Abstract
Activated pulmonary CD4+ T lymphocytes of the Th-1 type are essential for the inflammatory process in sarcoidosis, and IFN-γ production is crucial for the characteristic granuloma formation. Both the T cells and their inflammatory mediators may constitute possible targets for immunotherapy. A particular T-cell subset, the T-cell receptor (TCR) AV2S3+ bronchoalveolar lavage (BAL) CD4+ T cells, is found at dramatically increased levels in the BAL fluid of human leukocyte antigen (HLA)-DRB1*0301–positive and/or HLA-DRB3*0101–positive patients with sarcoidosis. The AV2S3+ BAL CD4+ T cells strongly associate with the sarcoid inflammation, and future studies on this particular T-cell subset to reveal their specificity may lead to the identification of sarcoidosis-specific antigen(s). T-cell subpopulations with regulatory functions (i.e., natural killer T cells and T regulatory cells) have recently been described as abnormal in sarcoidosis. Dysfunctional regulatory T cells may allow T effector cells to contribute to the formation of granulomas, and they may thus be relevant for the inflammatory process in this disease. These findings are exciting news and will be of help in designing new treatment strategies.
Keywords: T cells, sarcoidosis, granuloma
Sarcoidosis is a granulomatous disease of unknown etiology, characterized by the formation of noncaseating granulomas, and most commonly affecting the lungs. A distinct compartmentalization to the lungs of CD4+ T cells (1), spontaneously releasing IL-2 (2) and expressing the activation marker, human leukocyte antigen (HLA)-DR (3), was found already in the early 1980s through the use of bronchoscopy and bronchoalveolar lavage (BAL). Since then, lung compartmentalized T cells have been considered to be of central importance for the inflammatory process in sarcoidosis. Moreover, IFN-γ (later known as a typical Th1 cytokine) was shown in 1985 to be released by lung mononuclear cells to a substantially higher degree in patients with pulmonary sarcoidosis than from healthy control subjects (4).
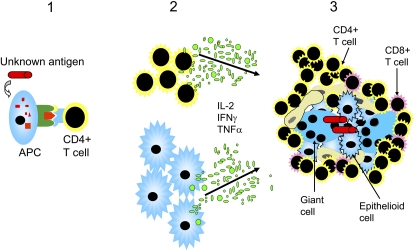
The characteristic finding of lung-accumulated CD4+ T cells in sarcoidosis and the resulting increase in the BAL fluid CD4/CD8 ratio has come to be a clinically important marker of the disease, and is used for diagnostic purposes (5). Through their IFN-γ and tumor necrosis factor (TNF)-α production, the lung accumulated T cells contribute to granuloma formation (Figure 1). A crucial importance of IFN-γ for granuloma formation was suggested, because IFN-γ knockout mice could not form granulomas in an animal model of hypersensitivity pneumonitis (6). Also, in studies of HIV-infected patients, the importance of T cells for the development of granulomas in sarcoidosis has been highlighted (7). HIV-infected patients recover from sarcoidosis, with disappearance of granulomas, when their T-lymphocyte counts are below 200/μl. Interestingly, when T-lymphocyte numbers increase as a result of appropriate treatment, sarcoidosis reappears (7).
Figure 1.
Unknown antigens are presented by antigen-presenting cells (APC) and recognized by CD4+ T helper cells (1). As a result, CD4+ T cells and macrophages are stimulated to proliferate and to produce inflammatory mediators, such as IL-2, IFN-γ, and tumor necrosis factor (TNF)-α (2), which are considered pivotal for granuloma formation (3). The granulomas consist of epithelioid macrophages, giant cells, and lymphocytes, which are mostly CD4+ T cells.
