Abstract
Background and Purpose
The potential for successful treatment of intracranial aneurysms by flow diversion is gradually being recognized in the clinical setting; however, the devices currently available (stents) are not designed for flow diversion. We evaluate the long-term response of an appropriately designed flow diversion device in producing thrombotic occlusion of experimental aneurysms.
Methods
Three different configurations of an original flow diversion device were implanted across thirty elastase-induced aneurysm models in rabbits. Ten animals per device configuration were followed-up for 3 weeks (n=3), 3 months (n=3), or 6 months (n=4) and tissue explanted post-sacrifice was sent for histology. The temporal variation in angiographic contrast intensity within each aneurysm was fitted with a mathematical model to quantify the alteration in local hemodynamics caused by the implanted device. A predictive index, called the washout coefficient, was constructed to estimate long-term aneurysm occlusion probabilities immediately after treatment with any flow diversion device.
Results
The device with a porosity of 70% and pore density of 18 pores/mm2 performed better at occluding aneurysms than devices with 70% porosity, 12 pores/mm2 and 65% porosity, 14 pores/mm2. A value of the washout coefficient less than 30 predicted greater than 97% angiographic aneurysm occlusion over a period of six months with a sensitivity of 73% and specificity of 82%.
Conclusions
The flow diversion devices effected successful and stable aneurysm occlusion. Pore density, rather than porosity, may be the critical factor modulating efficacy of such devices.
Keywords: Stents, Histology, Scanning electron microscopy, Washout coefficient, Side-branch patency
Approximately five percent of the 700,000 strokes occurring in the United States each year are subarachnoid hemorrhages (SAH) caused by the rupture of intracranial aneurysms.1 Although only 0.2% of the aneurysms carried by the general population rupture every year, the prognosis after SAH is dismal; 10 to 20% of patients are dead upon admission and fatality rates range from 32 to 67% per calendar year.2 Current treatment of intracranial aneurysms is either by surgical clipping or endovascular coiling and a comparison between these two modalities in the International Subarachnoid Aneurysm Trial showed a 7% absolute reduction in risk with coiling at 1 year post-treatment with the benefit being maintained until 7 years.3 The requirement for late re-treatment may, however, be as much as 7 times higher in the endovascular cohort.4 Flexible, intracranial stents have consequently been developed to assist the endovascular coiling of wide-necked aneurysms (ratio of aneurysm height to neck less than 1.5 or 2) with better overall outcomes as compared to coil-embolization alone.5–7
Seminal studies in the early- to mid-90s introduced the notion that intracranial aneurysms could be successfully treated solely by the deployment of stents within the parent artery.8–11 Their hypothesis was that stents would divert flow away from the aneurysm and into the parent artery and the resultant sluggish flow within the aneurysm would promote thrombus formation that would eventually organize into scar tissue and thus exclude the aneurysm from the circulation. Moreover, the stent implanted in the parent artery would, over time, be incorporated into the vessel wall thereby precluding regrowth of the aneurysm by hemodynamic mechanisms. Their results showed complete and stable occlusion of sidewall venous pouch canine carotid aneurysms treated with stents only over follow-up periods ranging from 1 to 9 months.9–11 These initial experimental results have been corroborated by subsequent clinical evidence showing progressive thrombosis or complete occlusion of aneurysms (mostly sidewall) at follow-up when treated with stents alone.12–14 On the other hand, other clinical reports have shown aneurysms treated with stents alone to have persistent filling at follow-up.14,15 It is important to note that none of these devices was designed to act as a flow diversion device, but instead as a scaffold for coils or stenotic arteries. Moreover, the follow-up times in the clinical studies noting incomplete aneurysm occlusion is less (average of about 1 month) than those that report complete aneurysm occlusion (average around 4 months).
The primary intent of a flow diversion device (as opposed to a stent) is to optimally alter the flow exchange between the parent artery and the aneurysm so as to promote complete thrombosis of the sac as rapidly as possible while eliciting minimal neointimal hyperplasia. There are no commercially available flow diversion devices as yet although recent reports16–18 suggest that the development of such devices is being actively pursued. Based on our previous experience with flow diversion for treating intracranial aneurysms,10,19–22 we have constructed a flow diversion device of original design. Six potentially-optimal device configurations with varying porosities and pore densities were constructed with the intent of homing in on the optimal configuration through experimentation. Detailed particle image velocimetry21 and preliminary in vivo22 studies were subsequently performed on the rabbit elastase-induced aneurysm model, which indicated three device configurations as having the best performance of the six. We proceeded to implant these three devices in a large cohort of experimental aneurysms, the results of which are presented here.
METHODS
Flow Diversion Devices
The devices were constructed of a cobalt-based alloy and were self-expanding in nature; Figure 1 compares one of the device configurations to a coronary stent (Wallstent®, Boston Scientific, Natick, MA). The two important characteristics of any such device are the porosity, which is defined as the ratio of the metal free surface area to the total surface area, and the pore density, which is defined as the number of pores per unit surface area. All three configurations (labeled as devices I, II, and III) had an open device diameter of 4 mm and the same surface pattern; nominal porosities of devices I, II, and III were 70%, 65%, and 70%, respectively; pore densities of devices I, II, and III were approximately 12, 14, and 18 pores/mm2, respectively.
