Abstract
Objectives
Thyrotropin-releasing hormone (TRH) is expressed in rodent and human adult pancreas, and in mouse pancreas during embryonic development. However, expression of TRH receptors (TRHRs) in the pancreas is controversial. We sought to provide evidence that the TRH/TRHR system might play a role in fetal development.
Methods
We used quantitative RT-PCR to measure TRH and TRHR mRNA. To study the effects of TRHR expression in a pancreatic progenitor population, we expressed TRHRs in human islet-derived precursor cells (hIPCs) by infection with adenoviral vector AdCMVmTRHR. TRHR signaling was measured as inositol phosphate production and intracellular calcium transients. TRHR expression was measured by [3H]methyl-TRH binding. Apoptosis was monitored by release of cytochrome C from mitochondria.
Results
We show that TRH mRNA is expressed in human fetal and adult pancreas, and that TRHR mRNA is expressed in fetal human pancreas but not in adult human pancreas. TRHRs expressed in hIPCs were shown to signal normally. Most importantly, TRH treatment for several days stimulated apoptosis in hIPCs expressing approximately 400,000 TRHRs per cell.
Conclusions
These findings suggest a possible role for TRH/TRHR signaling in pancreatic precursors to promote programmed cell death, a normal constituent of morphogenesis during embryonic development in humans.
Keywords: thyrotropin-releasing hormone, thyrotropin-releasing hormone receptor, programmed cell death, embryonic development
Introduction
Pancreatic islet precursor cells that may be used for cell replacement therapy for treatment of patients with type 1 diabetes mellitus are being studied intensively (for review, see (1)). Identification of cell surface receptors and their signaling pathways that contribute to the development of pancreatic islets of Langerhans and may be active in precursor cells is important because it may provide new insights into ways of controlling expansion, survival and differentiation of precursor cells in vitro. A number of seven transmembrane-spanning (or G protein-coupled) receptors have been shown to be expressed on islets and to regulate islet function (2). Thyrotropin-releasing hormone (TRH) is a tripeptide hormone originally identified in hypothalamic neurons (3,4) and secreted into the hypothalamic-hypophyseal portal blood. TRH binds to cell-surface TRH receptors (TRHRs), which are seven-transmembrane spanning receptors, on thyrotrophs and mammotrophs in the anterior pituitary gland and activates G proteins Gq/G11 leading to activation of phospholipase C and a subsequent increase in intracellular free calcium. In the anterior pituitary, TRHR activation stimulates the production and secretion of thyroid-stimulating hormone and prolactin (5,6). However, TRH is also expressed in many extrahypothalamic tissues including other areas of the brain, the cardiovascular and reproductive systems, and the gastrointestinal tract (7,8). TRHR expression has also been identified outside the pituitary gland in several regions of the central nervous system and outside the central nervous system in the retina and testis (9–12).
TRH has been shown to be expressed in rodent and human fetal and adult pancreas (12–17). Indeed, electron microscopy has revealed that TRH is co-localized with insulin in secretory granules in β cells in fetal rat islets (17,18). In contrast, it is controversial whether TRHRs are expressed in the normal pancreas (12,13) even though several lines of evidence suggest a possible pancreatic role for TRHR signaling (19). Perhaps the most compelling data in this regard are the alterations in glucose homeostasis that were observed in TRH-knockout mice (20) that are consistent with the idea that the TRH/TRHR system plays a role in embryonic development or adult function of β cells, or both.
We (21) and others (22–24) have shown that cells can be derived from adult human islets to produce a population of proliferating precursors cells. We named the cells derived using our protocol human islet-derived precursor cells (hIPCs) (25). hIPCs can be induced by serum deprivation to differentiate into hormone-expressing cells. Because in vitro formation, expansion and differentiation of hIPCs are currently not optimal, methods to improve the viability and endocrine differentiation of these cells are being sought.
In this study, we show that both TRH and TRHR transcripts are expressed in human fetal pancreas but only TRH transcripts were found in adult pancreas. We hypothesize that TRHR signaling may be involved in embryonic development of islets. In an effort to assess the potential effects of TRHR signaling in pancreatic precursor cells, we expressed TRHRs in hIPCs. We show that TRH stimulates programmed cell death (PCD) of hIPCs expressing high levels of TRHRs.
Materials and Methods
Cells and media
hIPCs were derived from islets isolated from human cadaveric pancreas and cultured in CMRL-1066 (GIBCO) supplemented with 10% fetal bovine serum as previously described (21). For survival assays, serum-free medium (SFM) was composed of CMRL-1066 medium supplemented with 1% bovine serum albumin, insulin (10 µg/ml), transferrin (5.5 µg/ml) and sodium selenite (6.7 ng/ml). Human fetal pancreases at 19–22 weeks of gestation were obtained from Advanced Bioscience Resources (Oakland, Ca). Informed consent for tissue donation was obtained by the procurement center.