IMMUNOREGULATORY NATURAL KILLER T CELLS IN SARCOIDOSIS
A recently recognized immunoregulatory lymphocyte, the natural killer T (NKT) cell, is unique in its capacity to rapidly produce large quantities of both Th1 (IFN-γ) and Th2 (IL-4) cytokines. In mice, NKT cells have been shown to prevent the progression of Th1-mediated autoimmune diseases (8, 9), and, in humans, studies on identical twins showed diabetics to have reduced numbers and dysfunctional NKT cells (10). The majority of NKT cells are CD4 positive, and they express an invariant T-cell receptor (TCR) (in humans, Vα24-Jα18/Vβ11). They recognize glycolipid antigens, rather than peptide antigens, presented by CD1d molecules (11). The glycolipid, α-galactosylceramide, is a potent NKT-cell stimulus, and has been used in CD1d-tetramers for staining CD1d-restricted NKT cells.
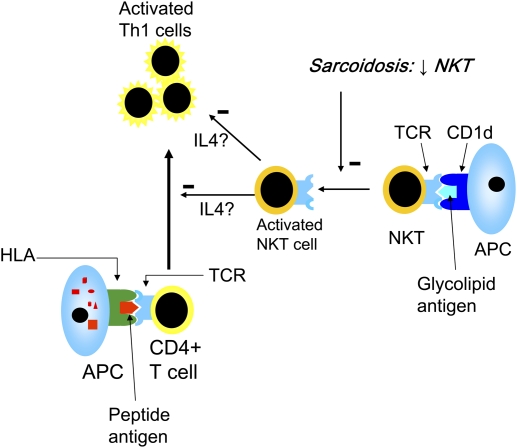
In sarcoidosis, NKT cells have been found at reduced levels in blood and BAL fluid (12, 13). In addition, when patients blood NKT cells were stimulated with α-galactosylceramide, they were shown to have impaired IFN-γ production (12). Interestingly, patients with Löfgren's syndrome, with a good prognosis, had normal levels of NKT cells in blood (13). Regarding NKT cells in granulomas of patients with sarcoidosis, the results are conflicting (12–14), and require additional investigation. Altogether, a reduction of regulatory NKT cells in sarcoidosis could help to explain the exaggerated T-cell response in this disease (Figure 2). These results are in sharp contrast to new findings in asthma (i.e., a Th2-mediated disease), in which NKT cells recently were shown to make up the majority of all CD4+ lung T cells (15).
Figure 2.
Natural killer T (NKT) cells recognize glycolipids presented by CD1d molecules and are capable of producing large amounts of IFN-γ and IL-4. They are considered to have immunoregulatory effects. The reduced levels and/or dysfunctional NKT cells that have been described in sarcoidosis (12–14) may lead to a loss of control of the activated Th1 proinflammatory cells in sarcoidosis, theoretically through reduced NKT cell production of IL-4 (42), which has down-regulatory effects on Th1 cells. HLA = human leukocyte antigen.
T REGULATORY CELLS IN SARCOIDOSIS
Naturally occurring T regulatory (Treg) cells coexpress CD4 and CD25bright (CD25 strongly expressed on the cellular surface) and are capable of suppressing proliferation and cytokine production of activated T cells via cell–cell contact. Treg cells can also be defined through their expression of the transcription factor, forkhead box P3 (FOXP3) (16), which may be a more reliable marker, as CD25 is also expressed by activated T cells. Recently, coexpression of CD25 and CD27 was suggested to delineate FOXP3-positive Treg cells (17). In mice, Treg cells can inhibit autoimmune diseases (18), and mice and humans that lack FOXP3 die of severe autoimmune disorders (19, 20).
Increased numbers of CD4+ CD25bright Treg cells were described in peripheral blood and BAL fluid of patients with sarcoidosis (21, 22). In the study by Miyara and colleagues, the sarcoid Treg cells were unable to completely downregulate the production of inflammatory cytokines, such as TNF-α, thus allowing granuloma formation. However, they had strong antiproliferative activity, which was suggested to be related to the typical state of anergy seen in patients with sarcoidosis (22). We recently analyzed the Treg marker, FOXP3, in BAL CD4+ T cells and, intriguingly, we found a down-regulation of FOXP3 in patients with sarcoidosis as compared with control subjects. As opposed to previous results (21, 22), this would indicate reduced levels of Treg cells in sarcoidosis. Alternatively, the reduced FOXP3 levels could suggest a qualitative abnormality of Treg cells in this disease. Future studies including functional aspects of Treg cells will undoubtedly shed more light on this interesting and important CD4+ T-cell population.