Figure 1.

Image of one of the flow diversion device configurations (right) in comparison to a coronary stent (Wallstent, left).
Animal Experiments
All experimental protocols were approved by the Animal Care and Use Committee at our institution. Initiation and maintenance of anesthesia were identical for all procedures. New Zealand white rabbits were pre-anesthetized with an intramuscular injection of Glycopyrrolate (0.1 mg/kg) and sedated with intramuscular injections of Xylazine (5 mg/kg) and Ketamine (35 mg/kg); sedation was maintained with 1–2% Isoflurane throughout the procedure. After completion of survival procedures, the animals were given intramuscular Buprenorphine (0.04 mg/kg) for pain relief and subcutaneous Ketoprofen (2 mg/kg) as anti-inflammatory and antipyretic immediately after surgery and twice a day for three days post-surgery. The procedure for creating elastase-induced aneurysms in rabbits is described elsewhere;23 model aneurysms were created in 40 rabbits.
After allowing sufficient time for the aneurysm to mature (nominally 3 weeks), the animals were brought back to the angiography suite for flow diversion device implantation. Ten animals were implanted with each of the three device configurations and the remainder ten animals served as controls. Treated animals were orally given 20 mg of aspirin once a day beginning 2 days before the procedure and continuing till 10 days after the procedure to mitigate acute thrombotic events. Through a right transfemoral approach, the devices were implanted in the innominate-to-subclavian artery with the intent of maintaining equal lengths on either side of the aneurysm while also covering the vertebral artery ostium. High-speed angiographic sequences were acquired at 30 frames/second over 20 seconds while injecting 5 cc of angiographic contrast (VisipaqueTM, 320 mgI/ml, GE Healthcare, UK) at a rate of 2 cc/s both before, and immediately after, device implantation. Identical imaging (image to source distance, C-arm orientation, acquisition mode) and injection (injection volume, injection rate, preset delay) parameters were maintained during both acquisitions. The choice of the device to be implanted in any given animal was solely dependent on the study schedule and was otherwise arbitrary.
Animals were followed-up at 21 days (n = 12, 3 animals per treatment group), 90 days (n = 12, 3 animals per treatment group), or 180 days (n = 16, 4 animals per treatment group) after the implantation phase of the study. High-speed follow-up angiograms were acquired via left transfemoral access. The animal was euthanized with an overdose of sodium pentobarbital (1 ml intravenous injection of Euthasol®, Delmarva Laboratories, Midlothian, VA), flushed with saline and 10% formalin, after which the aneurysm-parent vessel complex including the aorta, vertebral artery, superficial cervical and costocervical trunk, and distal subclavian artery was explanted. Explanted tissue was pressure-fixed with 10% formalin under approximately 50 mmHg for about 20 minutes.
Tissue Processing
Tissue samples evaluated by histology were processed through a graded series of ethanol, xylene, and were embedded in methyl methacrylate. Sections were taken at approximately 600 μm intervals, polished down to 6 μm, and stained with a metachromatic (toluidine blue-basic fuchsin) stain. Neointimal thicknesses at any section were calculated as the difference between device and lumen radii based on measurements of internal elastic membrane area (device area) and lumen area. Tissue samples obtained at 180 days follow-up from one animal per treatment group were evaluated by scanning electron microscopy (SEM). Prior to tissue harvesting for SEM evaluation, the vasculature was sequentially perfused with 5% dextrose in water, 0.25% silver nitrate, 5% dextrose in water, and formalin. The implanted parent artery was cut longitudinally to obtain two half-cylinder shape segments and the silver nitrate was developed by exposing the endothelial cells to Kodak T-Max solution. The tissue was then placed in 1% Zetterquist’s Osmium, rinsed in Zetterquist’s Buffer, washed in water, dehydrated in a graded series of ethanol and acetone solutions and dried in hexamethyldisilazane (HMDS). Samples were mounted and sputter-coated with gold or palladium for SEM observation.
Angiographic Analysis
The angiographic image with maximal opacification of the aneurysm was selected in each of the acquired sequences and the aneurysm was manually delineated as a region of interest (ROI). The percentage angiographic aneurysm occlusion at follow-up was calculated as , where A1 was the aneurysm area (in pixels) before device implantation A1 and A2 was the aneurysm area at follow-up. The flow exchange between the parent artery and the aneurysm was measured by calculating the temporal variation in average grayscale intensity (ratio of the sum of grayscale intensities of all pixels to total number of pixels) within the ROI. These aneurysmal washout curves were then normalized and fit to a mathematical model described previously24 in the least squares sense. The model demarcates the aneurysm-parent vessel flow exchange into convective and diffusive components as characterized by amplitudes and time constants. The alteration in local hemodynamics produced by a flow diversion device could then be quantified by the extent to which the device increased the diffusive mode of flow exchange as seen by an increase in the amplitude of the diffusive component (ρdiff) and the diffusive time constant (τdiff).