Real time quantitative RT-PCR (qRT-PCR)
Total RNA was purified, reverse-transcribed and transcript levels of selected genes were measured by qRT-PCR as described (21). Briefly, total RNA was purified using Trizol (Invitrogen) and first strand cDNA was prepared using a High Capacity cDNA Archive Kit (Applied Biosystems). Finally, PCR was performed in 25 µl reactions in 96-well plates using cDNA prepared from 100 ng of total RNA and Universal PCR Master Mix (Applied Biosystems). Primers and probes were Assay-on-Demand (Applied Biosystems).
Infection with AdCMVmTRHR and Ad5GFP
To achieve efficient infection, monodispersed hIPCs at 400,000 cells/ml were incubated with viral constructs AdCMVmTRHR expressing mouse TRHR1 (mTRHR) or Ad5GFP expressing green fluorescent protein (GFP) in SFM without bovine serum albumin. We used mouse TRHR1 because it is the mouse homolog of human TRHR and we find it at higher levels than human TRHRs when expressed ectopically in mammalian cells in culture. Infection multiples ranged from 100 to 10,000 viral particles per cell. The cells were maintained in suspension for 1 h by agitation on a rotating plate shaker, after which 3 volumes of medium with fetal bovine serum was added to the dish to promote cell adhesion. Expression of TRHR was maximal 2–3 days after infection.
TRH receptor binding assay
hIPCs were infected with AdCMVmTRHR and seeded in 6-well plates at 200,000 cells per well. After 2–3 days, the medium was aspirated and wells were washed 3 times with HBSS with 10 mM HEPES, pH 7.4, and incubated at 37°C for 90 min in buffered HBSS containing 1.5 ml of 2 nM [3H]methyl TRH (MeTRH) (87 Ci/mmol). Subsequently, dishes were placed on ice, washed 3 times with cold HBSS and cells dissolved in 0.4 N NaOH. Bound [3H]MeTRH was determined by scintillation counting using an external standard to assess efficiency. Nonspecific binding was determined by assaying cells which had not been infected with AdCMVmTRHR.
Determination of cell number
Adherent viable cells in 6-well plates were assessed as follows. Media and floating debris was aspirated and 1 ml of 0.05% trypsin/EDTA (Cellgro) was added to each well and cells were incubated at 37°C for 2–5 min. Detached cells were removed and the wells were rinsed with an additional aliquot of serum-containing medium (SCM). After centrifugation for 5 min, cells were resuspended in 0.6 ml of HBSS. Total and viable cell counts were determined using a Beckman Coulter Vi-cell.
Determination of average receptor number per cell
For each dose of viral particles, triplicate wells were assayed to determine the number of viable cells per well and triplicate wells were assayed for specifically bound [3H]MeTRH. To determine the average receptors per cell, the number of molecules of [3H]MeTRH bound were calculated from the specific activity of MeTRH, adjusted for the expected occupancy of TRHR with 2 nM MeTRH (Kd ~ 2 nM) and divided by the average number of viable cells per well.
Measurement of inositol phosphates
Cells were labeled for 2 days with myo-[3H]inositol in SCM beginning at the time of infection with adenovirus, washed 3 times in buffered HBSS and incubated in the same solution with 10mM LiCl without or with 1µM TRH for 2 h at 37°C. [3H]inositol phosphates were measured as previously described (26).
Determination of intracellular free calcium
Cells were infected in suspension and then allowed to adhere overnight at 50% confluence on #1.5 coverglass slides placed in 6-well plates. Cells were loaded with Fura 2-AM (5 µM, Molecular Probes) for 30 to 60 min in SCM. Acquisition and analysis of 340 nm/380 nm excitation ratio at 510 nm emission was performed as described (27). The rate of acquisition was 2–4 ratio images/s. Data are presented as changes in the fluorescence ratio of individual cells.