RESTRICTED TCR EXPRESSION BY BAL T CELLS
A restricted TCR repertoire of lung-accumulated TCR-αβ–expressing T cells was described in the 1980s by Moller and colleagues (23). Several studies subsequently confirmed that BAL CD4+ T cells had a biased TCR-Vα or -Vβ usage, and, in many cases, were oligoclonal (i.e., derived from a limited number of T cells), indicating T-cell reactivity against a few sarcoidosis-associated antigens in the lungs of these patients (24–28). Oligoclonal T cells with specific TCR sequence motifs were identified both in the lungs and blood of patients with sarcoidosis and, interestingly, after intradermal injections with Kveim's antigen (which was previously used for diagnostic purposes), also in the skin (29). In most cases, however, the TCR-Vα or -Vβ gene expression differed between patients, with one distinct exception, i.e., the TCR AV2S3–expressing T cells found in HLA-DRB1*0301–positive patients with sarcoidosis.
TCR AV2S3–EXPRESSING BAL T CELLS IN HLA-DRB1*0301–POSITIVE PATIENTS
Our own group has shown that DRB1*0301-positive patients have dramatic expansions of TCR AV2S3+ CD4+ T cells in their lungs (25, 30). Such T cell expansions are found in virtually every DRB1*0301-positive patient with sarcoidosis, but not in patients with other inflammatory pulmonary diseases, such as allergic alveolitis or asthma, or in healthy individuals. Sequencing of the TCR of AV2S3+ CD4+ lung T cells demonstrated (1) oligoclonality and (2) different nucleotide combinations coding for the same amino acid sequence in the CDR3 region of the TCR (i.e., the most hypervariable part of the TCR and directly interacting with the antigenic peptide), indicating that they had been selected by interaction with specific antigens (31). AV2S3+ CD4+ lung T cells also express activation markers to a higher degree than other lung CD4+ T cells (32), strongly supporting our hypothesis that they have recognized and proliferated in response to a sarcoidosis-associated antigen. Moreover, the number of AV2S3+ CD4+ lung T cells correlates with disease activity (33), further linking them to the pathogenesis of sarcoidosis. There is also a correlation between the magnitude of accumulated AV2S3+ BAL T cells at disease onset and clinical features of the disease, with higher numbers of AV2S3+ BAL T cells correlating with a better prognosis, indicating a protective role for this particular T-cell subset (34). Our most recent studies indicate that the AV2S3+ BAL T cells are effector cells rather than Treg cells (i.e., they do not seem to express FOXP3). Moreover, AV2S3+ CD4+ BAL T cells display a significantly reduced expression of both CD25 and CD27 as compared with the remaining (AV2S3-negative) CD4+ BAL T cells of the same patients (32). This once again indicates an effector function of this particular T-cell subset, as coexpression of CD25 and CD27 has been suggested to define FOXP3+ Treg cells in humans (17).
In certain other ethnic groups, such as the Japanese, HLA-DRB1*0301 is rare, and, accordingly, there are usually no AV2S3+ BAL T cells accumulated in the lungs of these patients (35). However, lung accumulated AV2S3+ BAL T cells can be found in HLA-DRB1*0301–positive patients other than whites, indicating the importance for the HLA-DRB1*0301 gene to generate this local immune response (36). An African-born black woman adopted at an early age by Swedish parents and raised in Sweden, diagnosed with sarcoidosis after presentation with bilateral hilar lymphadenopathy and erythema nodosum, was found to harbor a large population of lung CD4+ T cells expressing TCR AV2S3 at the onset of disease. This patient was found to be HLA-DRB1*0301–positive, suggesting that certain HLA genes are strongly linked to specific immune responses that are identical, irrespective of racial background (36). On the other hand, environmental factors are also important, as shown by the finding of a pair of HLA-DRB1*0301–positive monozygotic twins, where one had sarcoidosis and also lung accumulated AV2S3+ T cells at disease onset, but not after clinical recovery, whereas her healthy twin sister had normal AV2S3+ T-cell numbers in both BAL and blood (37).