In addition to increasing the proportion and washout time of the diffusive region within the aneurysm, a flow diversion device would also reduce the net amount of dye entering the aneurysm, as reflected by changes in the amplitude of the aneurysmal washout curve. To account for these different mechanisms in a single quantity, an index of the effectiveness of the device could be constructed. This index, called the washout coefficient here, was given as
where, δ% was the washout curve amplitude and ρdiff% and τdiff% were the amplitude and time constant of the diffusive component of the model obtained immediately after device implantation expressed as percentages of the corresponding values before device implantation. Such a parameter obtained immediately after device implantation could be used to assess whether or not any given device will occlude the treated aneurysm at follow-up. A prognostic test for aneurysm occlusion could then be developed based on a suitable threshold value for W. A threshold was chosen so as to maximize the sensitivity and specificity of the test based on the receiver operating characteristic (ROC) curve for the data and statistical significance of the test was assessed by Fisher’s exact test for the resulting contingency table. Statistical significance of the differences in angiographic aneurysm occlusion and neointimal thickness between devices was compared by analysis of variance or Student’s t tests. All statistical evaluations were made with the InStat software (version 5.1, GraphPad Software, San Diego, CA).
RESULTS
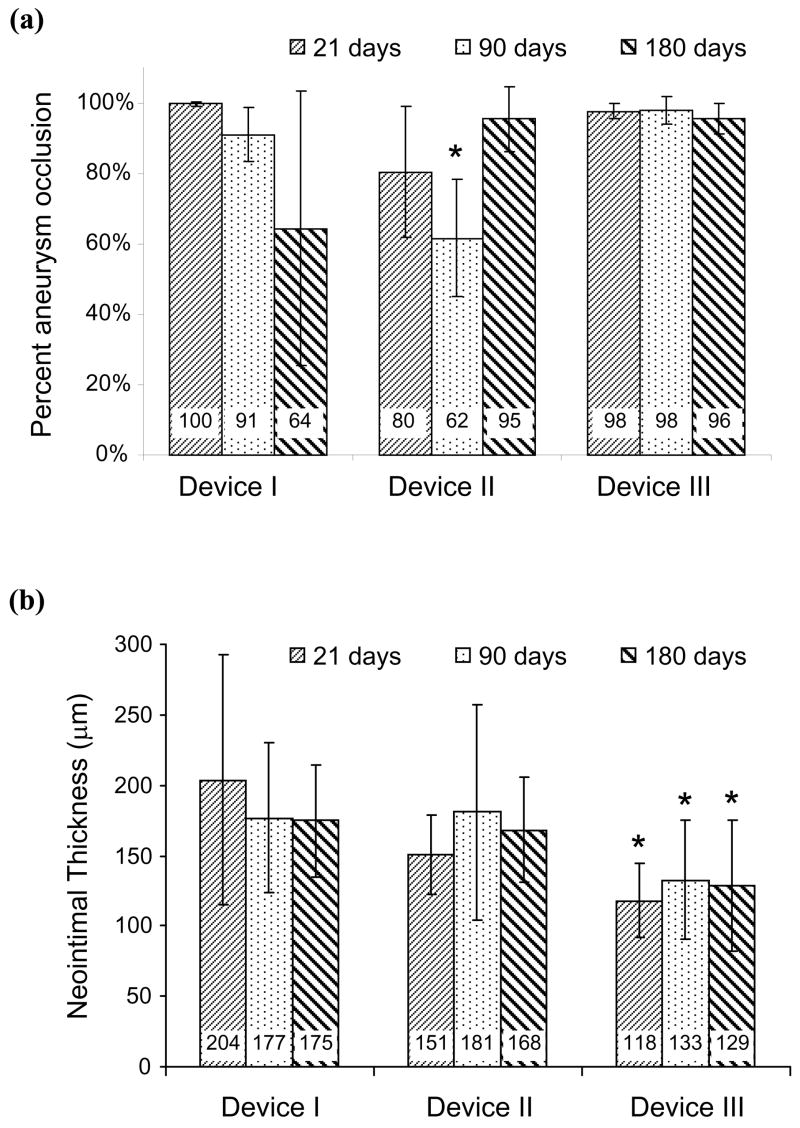
The average (± standard deviation) neck, width, and height of the 40 aneurysms were 4.1±1.4, 3.7±0.6, and 7.9±1.9 mm, respectively, and were similar to those reported by other investigators.25 The neck (p = 0.5), width (p = 0.9), or height (p = 0.3) of the aneurysms were not significantly different when comparing the four treatment groups. There were no cases of device migration or device fracture at any follow-up time. Figure 2 shows the angiograms from one animal treated with device III and followed-up at 180 days. Angiographically, the aneurysm was observed to be completely occluded in this case. Twenty four of the 30 treated animals had greater than 75% occlusion of the aneurysm, while 17 of the 30 animals had greater than 95% occlusion of the aneurysm at follow-up. All control aneurysms were patent at follow-up with an average patency rate of 95±3%. The degree of aneurysm occlusion produced by any of the devices was significantly higher than the occlusion of control aneurysms (p<0.0001). Figure 3a shows the aneurysm occlusion rates produced by the 3 devices at the 3 different follow-up time points. Device III was seen to consistently produce greater degrees (statistically non-significant) of aneurysm occlusion as compared to the other 2 devices.
