Abstract
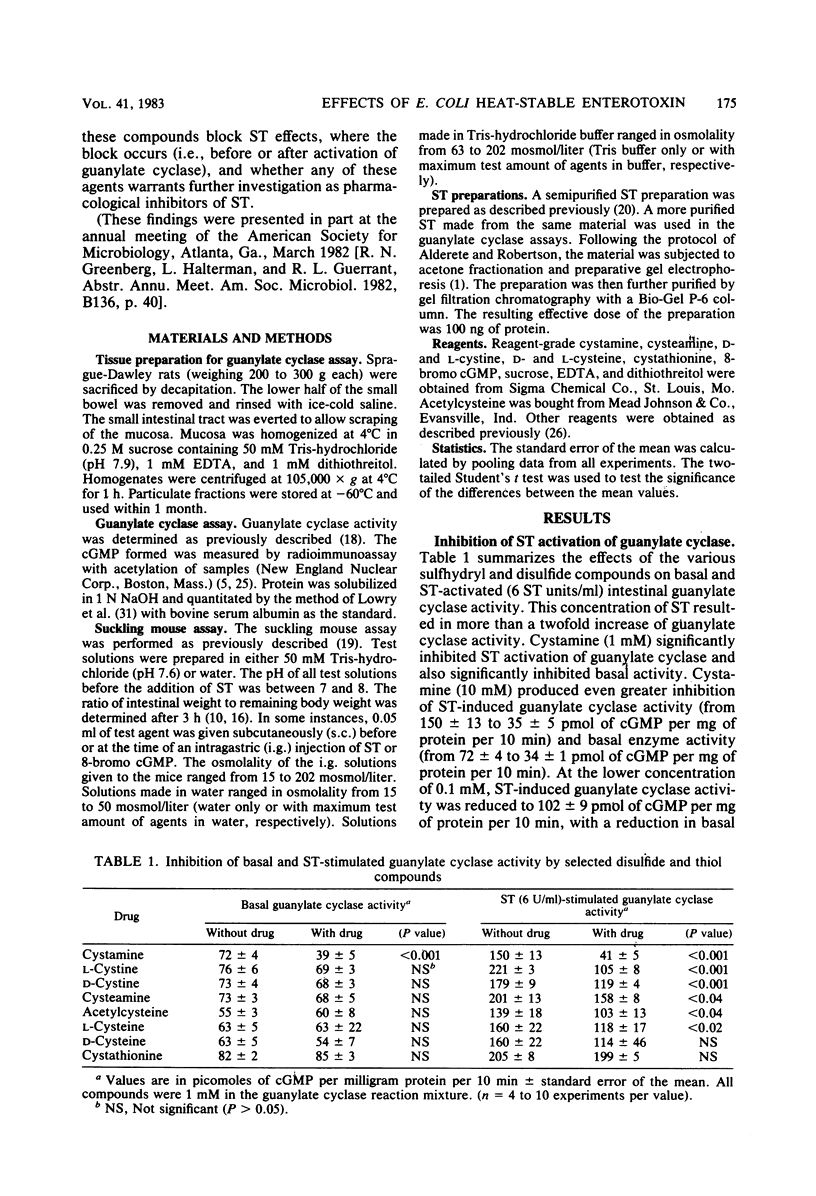
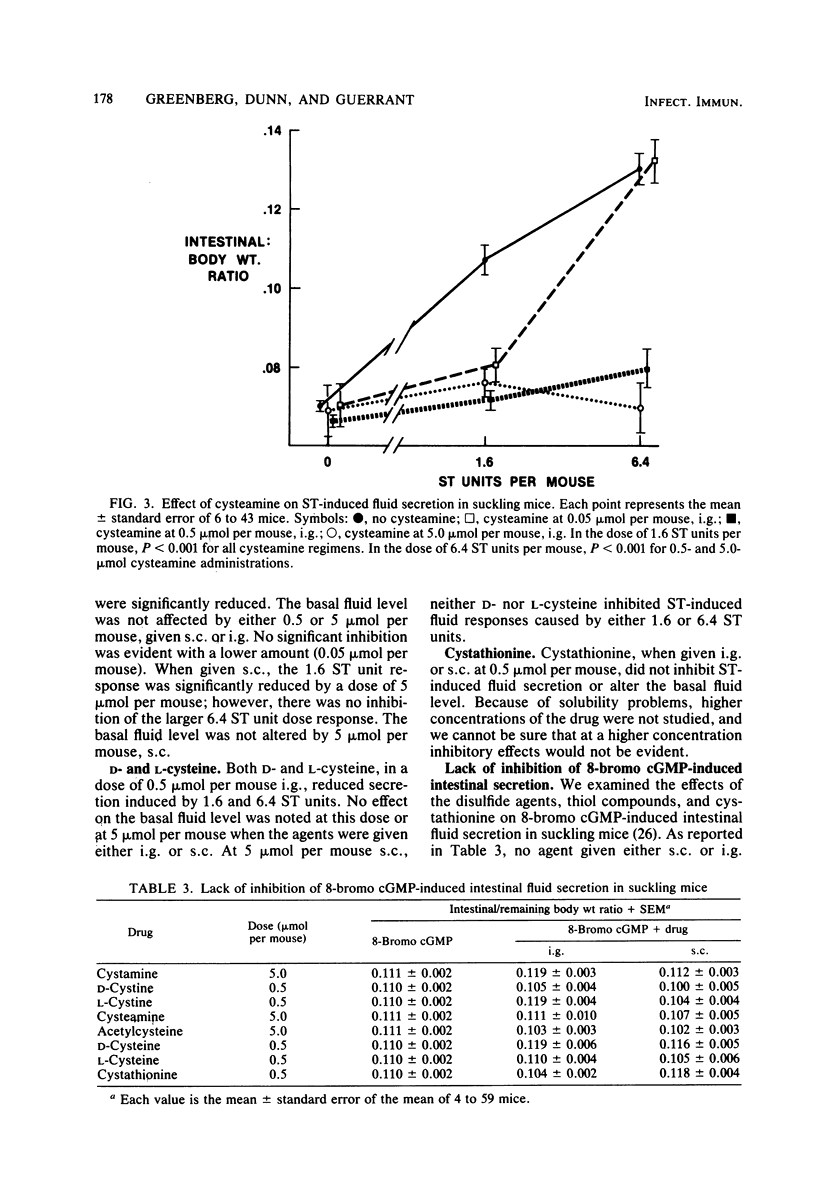
We examined the effects of disulfide and thiol compounds on Escherichia coli heat-stable enterotoxin (ST) and cyclic GMP-induced secretion. Both cystamine and cystine (disulfide compounds) reduced the secretory responses to submaximal doses of ST in suckling mice (at 0.5 mumol per mouse) and reduced ST activation of guanylate cyclase (by 33 to 73% at 1 mM). In higher doses, cystamine completely eradicated a maximally effective ST dose as well. In addition, the sulfhydryl (thiol) compounds cysteamine, cysteine, and acetylcysteine strikingly reduced the secretory response and the guanylate cyclase response to ST. Neither the disulfide nor the thiol compounds tested reduced cyclic GMP-induced secretion. These studies suggest that disulfide and thiol compounds both block ST-induced secretion before its activation of guanylate cyclase. Taken with the work of others, these findings suggest that disulfide compounds may alter the oxidation reduction state of a cell or act directly on the guanylate cyclase enzyme, whereas thiol compounds may inactivate ST itself by breaking its disulfide bridges, or it may alter guanylate cyclase activation by ST. Both families of compounds deserve further consideration among potential antisecretory agents for application in the control of ST-induced diarrhea.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Robertson D. C. Purification and chemical characterization of the heat-stable enterotoxin produced by porcine strains of enterotoxigenic Escherichia coli. Infect Immun. 1978 Mar;19(3):1021–1030. doi: 10.1128/iai.19.3.1021-1030.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameer B., Greenblatt D. J. Acetaminophen. Ann Intern Med. 1977 Aug;87(2):202–209. doi: 10.7326/0003-4819-87-2-202. [DOI] [PubMed] [Google Scholar]
- Apffel C. A., Walker J. E., Issarescu S. Tumor rejection in experimental animals treated with radioprotective thiols. Cancer Res. 1975 Feb;35(2):429–437. [PubMed] [Google Scholar]
- Brandwein H. J., Lewicki J. A., Murad F. Reversible inactivation of guanylate cyclase by mixed disulfide formation. J Biol Chem. 1981 Mar 25;256(6):2958–2962. [PubMed] [Google Scholar]