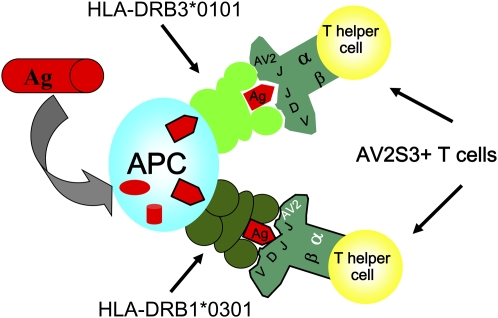
A second group of patients with sarcoidosis (i.e., HLA-DRB1*0301 negative and DRB3*0101 positive) was found with lung accumulated AV2S3+ T cells (38). The HLA-DRB1*0301 and HLA-DRB3*0101 molecules have distinct similarities, and can present identical antigen peptides, suggesting that a hypothetical sarcoidosis-specific antigen could be presented either by the HLA-DRB1*0301 or the DRB3*0101 molecule, and, in both cases, be recognized preferentially by AV2S3 lung T cells (Figure 3) (38, 39). Mycobacterium tuberculosis has long been suggested as a possible pathogen in sarcoidosis, in accordance with recent findings by Song and colleagues (40). It was therefore interesting that M. tuberculosis extracts could preferentially stimulate peripheral blood AV2S3+ CD4+ T cells of healthy HLA-DRB1*0301–positive individuals (41). The specificity of AV2S3+ lung T cells in sarcoidosis, however, remains to be established.
Figure 3.
An antigen is processed by an antigen-presenting cell (APC) and presented in the form of a peptide in the context of an HLA molecule. According to our hypothesis, both HLA-DRB1*0301 and HLA-DRB3*0101 molecules can present identical peptides, and be recognized preferentially by T-cell receptor AV2S3+ CD4+ T lymphocytes. Ag = antigen.
CONCLUSIONS
Sarcoidosis involving the lung continues to cause significant morbidiy and mortality. Data concerning the importance of T cells and, more recently, the roles of regulatory T cells and ASV2S3+ CD4+ T cells, provide insight into pathogenesis of the disease. The existence of a sarcoid-specific antigen presented by HLA subtypes found in patients with sarcoidosis and preferentially recognized by AV2S3+ T cells provides a potential mechanistic focus for therapeutic targets.
Acknowledgments
The authors thank Jan Wahlström, Anders Planck, Mary Berlin, Olle Olerup, Hans Wigzell, Kia Katchar, Farah Idalih, Benita Dahlberg, Berit Olsson, Lotta Müller-Suur, Margita Dahl, Gunnel De Forest, Heléne Blomquist, and Eva-Marie Karlsson for their contributions to this work.
Supported by the Swedish Heart-Lung Foundation, the King Oscar II Jubilee Foundation, the Swedish Medical Research Council, Torsten and Ragnar Söderbergs Foundation, National Institutes of Health grant R21HL077579-02 (HL04-009), the Karolinska Institutet, and the Stockholm County Council.