Figure 2.
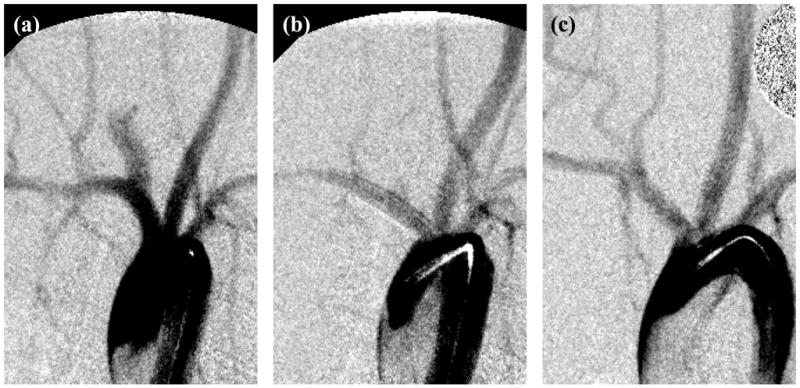
Angiograms from one animal treated with device III showing the aneurysm (a) before device implantation, (b) immediately after device implantation, and (c) at 180 days follow-up.
Figure 3.
(a) Mean percentage angiographic occlusion of the aneurysm at different follow-up time points for the 3 devices. (b) Mean thickness of the neointima formed on the luminal surface of the devices at different follow-up time points. * indicates significance (p<0.05) with respect to the other 2 devices at corresponding follow-up time; error bars represent one standard deviation.
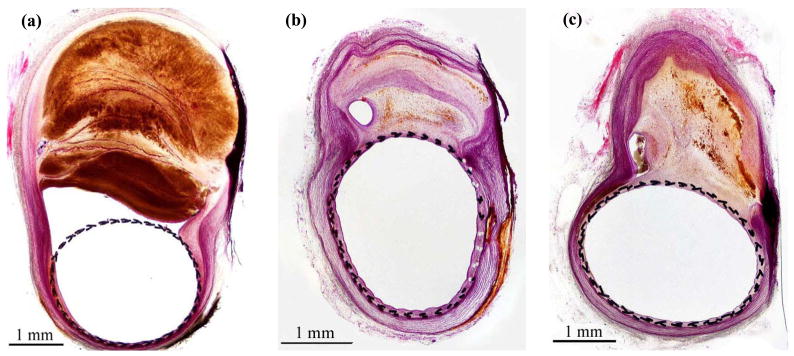
Histological sections of the aneurysms showed a progression in maturity of intraaneurysmal thrombus from acute, unorganized clot at 21 days through to complete organization with neovascularization at 6 months (Figure 4a through 4c). A thin, smooth neointima was observed in all samples at all time points in histological evaluations. The average neointimal thicknesses formed within each device at different follow-up time points are graphed in Figure 3b. Device III consistently had significantly lower neointimal thicknesses as compared to the other 2 devices. For a given device, there were no significant differences over various follow-up times (p>0.59). Endothelial cells were seen to cover the entire luminal surface of all the devices in scanning electron microscope (SEM) sections of samples explanted at 6 months (Figure 5a). The vertebral artery and the costocervical trunk jailed by the flow diversion devices remained angiographically patent in all cases at all follow-up time points (Figure 2c). SEM sections at the vertebral artery corroborated side branch patency in all processed samples (Figure 5b).
Figure 4.
(a) to (c): histological sections of the aneurysm from 3 animals treated with device III and followed-up at 21, 90 and 180 days post-treatment, respectively. Mature thrombus shows as a lighter shade as compared to acute thrombus with the staining method employed.
Figure 5.