- Burgess M. N., Bywater R. J., Cowley C. M., Mullan N. A., Newsome P. M. Biological evaluation of a methanol-soluble, heat-stable Escherichia coli enterotoxin in infant mice, pigs, rabbits, and calves. Infect Immun. 1978 Aug;21(2):526–531. doi: 10.1128/iai.21.2.526-531.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. C., Rohde J. E., Sharp G. W. Intestinal adenyl-cyclase activity in human cholera. Lancet. 1971 May 8;1(7706):939–941. doi: 10.1016/s0140-6736(71)91443-7. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Inhibition by retinol and butylated hydroxyanisole of carcinogen-mediated increases in guanylate cyclase activity and guanosine 3':5'-monophosphate accumulation. Cancer Res. 1977 Nov;37(11):4088–4097. [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Modes of action of enterotoxins from Vibrio cholerae and EScherichia coli. Rev Infect Dis. 1979 Nov-Dec;1(6):918–926. doi: 10.1093/clinids/1.6.918. [DOI] [PubMed] [Google Scholar]
- Frantz J. C., Robertson D. C. Immunological properties of Escherichia coli heat-stable enterotoxins: development of a radioimmunoassay specific for heat-stable enterotoxins with suckling mouse activity. Infect Immun. 1981 Jul;33(1):193–198. doi: 10.1128/iai.33.1.193-198.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. N., Guerrant R. L., Chang B., Robertson D. C., Murad F. Inhibition of Escherichia coli heat-stable enterotoxin effects on intestinal guanylate cyclase and fluid secretion by quinacrine. Biochem Pharmacol. 1982 Jun 1;31(11):2005–2009. doi: 10.1016/0006-2952(82)90413-0. [DOI] [PubMed] [Google Scholar]
- Greenberg R. N., Guerrant R. L. E. coli heat-stable enterotoxin. Pharmacol Ther. 1981;13(3):507–531. doi: 10.1016/0163-7258(81)90027-9. [DOI] [PubMed] [Google Scholar]
- Greenberg R. N., Murad F., Chang B., Robertson D. C., Guerrant R. L. Inhibition of Escherichia coli heat-stable enterotoxin by indomethacin and chlorpromazine. Infect Immun. 1980 Sep;29(3):908–913. doi: 10.1128/iai.29.3.908-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. N., Murad F., Guerrant R. L. Lanthanum chloride inhibition of the secretory response to Escherichia coli heat-stable enterotoxin. Infect Immun. 