Confocal Microscopy
hIPCs were infected and seeded on plastic Labtec slides at a density of 50,000–100,000 cells per chamber. After 24 h incubation in SFM without or with 1 µM TRH, cells were washed with DPBS and fixed in 4% paraformaldehyde and stored at 4°C for at least 4 h. Fixed hIPCs were washed in DPBS and permeabilized with chilled 50% methanol. After a 15 minute permeabilization, hIPCs were washed with DPBS, incubated in donkey serum for 30 min to block non-specific binding and then incubated overnight in primary mouse anti-cytochrome C (Zymed Laboratories inc.) at 4°C. The following day, cells were rinsed in DPBS and incubated at 37°C for 1.5 h in secondary antibody Alexa-Fluor 488 for cytochrome c and in primary Alexa-Fluor 633-phalloidin (Molecular Probes, OR) for f-actin staining. Slides were then washed extensively in DPBS and mounted in Mowiol containing Hoechst-33342 for nuclear staining. Confocal images were captured with a Zeiss LSM 510 Meta laser scanning inverted microscope using a 25X/1.3 oil objective with optical slices less than 0.6 µm. Magnification, laser and detector gains were identical across samples. Results presented are representative fields confirmed from at least 6 biological duplicates.
Results
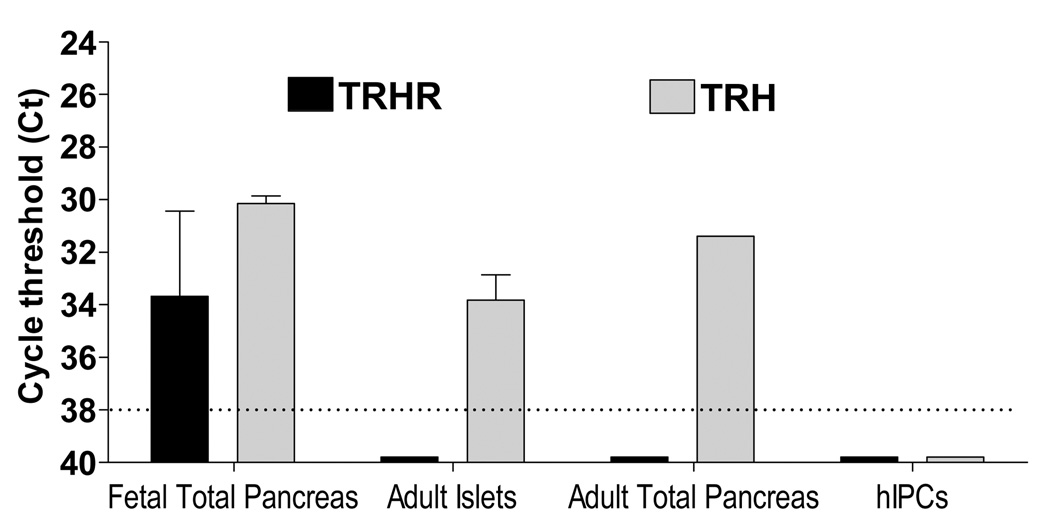
mRNAs for TRHR and TRH were measured by quantitative RT-PCR (qRT-PCR) in tissue samples from human fetal and adult pancreas (Fig. 1). TRHR transcript was detected at cycle threshold (Ct) of 33.7 ± 3.2 in 100 ng of total RNA isolated from samples of fetal pancreas ranging from 14 to 21 weeks of gestation. In contrast, TRHR mRNA was not detected (Ct ≥ 38) in several human adult islet preparations from cadaveric donor pancreases, from adult human total pancreas, from several populations of hIPCs derived from different donors and from human pancreatic cancer Panc-1 cells (not shown). Unlike the receptor transcript, TRH mRNA was present at Ct values of 30.2 ± 0.7 in fetal pancreas, 33.8 ± 1.9 in adult human islets and 31.4 in adult total pancreas; no TRH mRNA was detected in hIPCs.
Figure 1. TRHR and TRH mRNA levels in human fetal and adult pancreas, and hIPCs.
TRHR and TRH transcripts were measured by qRT-PCR using 100 ng total RNA from different tissue samples of human fetal and adult pancreas, and hIPCs. Fetal total pancreas is an average from assays performed on pancreas samples from fetuses between 14 to 21 weeks of gestation. The dotted line represents the limit of sensitivity of the assay. The data shown are mean ± SD of samples measured in duplicate and normalized to expression of the housekeeping gene GAPDH.