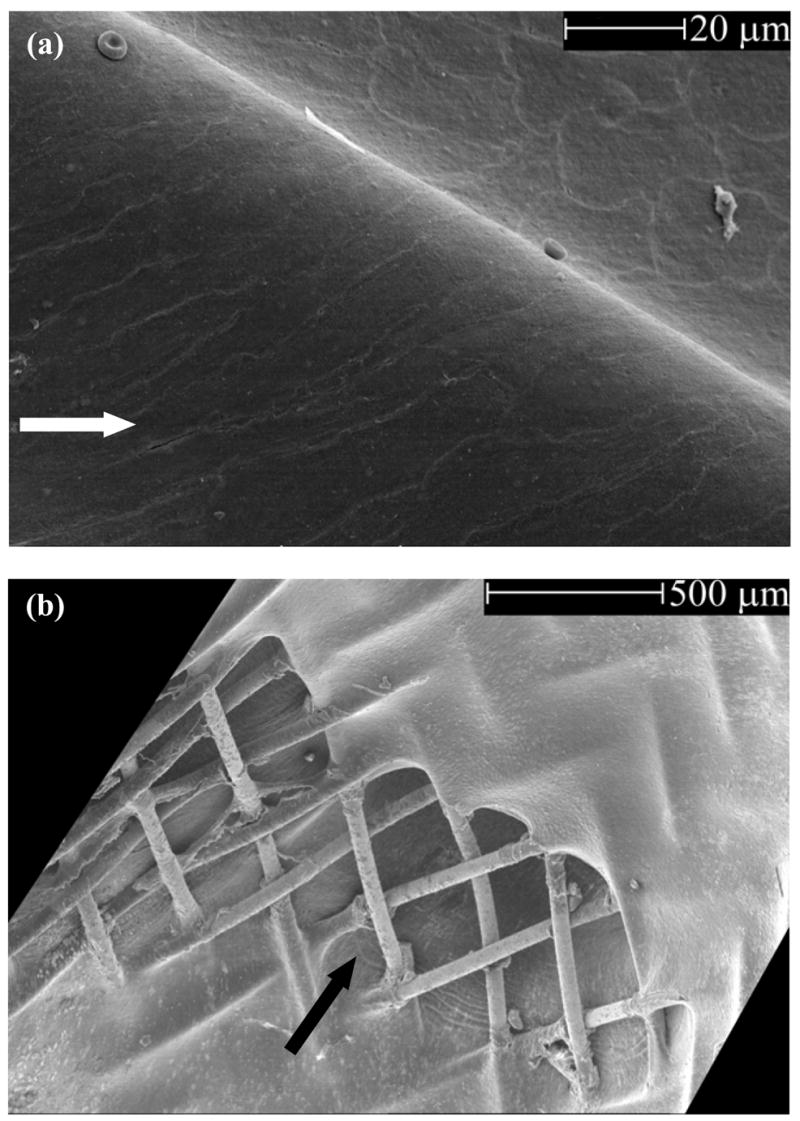
Scanning electron microscope images (a) Endothelial cells covering one of the device struts; the entire luminal surface of the devices was observed to have an endothelial lining; arrow shows flow direction in the parent artery (b) Patent vertebral artery ostium; arrow shows flow direction into the vertebral artery. The longitudinal axis of the vertebral is at an angle to the cut section of the subclavian. Both images of tissue obtained 180 days post-treatment.
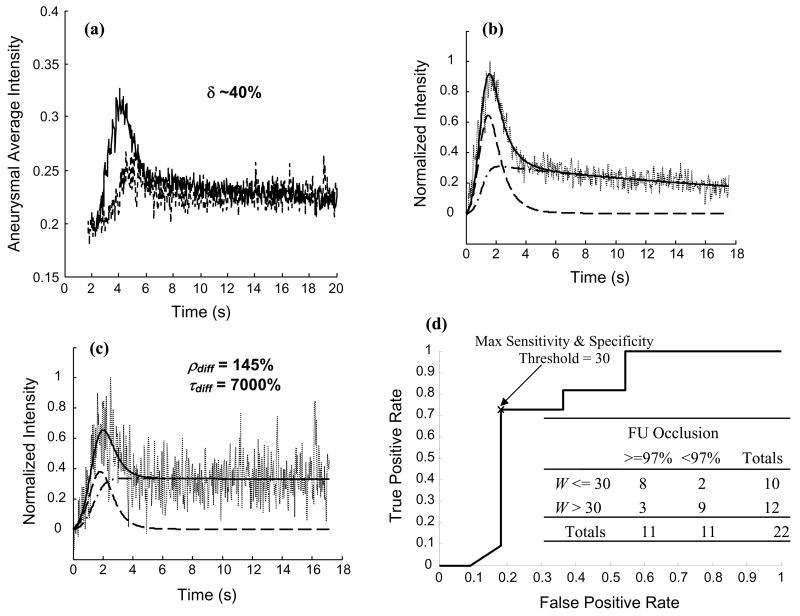
Aneurysmal washout curves from one case obtained before device implantation and immediately after implantation of device III are shown in Figure 6a. In this case, the amount of dye entering the aneurysm (amplitude of the washout curve) after device implantation was reduced to approximately 40% of the corresponding value before device implantation. The mathematical model faithfully captured the trend of all the aneurysmal washout curves and the model-fits to the 2 curves in Figure 6a are shown in Figures 6b and 6c as examples. The convective and diffusive components of the model are superposed on the plots. The proportion of the aneurysm-parent vessel flow exchange dominated by diffusion was increased by about 45% due to the flow diversion device in this case; the time constant of this diffusive flow exchange was concomitantly increased to 70 times the corresponding value before device implantation.
Figure 6.