1982 Feb;35(2):483–488. doi: 10.1128/iai.35.2.483-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Chen L. C., Sharp G. W. Intestinal adenyl-cyclase activity in canine cholera: correlation with fluid accumulation. J Infect Dis. 1972 Apr;125(4):377–381. doi: 10.1093/infdis/125.4.377. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Ganguly U., Casper A. G., Moore E. J., Pierce N. F., Carpenter C. C. Effect of Escherichia coli on fluid transport across canine small bowel. Mechanism and time-course with enterotoxin and whole bacterial cells. J Clin Invest. 1973 Jul;52(7):1707–1714. doi: 10.1172/JCI107352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Hughes J. M., Chang B., Robertson D. C., Murad F. Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: studies of tissue specificity, potential receptors, and intermediates. J Infect Dis. 1980 Aug;142(2):220–228. doi: 10.1093/infdis/142.2.220. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Limitations of the infant mouse test for Escherichia coli heat stable enterotoxin. Can J Comp Med. 1979 Oct;43(4):371–379. [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Hughes R. D., Gazzard B. G., Hanid M. A., Trewby P. N., Murray-Lyon I. M., Davis M., Williams R., Bennet J. R. Controlled trial of cysteamine and dimercaprol after paracetamol overdose. Br Med J. 1977 Nov 26;2(6099):1395–1395. doi: 10.1136/bmj.2.6099.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitany R. A., Scoot A., Forsyth G. W., McKenzie S. L., Worthington R. W. Evidence for two heat-stable enterotoxins produced by enterotoxigenic Escherichia coli. Infect Immun. 1979 Jun;24(3):965–966. doi: 10.1128/iai.24.3.965-966.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Kimura H., Mittal C. K., Murad F. Activation of guanylate cyclase from rat liver and other tissues by sodium azide. J Biol Chem. 1975 Oct 25;250(20):8016–8022. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mittal C. K., Murad F. Activation of guanylate cyclase by superoxide dismutase and hydroxyl radical: a physiological regulator of guanosine 3',5'-monophosphate formation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4360–4364. doi: 10.1073/pnas.74.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F., Arnold W. P., Mittal C. K., Braughler J. M. Properties and regulation of guanylate cyclase and some proposed functions for cyclic GMP. Adv Cyclic Nucleotide Res. 1979;11:175–204. [PubMed] [Google Scholar]