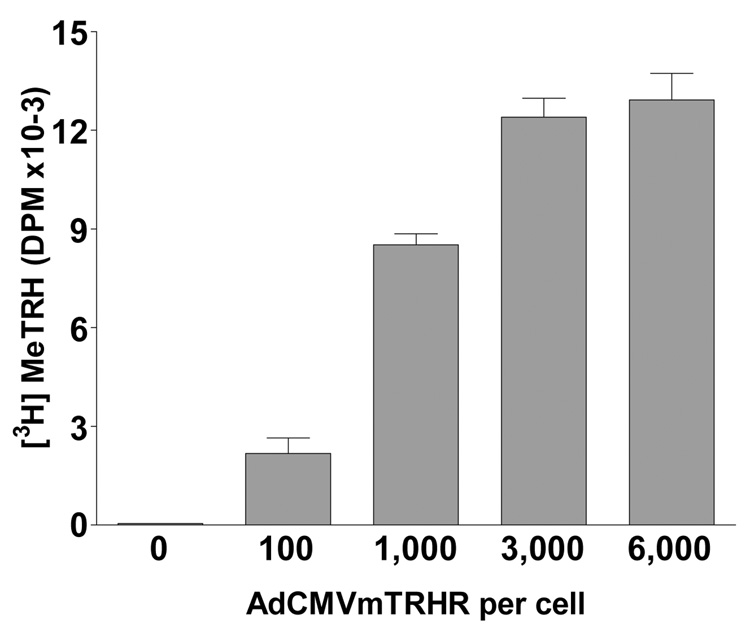
Because we found TRHR transcripts in human fetal pancreas, we hypothesized that TRHR signaling may play a role in the development of islets. As hIPCs did not express TRHRs, TRHRs were ectopically expressed using AdCMVmTRHR (Fig. 2). TRHR expression was time-dependent and was easily detected at 20 h after infection (not shown). Analysis by flow cytometry of hIPCs infected with a GFP-expressing adenovirus revealed that greater than 75% of cells expressed GFP within 48 h of infection and that 90% of the cells were GFP-positive 6 days after infection when maintained in a growth inhibitory serum-free media (data not shown). Binding assays with [3H]MeTRH, an analog with greater affinity for TRHR than TRH, demonstrated specific binding that was dependent on the dose of AdCMVmTRHR particles. hIPCs not infected or infected with a control vector showed no specific binding. Approximately 400,000 TRHRs were expressed per cell following infection with 3000 AdCMVmTRHR particles per cell.
Figure 2. AdCMVmTRHR-mediated expression of TRHRs in hIPCs.
Binding of 2 nM [3H]MeTRH was measured in hIPCs 48 h after infection with AdCMVmTRHR. The data represent the mean ± SD of triplicate measurements in a representative experiment.
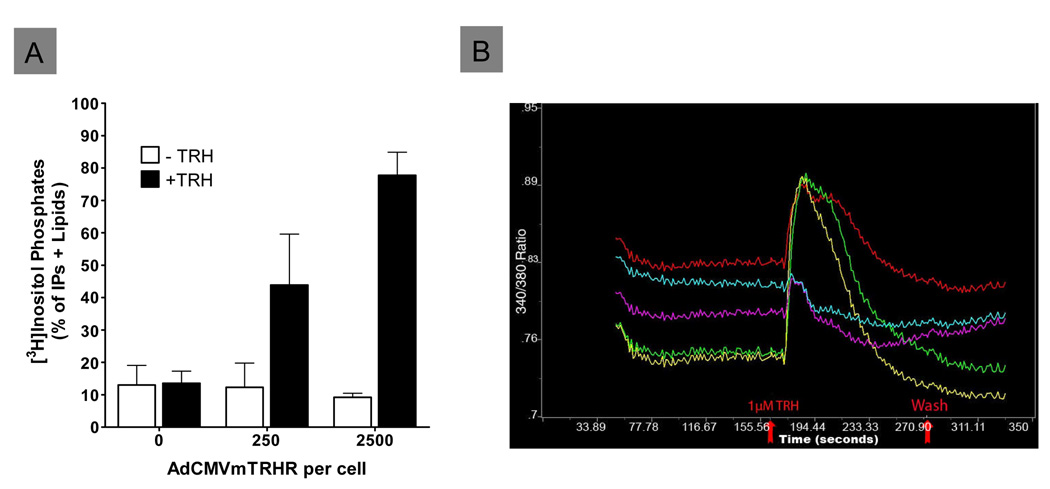
hIPCs infected with AdCMVmTRHR and stimulated with TRH exhibited activation of the phosphoinositide pathway leading to accumulation of [3H]inositol phosphates when cells were pre-incubated with myo-[3H]inositol to label phosphoinositide substrates (Fig. 3A). Increases in accumulation of inositol phosphate second messengers of 3- or 5-fold over basal levels were observed in response to TRH in hIPCs infected with 250 or 2500 particles per cell, respectively. TRH-stimulated inositol phosphate accumulation may have been limited by the available pool of labeled phosphatidylinositol-4,5-bisphosphate. Consistent with the low basal signaling of TRHR, cells expressing high levels of receptor did not exhibit increased inositol phosphate accumulation in the absence of TRH. hIPCs infected with AdCMVmTRHR demonstrated TRH-stimulated increases in intracellular free calcium concentration also (Fig. 3B). Only cells infected with AdCMVmTRHR exhibited calcium mobilization after TRH treatment (not shown). These results demonstrate that TRHRs expressed in hIPCs following infection with AdCMVmTRHR are functional and signal appropriately through Gq.