(a) Aneurysmal washout curves for one case obtained before device III implantation (solid line) and immediately after device implantation (dashed line); after device implantation the amplitude of the curve is reduced to approximately 40% of the value before device implantation. Mathematical model-fits to the normalized washout curves obtained (b) pre-implantation and (c) immediately post-implantation quantify the increase in diffusive flow exchange produced by the device. Washout curves (dotted lines); model-fits (solid lines); convective components (dashed lines); diffusive components (dash-dot lines); after device implantation the amplitude and the time constant of the diffusive component are increased to 1.45 and 70 times the respective values before device implantation. (d) The receiver operating characteristic curve for the washout coefficient (W) data grouped based on angiographic aneurysm occlusion at follow-up (FU) of greater or less than 97%. Inset shows the contingency table based on the threshold value of 30.
There was a statistically significant difference in the washout coefficient (W) values when correlated with the angiographic aneurysm occlusion at follow-up. The average W value for cases with >= 97% angiographic occlusion of the aneurysm at follow-up (21±15) was significantly lower than the average value for cases with <97% angiographic aneurysm occlusion at follow-up (51±34); Welch-corrected t test p=0.02. The threshold value of W which gave the maximum sensitivity and specificity for the prognostic test was 30. Figure 6d shows the ROC curve for the washout coefficient data with the inset showing the contingency table based on a threshold value of 30. The sensitivity and the specificity of this test were 73% and 82%, respectively. The Fisher’s exact test two-sided p value was 0.03, suggesting that the row-column association was statistically significant.
DISCUSSION
The endovascular treatment of intracranial aneurysms has progressed enough to consider it as a valid line of defense against most aneurysms. The phrase “endovascular treatment of aneurysms” currently implies the deposition of micro-coils into the sac with the goal of establishing complete thrombosis and exclusion of the aneurysm. Limitations remain, however, with regard to the long-term durability of aneurysm occlusion with coiling because of two inherently related phenomena. The repeated pulsatile impingement of blood flow against the embolic mass tends to compact it and/or the aneurysm recanalizes as its neck remains exposed to parent artery flow. The placement of a stent within the parent vessel and across the aneurysm neck as a support for coils was proposed as the next treatment measure in order to overcome these drawbacks. Intracranial stents were consequently developed and marketed and short-term results with these devices are now considered promising.5,6 The potential benefit of stents (in addition to the effect of coils) is that they reduce the flow within the aneurysms while providing a matrix for neointimal formation. The aneurysm becomes occluded, the stent gets encapsulated within the vessel wall, the aneurysm neck is not “open” to flow any further, and thus successful exclusion of the sac is effected. As the burden of treatment seems to be largely carried by the stents in this conceptual argument, it may be reasonable to question the need for coil deposition within the sac. This line of reasoning (treatment by stents only) was followed by seminal reports in the literature approximately fifteen years ago.9–11 Preliminary testing of the hypothesis in experimental animals and arbitrarily gathered clinical data suggested that stents alone could indeed effect successful aneurysm exclusion.9–14 Through the course of these results being accumulated, however, it was observed that commercially available stents did not have the capacity to induce a large enough flow modification within the aneurysm to promote complete aneurysm thrombosis. This was supported by the fact that sometimes, two or three prevalent stents needed to be deployed12 in order to approach the intraaneurysmal flow modifications that the ideal device would engender.
In contrast to an ideal stent, an ideal flow diversion device has low porosity and high pore density values optimized to promote intraaneurysmal thrombosis while maintaining patency of the parent vessel and side branches. Moreover, lower radial forces are required of this device as compared to a stent, which facilitates the optimization of other device characteristics such as longitudinal flexibility, trackability, and conformability. Recognition of the potential for aneurysm treatment by flow diversion is evidenced by the recent development of these devices by various groups. One such device that is furthest in development (Pipeline Neuroendovascular Device, Chestnut Medical Technologies, Menlo Park, CA) consists of 16 platinum and 16 stainless steel filaments that are braided to a 70% porosity. The treatment of 17 rabbit elastase-induced aneurysms with this device showed complete to near-complete angiographic occlusion in 5 of 6, 5 of 5, and 5 of 6 aneurysms followed-up at 1 month, 3 months, and 6 months, respectively.17 Jailed vertebral arteries were patent in all cases. Histological sections of the aneurysm showed acute unorganized thrombus at the dome in all animals at 1 month, poorly organized thrombus in 3/4 animals and organized connective tissue in 1/4 animals at 3 months, and poorly organized thrombus in 4/6 and organized connective tissue in 2/6 animals at 6 months. By comparison, the flow diversion devices used in this report resulted in complete organization of aneurysm contents in 2/4, 2/5, and 3/6 of animals at 3 weeks, 3 months, and 6 months, respectively. The flow diversion devices seem to perform better than the Pipeline device based on these data. Neointimal thicknesses within both devices are in the same range. Further studies specifically aimed at device comparisons are required to judge the equivalency of our flow diversion devices and these other devices.