- Pomerantseva M. D., Viklina G. A. Vliianie tsistamina na vykhod dominantnykh letal'nykh mutatsii i retsiproknykh translokatsii v polovykh kletkakh myshei, podvergshikhsia gamma-oblucheniiu v raznykh dozakh. Genetika. 1975;10(7):55–61. [PubMed] [Google Scholar]
- Prescott L. F., Newton R. W., Swainson C. P., Wright N., Forrest A. R., Matthew H. Successful treatment of severe paracetamol overdosage with cysteamine. Lancet. 1974 Apr 6;1(7858):588–592. doi: 10.1016/s0140-6736(74)92649-x. [DOI] [PubMed] [Google Scholar]
- Solomon A. E., Briggs J. D., Knepil J., Henry D. A., Winchester J. F., Birrell R. Therapeutic comparison of thiol compounds in severe paracetamol poisoning. Ann Clin Biochem. 1977 Jul;14(4):200–202. doi: 10.1177/000456327701400155. [DOI] [PubMed] [Google Scholar]
- Staples S. J., Asher S. E., Giannella R. A. Purification and characterization of heat-stable enterotoxin produced by a strain of E. coli pathogenic for man. J Biol Chem. 1980 May 25;255(10):4716–4721. [PubMed] [Google Scholar]
- Thoene J. G., Oshima R. G., Crawhall J. C., Olson D. L., Schneider J. A. Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest. 1976 Jul;58(1):180–189. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos O., Budke L., Grant G. A. In vitro evaluation of some latent radioprotective compounds. Int J Radiat Biol Relat Stud Phys Chem Med. 1976 Nov;30(5):433–448. doi: 10.1080/09553007614551251. [DOI] [PubMed] [Google Scholar]
- White A. A., Crawford K. M., Patt C. S., Lad P. J. Activation of soluble guanylate cyclase from rat lung by incubation or by hydrogen peroxide. J Biol Chem. 1976 Dec 10;251(23):7304–7312. [PubMed] [Google Scholar]
- Yudkoff M., Foreman J. W., Segal S. Effects of cysteamine therapy in nephropathic cystinosis. N Engl J Med. 1981 Jan 15;304(3):141–145. doi: 10.1056/NEJM198101153040303. [DOI] [PubMed] [Google Scholar]
- de Ferreyra E. C., de Fenos O. M., Bernacchi A. S., de Castro C. R., Castro J. A. Therapeutic effectiveness of cystamine and cysteine to reduce liver cell necrosis induced by several hepatotoxins. Toxicol Appl Pharmacol. 1979 Apr;48(2):221–228. doi: 10.1016/0041-008x(79)90027-9. [DOI] [PubMed] [Google Scholar]