Figure 3. Functional analysis of TRHRs in hIPCs.
A) [3H]Inositol phosphate accumulation was measured in hIPCs prelabeled with [3H]inositol and infected with 250 or 2500 AdCMVmTRHR particles per cell during a 60 min incubation without or with 1 µM TRH. B) Intracellular free Ca++ concentration was measured in real-time by epifluorescence microscopy using the fluorescent Ca++ indicator Fura-2. The sharp rise in Ca++ levels after TRH stimulation followed by a staggered fall to below basal levels is typical of Gq signaling.
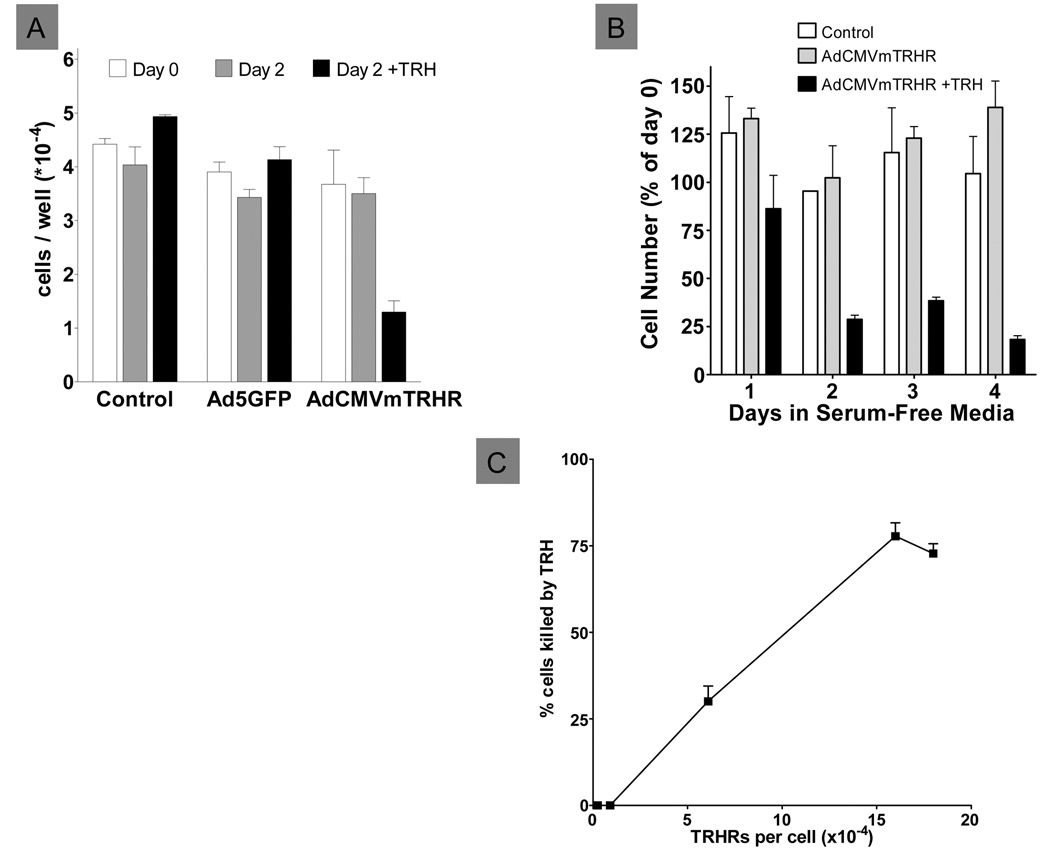
The effects of TRH on cell viability of hIPCs expressing TRHRs were assessed in SFM. Uninfected hIPCs or hIPCs infected with Ad5GFP or AdCMVmTRHR were incubated in SFM for 2 days (Fig. 4A). Without TRH treatment, the number of adherent viable cells remained roughly constant over the 2 day incubation in all cells. In contrast, treatment with TRH for 2 days reduced the number hIPCs expressing TRHRs but had no effect on uninfected cells or in cells expressing GFP. A time course study (Fig. 4B) revealed that TRH reduced TRHR-expressing cells significantly within one day and further reduced the number of surviving cells at day 2 to a level that remained similar through day 4. In contrast, cells expressing TRHRs but not treated with TRH did not decline and remained similar in number to control uninfected cells throughout the 4 day period. Cell death in response to TRH treatment in SFM was found to be dependent on the level of TRHR expression (Fig. 4C).