The jailing of perforators, which are side branches of intracranial arteries ranging from 100 μm to 1 mm, by device struts was simulated by implanting the devices across the vertebral artery ostium originating adjacent to the aneurysm. This entrance diameter is probably on the higher side of actual perforator ostia, but none of the vertebral arteries were occluded angiographically or histologically through 180 days after device implantation. An in vitro study with flow diversion devices having the same nominal porosities as the ones used here showed that the maximal reduction in mean flow rate through the vertebral artery was about 15%.21 Although it has been suggested that perforators remain patent if less than 50% of their ostial diameter is covered by the device mesh,26 methodological (possibly bench-top) studies on the effect of varying device porosities covering 100–200 μm diameter side branches are required to identify the critical ranges of device parameters vis-à-vis perforator filling and/or occlusion. More than three-quarters of the luminal surface of all devices was noted to be lined with endothelial cells on all histological sections at all follow-up time points, which is evidence of minimal injury to the arterial wall produced by these self-expanding devices. Further, the neointimal thickness within a given device did not change significantly over the follow-up period of three weeks to six months. These results suggest stable incorporation of the flow diversion device within the arterial lumen within a few weeks.
Our results suggest that the device with the highest pore density (device III) performed the best, even in comparison with devices with the same (device I) or lower (device II) nominal porosities. Presumably, the finer meshes implied by higher pore densities further stratify the flow exchange (flow movement perpendicular to the mesh) between the parent vessel and the aneurysm, which leads to delayed washout of blood and increased probability of intraaneurysmal thrombosis. Further refinement of the best device by decreasing its filament diameter and concomitantly maximizing its pore density to an optimum value may lead to yet superior success rates. Patency of cerebral perforators must, however, be considered while increasing device pore density because pore size reductions below orders of cellular lengths may allow neointimal cells to bridge the gap between device struts and completely cover perforator ostia.
The 97% aneurysm occlusion rate used to stratify the prognostic test for treatment success based on the washout coefficient was a post-hoc measure chosen to group enough number of animals with complete to near-complete occlusion and reach a significant difference between groups. Because occlusion percentages were grouped over the follow-up times, the test only predicts treatment success at 180 days (longest follow-up time used in this study). Also, the washout coefficient value for a control aneurysm (no flow diverter) would be 1, so the test cannot be used to evaluate cases for which the value comes to be <=1 after flow diverter implantation. The sensitivity of the test (73%) is probably not high enough to guide clinical course of management. The true negative rate (82%) is less crucial because if the aneurysm actually occludes after the test predicts that it will not (W > 30), there is negligible loss in patient care. The physician may choose to take additional treatment steps (multiple devices or coiling) if the test is negative. In either case, the test can at least supplement the physician’s experience in evaluating the next course of action. As mentioned previously, optimization of the washout curves and the prognostic test based on the washout coefficient can be performed immediately after device deployment, while the patient is on the angiographic table. This test is based on a data set of experimental aneurysms and its validity will have to be evaluated on large sets of clinical data.
SUMMARY
Three novel flow diversion device configurations developed and refined based on past experience were tested in a large cohort of experimental aneurysms. Successful and stable aneurysm occlusion was effected by the devices over the study period of six months. Results suggest that the device with a porosity of 70% and pore density of 18 pores/mm2 performed better than devices of 65% porosity, 14 pores/mm2 and 70% porosity, 12 pores/mm2. The pore density of flow diversion devices may thus be the critical factor modulating treatment success. Angiographic quantification suggested a parameter, called the washout coefficient here, which could be employed to estimate aneurysm occlusion probabilities immediately after the treatment and thus preclude unnecessary additional treatment manipulations. Based on the aneurysms treated and the devices used, a value of this parameter less than 30 predicts greater than 97% angiographic occlusion of the aneurysm over a period of six months. Generalization of this threshold value to the treatment of any given aneurysm by flow diversion requires further testing.
Acknowledgments
SOURCES OF FUNDING
The study was supported by a National Institutes of Health grant (NS045753-01A1) and Siemens Medical Solutions.