Conflict of Interest Statement: Neither author has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hunninghake GW, Crystal RG. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med 1981;305:429–434. [DOI] [PubMed] [Google Scholar]
- 2.Pinkston P, Bitterman PB, Crystal RG. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med 1983;308:793–800. [DOI] [PubMed] [Google Scholar]
- 3.Saltini C, Spurzem JR, Lee JJ, Pinkston P, Crystal RG. Spontaneous release of interleukin 2 by lung T lymphocytes in active pulmonary sarcoidosis is primarily from the Leu3+DR+ T cell subset. J Clin Invest 1986;77:1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson BW, McLemore TL, Crystal RG. γ Interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest 1985;75:1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ATS/ERS/WASOG. Statement on sarcoidosis: joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS), and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736–755. [DOI] [PubMed] [Google Scholar]
- 6.Gudmundsson G, Hunninghake GW. Interferon-γ is necessary for the expression of hypersensitivity pneumonitis. J Clin Invest 1997;99:2386–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris DG, Jasmer RM, Huang L, Gotway MB, Nishimura S, King TE Jr. Sarcoidosis following HIV infection: evidence for CD4+ lymphocyte dependence. Chest 2003;124:929–935. [DOI] [PubMed] [Google Scholar]
- 8.Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, Kronenberg M, Koezuka Y, Delovitch TL, Gombert JM et al. Activation of natural killer T cells by α-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med 2001;7:1057–1062. [DOI] [PubMed] [Google Scholar]
- 9.Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, et al. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med 2001;7:1052–1056. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature 1998;391:177–181. [DOI] [PubMed] [Google Scholar]
- 11.Brutkiewicz RR. CD1d ligands: the good, the bad, and the ugly. J Immunol 2006;177:769–775. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, Kaneko Y, Seino K, Yamada Y, Motohashi S, Koike J, Sugaya K, Kuriyama T, Asano S, Tsuda T, et al. Impaired IFN-γ production of Vα24 NKT cells in non-remitting sarcoidosis. Int Immunol 2004;16:215–222. [DOI] [PubMed] [Google Scholar]
- 13.Ho LP, Urban BC, Thickett DR, Davies RJ, McMichael AJ. Deficiency of a subset of T-cells with immunoregulatory properties in sarcoidosis. Lancet 2005;365:1062–1072. [DOI] [PubMed] [Google Scholar]
- 14.Mempel M, Flageul B, Suarez F, Ronet C, Dubertret L, Kourilsky P, Gachelin G, Musette P. Comparison of the T cell patterns in leprous and cutaneous sarcoid granulomas: presence of Vα24-invariant natural killer T cells in T-cell–reactive leprosy together with a highly biased T cell receptor Vα repertoire. Am J Pathol 2000;157:509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med 2006;354:1117–1129. [DOI] [PubMed] [Google Scholar]
- 16.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 17.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 2005;201:1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity 1995;3:171–174. [DOI] [PubMed] [Google Scholar]
- 19.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 2001;27:68–73. [DOI] [PubMed] [Google Scholar]
- 20.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001;27:18–20. [DOI] [PubMed] [Google Scholar]
- 21.Planck A, Katchar K, Eklund A, Gripenback S, Grunewald J. T-lymphocyte activity in HLA-DR17 positive patients with active and clinically recovered sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2003;20:110–117. [PubMed] [Google Scholar]
- 22.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Kambouchner M, Valeyre D, Chapelon-Abric C, Debre P, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med 2006;203:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moller DR, Konishi K, Kirby M, Balbi B, Crystal RG. Bias toward use of a specific T cell receptor β-chain variable region in a subgroup of individuals with sarcoidosis. J Clin Invest 1988;82:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunewald J, Janson CH, Eklund A, Ohrn M, Olerup O, Persson U, Wigzell H. Restricted V α 2.3 gene usage by CD4+ T lymphocytes in bronchoalveolar lavage fluid from sarcoidosis patients correlates with HLA-DR3. Eur J Immunol 1992;22:129–135. [DOI] [PubMed] [Google Scholar]