Figure 4. Effects of TRH on the viability of hIPCs expressing TRHR.
A) Uninfected hIPCs (Control) or hIPCs infected with 3,000 particles of AdCMVmTRHR or Ad5GFP per cell were incubated in SFM without or with 1 µM TRH for 2 days. Data represent the mean ± SD of triplicate measurements in a representative experiment. B) Time course of the effect of TRH in uninfected hIPCs (Control) and in AdCMVmTRHR-infected hIPCs incubated without or with 1 µM TRH. Data represent the mean ± SD of triplicate measurements in a representative experiment. C) TRH-mediated cell death was dependent on the level of TRHR expression. Data represent the mean±SE of triplicate measurements in 4 experiments.
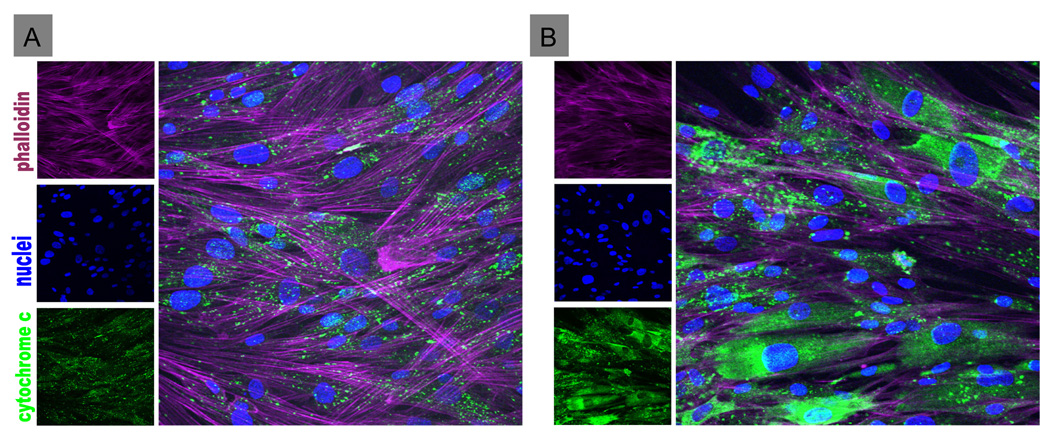
Lastly, we showed that the decrease in viable cell number was caused by TRH-induced apoptosis, one form of PCD. We monitored leakage of cytochrome C from mitochondria as an index of apoptosis. hIPCs were incubated in serum-free medium without or with TRH for 24 h. Control cells or untreated TRHR-expressing cells showed punctate cytochrome C staining, indicative of mitochondrial stores of cytochrome C (Fig. 5). By contrast, TRH-treated TRHRexpressing hIPCs demonstrated cytochrome C staining dispersed throughout the cytoplasm.
Figure 5. Effect of TRH on apoptosis of hIPCs as monitored by release of mitochondrial cytochrome C into the cytoplasm.
Uninfected hIPCs (A) or hIPCs infected with 3,000 particles of AdCMVmTRHR per cell (B) were incubated in serum-free medium without (A) or with 1 µM TRH (B) for 24 h and then fixed and stained for f-actin, cytochrome C and nuclei. TRHR-expressing cells not treated with TRH (not shown) were similar to uninfected hIPCs (A) demonstrating punctate cytochrome C staining indicating mitochondrial localization. TRHR-expressing hIPCs treated with TRH (B) showed dispersed cytoplasmic staining indicating leakage of cytochrome C from mitochondria.
Discussion
Our data show that TRHR-mediated PCD occurs only in cells expressing high levels of TRHRs. Our measurements of TRH expression in the fetal pancreas are consistent with the idea that there may be high expression of TRHR transcripts, which could lead to expression of functional TRHRs, in a subset of cells. Our conditions for qRT-PCR using 100 ng of total RNA, which corresponds to about 3,000 cells, results in a Ct value of approximately 18 for the housekeeping gene GAPDH. Any transcript with a Ct value greater than 37 is considered undetectable and can be interpreted to indicate that less than 800 copies of target gene mRNA were present in the reaction (28). Thus, the undetectable TRHR transcript in adult human islets or total pancreas suggests that there were fewer than 800 transcripts per 3,000 cells or less than 1 transcript per four cells. In contrast, TRHR transcript in fetal pancreas gave a Ct value of approximately 34 or 4 cycles above the undetectable level which reflects at least 24 = 16-fold more transcript in fetal human pancreas than in adult human islets. If distributed evenly in the 3,000 cell sample, this would yield an average transcript content of 800 × 16 / 3000 ≈ 4 copies per cell. However, if only a subset of the diverse population of exocrine, vascular and endocrine cells within the developing human pancreas expresses TRHRs, transcript levels would be proportionally higher and yield a level of TRHRs sufficient to cause PCD when activated by TRH.