References
- 1.de Rooij NK, Linn FH, van der Plas JA, et al. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365–72. doi: 10.1136/jnnp.2007.117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hop JW, Rinkel GJ, Algra A, et al. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–4. doi: 10.1161/01.str.28.3.660. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–17. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 4.Pelz DM, Levy EI, Hopkins LN. Advances in interventional neuroradiology 2007. Stroke. 2008;39:268–72. doi: 10.1161/STROKEAHA.107.510628. [DOI] [PubMed] [Google Scholar]
- 5.Higashida RT, Halbach VV, Dowd CF, et al. Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: the Cordis Enterprise stent. AJNR Am J Neuroradiol. 2005;26:1751–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms: results at initial (3–6-mo) follow-up. Neurosurgery. 2005;56:1191–201. doi: 10.1227/01.neu.0000159645.86823.af. [DOI] [PubMed] [Google Scholar]
- 7.Wakhloo AK, Mandell J, Gounis MJ, et al. Stent-assisted reconstructive endovascular repair of cranial fusiform atherosclerotic and dissecting aneurysms - Long-term clinical and angiographic follow-up. Stroke. 2008 doi: 10.1161/STROKEAHA.107.512996. Accepted. [DOI] [PubMed] [Google Scholar]
- 8.Wakhloo AK, Shellhammer F, de Vries J, et al. Coated and non-coated stents for vessel reconstruction and treatment of aneurysms and av-fistulas: An experimental study. ESNR 92; XVIIIth Congress of the European Society of Neuroradiology; 1992. p. S24. Abstract. [Google Scholar]
- 9.Turjman F, Acevedo G, Moll T, et al. Treatment of experimental carotid aneurysms by endoprosthesis implantation: preliminary report. Neurol Res. 1993;15:181–4. doi: 10.1080/01616412.1993.11740132. [DOI] [PubMed] [Google Scholar]
- 10.Wakhloo AK, Schellhammer F, de Vries J, et al. Self-expanding and balloon-expandable stents in the treatment of carotid aneurysms: an experimental study in a canine model. AJNR Am J Neuroradiol. 1994;15:493–502. [PMC free article] [PubMed] [Google Scholar]
- 11.Geremia G, Haklin M, Brennecke L. Embolization of experimentally created aneurysms with intravascular stent devices. AJNR Am J Neuroradiol. 1994;15:1223–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Doerfler A, Wanke I, Egelhof T, et al. Double-stent method: therapeutic alternative for small wide-necked aneurysms. J Neurosurg. 2004;100:150–4. doi: 10.3171/jns.2004.100.1.0150. [DOI] [PubMed] [Google Scholar]
- 13.Zenteno MA, Murillo-Bonilla LM, Guinto G, et al. Sole stenting bypass for the treatment of vertebral artery aneurysms: technical case report. Neurosurgery. 2005;57:E208. doi: 10.1227/01.neu.0000163683.64511.24. [DOI] [PubMed] [Google Scholar]
- 14.Szikora I, Berentei Z, Kulcsar Z, et al. Endovascular treatment of intracranial aneurysms with parent vessel reconstruction using balloon and self expandable stents. Acta Neurochir (Wien) 2006;148:711–23. doi: 10.1007/s00701-006-0785-6. [DOI] [PubMed] [Google Scholar]
- 15.Lanzino G, Wakhloo AK, Fessler RD, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91:538–46. doi: 10.3171/jns.1999.91.4.0538. [DOI] [PubMed] [Google Scholar]
- 16.Ionita CN, Paciorek AM, Hoffmann KR, et al. Asymmetric Vascular Stent. Feasibility Study of a New Low-Porosity Patch-Containing Stent. Stroke. 2008;39:2105–13. doi: 10.1161/STROKEAHA.107.503862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38:2346–52. doi: 10.1161/STROKEAHA.106.479576. [DOI] [PubMed] [Google Scholar]
- 18.Ahlhelm F, Roth C, Kaufmann R, et al. Treatment of wide-necked intracranial aneurysms with a novel self-expanding two-zonal endovascular stent device. Neuroradiology. 2007;49:1023–8. doi: 10.1007/s00234-007-0281-6. [DOI] [PubMed] [Google Scholar]
- 19.Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting: influence of stent porosity. Ann Biomed Eng. 1997;25:460–9. doi: 10.1007/BF02684187. [DOI] [PubMed] [Google Scholar]
- 20.Lieber BB, Livescu V, Hopkins LN, et al. Particle image velocimetry assessment of stent design influence on intra-aneurysmal flow. Ann Biomed Eng. 2002;30:768–77. doi: 10.1114/1.1495867. [DOI] [PubMed] [Google Scholar]
- 21.Seong J, Wakhloo AK, Lieber BB. In vitro evaluation of flow divertors in an elastase-induced saccular aneurysm model in rabbit. J Biomech Eng. 2007;129:863–72. doi: 10.1115/1.2800787. [DOI] [PubMed] [Google Scholar]
- 22.Lieber BB, Sadasivan C, Miskolczi L, et al. Flow divertors to treat cerebral aneurysms: preliminary results in the rabbit elastase-induced aneurysm model. Proceedings of the 2006 Summer Bioengineering Conference; 2006. [Google Scholar]
- 23.Miskolczi L, Gounis MJ, Onizuka M, et al. Elastase-induced saccular aneurysms in rabbits: Instructions 'for the rest of us'. Proceedings of the 42nd Annual Meeting American Society of Neuroradiology; 2004. p. 352. Abstract. [Google Scholar]
- 24.Sadasivan C, Lieber BB, Gounis MJ, et al. Angiographic quantification of contrast medium washout from cerebral aneurysms after stent placement. AJNR Am J Neuroradiol. 2002;23:1214–21. [PMC free article] [PubMed] [Google Scholar]
- 25.Seong J, Sadasivan C, Onizuka M, et al. Morphology of elastase-induced cerebral aneurysm model in rabbit and rapid prototyping of elastomeric transparent replicas. Biorheology. 2005;42:345–61. [PubMed] [Google Scholar]
- 26.Lopes DK, Ringer AJ, Boulos AS, et al. Fate of branch arteries after intracranial stenting. Neurosurgery. 2003;52:1275–8. doi: 10.1227/01.neu.0000064567.15270.27. [DOI] [PubMed] [Google Scholar]