinson P, Kuhn R. Taenia solium cysticercosis: Host-parasite interactions and the immune response. Chemical Immunolology. 1997;66:209–230. [PubMed] [Google Scholar]