PCD is a critical aspect of embryologic development that can be viewed as “eliminating unwanted cells… (during) 1) sculpting structures; 2) deleting unneeded structures; 3) controlling cell numbers; 4) eliminating abnormal, misplaced, nonfunctional or harmful cells; and 5) producing differentiated cells without organelles” (29). In many cases, PCD is initiated by signals from extracellular regulatory molecules such as hormones and growth factors. For example, induction of PCD in tadpoles at metamorphosis by thyroid hormones causes tail resorption and in developing chick embryos by nerve growth factor in cells of the early retina eliminates unwanted cells. Our findings of both TRH and TRHR expression in human fetal pancreas suggested that this ligand/receptor system might mediate PCD in developing islets. As there is no facile in vitro model of developing human islets that expresses TRHRs, we expressed TRHRs using adenovirus-mediated gene transfer in hIPCs. We show that TRH signals via the phosphoinositide/calcium transduction system and induces PCD in hIPCs expressing high levels of TRHRs. It is noteworthy that calcium signaling is an important pathway for transduction of signals leading to PCD (30). Although we were unable to measure TRHR number in fetal pancreatic cells, it is possible that TRHRs are expressed at levels sufficient to cause PCD in a subpopulation of fetal pancreas. Thus, we suggest that TRH may be an autocrine/paracrine factor that induces PCD during human islet development.
Acknowledgments
We thank the National Islet Cell Resource Program (Duarte, CA) for supplying human islets. This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
This work was supported by the Intramural Research Program, NIDDK, NIH.
References
- 1.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 2.Winzell MS, Ahren B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther. 2007;116:437–448. doi: 10.1016/j.pharmthera.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Boler J, Enzmann F, Folkers K, et al. The identity of chemical and hormonal properties of the thyrotropin releasing hormone and pyroglutamyl-histidyl-proline amide. Biochem Biophys Res Commun. 1969;37:705–710. doi: 10.1016/0006-291x(69)90868-7. [DOI] [PubMed] [Google Scholar]
- 4.Burgus R, Dunn TF, Desiderio D, et al. Molecular structure of the hypothalamic hypophysiotropic TRF factor of ovine origin: mass spectrometry demonstration of the PCA-His-Pro-NH2 sequence. C R Acad Sci Hebd Seances Acad Sci D. 1969;269:1870–1873. [PubMed] [Google Scholar]
- 5.Steinfelder HJ, Hauser P, Nakayama Y, et al. Thyrotropin-releasing hormone regulation of human TSHB expression: role of a pituitary-specific transcription factor (Pit-1/GHF-1) and potential interaction with a thyroid hormone-inhibitory element. Proc Natl Acad Sci U S A. 1991;88:3130–3134. doi: 10.1073/pnas.88.8.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris AR, Christianson D, Smith MS, et al. The physiological role of thyrotropin-releasing hormone in the regulation of thyroid-stimulating hormone and prolactin secretion in the rat. J Clin Invest. 1978;61:441–448. doi: 10.1172/JCI108955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson IM. Thyrotropin-releasing hormone. N Engl J Med. 1982;306:145–155. doi: 10.1056/NEJM198201213060305. [DOI] [PubMed] [Google Scholar]
- 8.Morley JE. Extrahypothalamic thyrotropin releasing hormone (TRH) -- its distribution and its functions. Life Sci. 1979;25:1539–1550. doi: 10.1016/0024-3205(79)90435-1. [DOI] [PubMed] [Google Scholar]
- 9.Leduque P, Bulant M, Dubois PM, et al. Processing of thyrotropin-releasing hormone prohormone (pro-TRH) in the adult rat pancreas: identification and localization of pro-TRH-related peptides in beta-cells of pancreatic islets. Endocrinology. 1989;125:1492–1497. doi: 10.1210/endo-125-3-1492. [DOI] [PubMed] [Google Scholar]
- 10.Satoh T, Feng P, Kim UJ, et al. Identification of thyrotropin-releasing hormone receptor messenger RNA in the rat central nervous system and eye. Brain Res Mol Brain Res. 1993;19:175–178. doi: 10.1016/0169-328x(93)90165-l. [DOI] [PubMed] [Google Scholar]