- 25.Grunewald J, Olerup O, Persson U, Ohrn MB, Wigzell H, Eklund A. T-cell receptor variable region gene usage by CD4+ and CD8+ T cells in bronchoalveolar lavage fluid and peripheral blood of sarcoidosis patients. Proc Natl Acad Sci USA 1994;91:4965–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellocq A, Lecossier D, Pierre-Audigier C, Tazi A, Valeyre D, Hance AJ. T cell receptor repertoire of T lymphocytes recovered from the lung and blood of patients with sarcoidosis. Am J Respir Crit Care Med 1994;149:646–654. [DOI] [PubMed] [Google Scholar]
- 27.Forman JD, Klein JT, Silver RF, Liu MC, Greenlee BM, Moller DR. Selective activation and accumulation of oligoclonal V β-specific T cells in active pulmonary sarcoidosis. J Clin Invest 1994;94:1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forrester JM, Wang Y, Ricalton N, Fitzgerald JE, Loveless J, Newman LS, King TE, Kotzin BL. TCR expression of activated T cell clones in the lungs of patients with pulmonary sarcoidosis. J Immunol 1994;153:4291–4302. [PubMed] [Google Scholar]
- 29.Klein JT, Horn TD, Forman JD, Silver RF, Teirstein AS, Moller DR. Selection of oligoclonal V β-specific T cells in the intradermal response to Kveim-Siltzbach reagent in individuals with sarcoidosis. J Immunol 1995;154:1450–1460. [PubMed] [Google Scholar]
- 30.Grunewald J, Eklund A, Hed J, Takahashi H, Wigzell H. Aspects on the alveolar accumulation of T cells in sarcoidosis. Sarcoidosis 1992;9:142–144. [PubMed] [Google Scholar]
- 31.Grunewald J, Hultman T, Bucht A, Eklund A, Wigzell H. Restricted usage of T cell receptor V α/J α gene segments with different nucleotide but identical amino acid sequences in HLA-DR3+ sarcoidosis patients. Mol Med 1995;1:287–296. [PMC free article] [PubMed] [Google Scholar]
- 32.Katchar K, Wahlstrom J, Eklund A, Grunewald J. Highly activated T-cell receptor AV2S3+ CD4+ lung T-cell expansions in pulmonary sarcoidosis. Am J Respir Crit Care Med 2001;163:1540–1545. [DOI] [PubMed] [Google Scholar]
- 33.Planck A, Eklund A, Grunewald J. Markers of activity in clinically recovered human leukocyte antigen-DR17–positive sarcoidosis patients. Eur Respir J 2003;21:52–57. [DOI] [PubMed] [Google Scholar]
- 34.Grunewald J, Berlin M, Olerup O, Eklund A. Lung T-helper cells expressing T-cell receptor AV2S3 associate with clinical features of pulmonary sarcoidosis. Am J Respir Crit Care Med 2000;161:814–818. [DOI] [PubMed] [Google Scholar]
- 35.Grunewald J, Shigematsu M, Nagai S, Mikuniya T, Wigzell H, Izumi T, Eklund AG. T-cell receptor V gene expression in HLA-typed Japanese patients with pulmonary sarcoidosis. Am J Respir Crit Care Med 1995;151:151–156. [DOI] [PubMed] [Google Scholar]
- 36.Grunewald J, Eklund A. Human leukocyte antigen genes may outweigh racial background when generating a specific immune response in sarcoidosis. Eur Respir J 2001;17:1046–1048. [DOI] [PubMed] [Google Scholar]
- 37.Grunewald J, Eklund A. Specific bronchoalveolar lavage fluid T cells associate with disease in a pair of monozygotic twins discordant for sarcoidosis. J Intern Med 2001;250:535–539. [DOI] [PubMed] [Google Scholar]
- 38.Grunewald J, Wahlstrom J, Berlin M, Wigzell H, Eklund A, Olerup O. Lung restricted T cell receptor AV2S3+ CD4+ T cell expansions in sarcoidosis patients with a shared HLA-DRβ chain conformation. Thorax 2002;57:348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagvekar N, Corlett L, Jacobson LW, Matsuo H, Chalkley R, Driscoll PC, Deshpande S, Spack EG, Willcox N. Scanning a DRB3*0101 (DR52a)-restricted epitope cross-presented by DR3: overlapping natural and artificial determinants in the human acetylcholine receptor. J Immunol 1999;162:4079–4087. [PubMed] [Google Scholar]
- 40.Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, Teirstein AS, Zhang Y, Cotter RJ, Moller DR. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 2005;201:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esin S, Batoni G, Saruhan-Direskeneli G, Harris RA, Grunewald J, Pardini M, Svenson SB, Campa M, Wigzell H. In vitro expansion of T-cell-receptor Vα2.3+ CD4+ T lymphocytes in HLA-DR17(3), DQ2+ individuals upon stimulation with Mycobacterium tuberculosis. Infect Immun 1999;67:3800–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing Th2 bias of natural killer T cells. Nature 2001;413:531–534. [DOI] [PubMed] [Google Scholar]