- 11.Satoh T, Feng P, Kim UJ, et al. Identification of thyrotropin-releasing hormone receptor in the rat testis. Neuropeptides. 1994;27:195–202. doi: 10.1016/0143-4179(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 12.Yamada M, Shibusawa N, Hashida T, et al. Expression of thyrotropin-releasing hormone (TRH) receptor subtype 1 in mouse pancreatic islets and HIT-T15, an insulin-secreting clonal beta cell line. Life Sci. 2000;66:1119–1125. doi: 10.1016/s0024-3205(00)00415-x. [DOI] [PubMed] [Google Scholar]
- 13.Luo LG, Yano N. Expression of thyrotropin-releasing hormone receptor in immortalized beta-cell lines and rat pancreas. J Endocrinol. 2004;181:401–412. doi: 10.1677/joe.0.1810401. [DOI] [PubMed] [Google Scholar]
- 14.Basmaciogullari A, Cras-Meneur C, Czernichow P, et al. Pancreatic pattern of expression of thyrotropin-releasing hormone during rat embryonic development. J Endocrinol. 2000;166:481–488. doi: 10.1677/joe.0.1660481. [DOI] [PubMed] [Google Scholar]
- 15.Benicky J, Strbak V. Glucose stimulates and insulin inhibits release of pancreatic TRH in vitro. Eur J Endocrinol. 2000;142:60–65. doi: 10.1530/eje.0.1420060. [DOI] [PubMed] [Google Scholar]
- 16.Martino E, Seo H, Lernmark A, et al. Ontogenetic patterns of thyrotropin-releasing hormone-like material in rat hypothalamus, pancreas, and retina: selective effect of light deprivation. Proc Natl Acad Sci U S A. 1980;77:4345–4348. doi: 10.1073/pnas.77.7.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leduque P, Aratan-Spire S, Czernichow P, et al. Ontogenesis of thyrotropin-releasing hormone in the human fetal pancreas. A combined radioimmunological and immunocytochemical study. J Clin Invest. 1986;78:1028–1034. doi: 10.1172/JCI112657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leduque P, Aratan-Spire S, Scharfmann R, et al. Coexistence of thyrotropin-releasing hormone and insulin in cultured fetal rat islets: a light and electron microscopic immunocytochemical study during islet neoformation. Biol Cell. 1989;66:291–296. [PubMed] [Google Scholar]
- 19.Kulkarni RN, Wang ZL, Akinsanya KO, et al. Pyroglutamyl-phenylalanyl-proline amide attenuates thyrotropin-releasing hormone-stimulated insulin secretion in perifused rat islets and insulin-secreting clonal beta-cell lines. Endocrinology. 1995;136:5155–5164. doi: 10.1210/endo.136.11.7588254. [DOI] [PubMed] [Google Scholar]
- 20.Yamada M, Saga Y, Shibusawa N, et al. Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc Natl Acad Sci U S A. 1997;94:10862–10867. doi: 10.1073/pnas.94.20.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gershengorn MC, Hardikar AA, Wei C, et al. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 22.Lechner A, Nolan AL, Blacken RA, et al. Redifferentiation of insulin-secreting cells after in vitro expansion of adult human pancreatic islet tissue. Biochem Biophys Res Commun. 2005;327:581–588. doi: 10.1016/j.bbrc.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 23.Gao R, Ustinov J, Korsgren O, et al. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia. 2005;48:2296–2304. doi: 10.1007/s00125-005-1935-8. [DOI] [PubMed] [Google Scholar]
- 24.Ouziel-Yahalom L, Zalzman M, Anker-Kitai L, et al. Expansion and redifferentiation of adult human pancreatic islet cells. Biochem Biophys Res Commun. 2006;341:291–298. doi: 10.1016/j.bbrc.2005.12.187. [DOI] [PubMed] [Google Scholar]
- 25.Gershengorn MC, Geras-Raaka E, Hardikar AA, et al. Are better islet cell precursors generated by epithelial-to-mesenchymal transition? Cell Cycle. 2005;4:380–382. doi: 10.4161/cc.4.3.1538. [DOI] [PubMed] [Google Scholar]
- 26.Imai A, Gershengorn MC. Measurement of lipid turnover in response to thyrotropin-releasing hormone. Methods Enzymol. 1987;141:100–101. doi: 10.1016/0076-6879(87)41059-8. [DOI] [PubMed] [Google Scholar]
- 27.Wei C, Geras-Raaka E, Marcus-Samuels B, et al. Trypsin and thrombin accelerate aggregation of human endocrine pancreas precursor cells. J Cell Physiol. 2006;206:322–328. doi: 10.1002/jcp.20459. [DOI] [PubMed] [Google Scholar]
- 28.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 30.Giorgi C, Romagnoli A, Pinton P, et al. Ca(2+) signaling, mitochondria and cell death. Curr Mol Med. 2008;8:119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]