Abstract
The availability of sensitive, reproducible, and feasible outcome measures for quantifying lung disease in children with cystic fibrosis (CF) younger than 6 years is critical to the conduct of clinical trials in this important population. Historically, identifying and quantifying the presence of lung disease in very young children with CF was hampered by a lack of reproducible measures of lung function or lung pathology. Over the past 10 years, significant progress has led to physiologic, anatomic, and bronchoscopic measures that may serve as endpoints for future intervention trials. These endpoints include infant and preschool lung function testing, computed tomography of the chest, and bronchoalveolar lavage markers of inflammation and infection. Much progress has occurred in standardizing lung function testing, which is essential for multicenter collaboration. Pulmonary exacerbation has the potential to serve as a clinical endpoint; however, there is currently no standardized definition in children with CF younger than 6 years. Further development of these outcomes measures will enable clinical trials in the youngest CF population with the objective of improving long-term prognosis.
Keywords: infant; child, preschool; respiratory function tests, computed tomography scanners, X-ray; bronchoalveolar lavage
In the past, clinical trials in young children with cystic fibrosis (CF) have been hindered by a lack of sensitive, reproducible outcome measures. Identifying and treating lung disease is critical during these early stages of airway growth. Recently, novel techniques have been developed that could potentially lead to earlier detection of CF lung disease and produce measurements that would serve as endpoints in multicenter clinical trials. These techniques include infant and preschool lung function tests, various imaging modalities, and bronchoalveolar lavage (BAL) markers of infection and inflammation. Pulmonary exacerbations have the potential to serve as a patient-oriented outcome for future clinical trials but currently lack a standardized definition in infants and toddlers. For each potential endpoint, this article reviews methodology, clinical and biologic relevance, sensitivity and specificity to treatment, feasibility, and safety.
INFANT PULMONARY FUNCTION TESTING
Background
Over the past 20 years, there has been much progress in measuring lung function during infancy, and the ability of a variety of measures to detect early physiologic abnormalities in infants with CF has been clearly established (1–22). Early CF airway disease has been detected by plethysmographic, gas dilution, and forced expiratory flow–volume techniques. All these techniques require sedation, generally with chloral hydrate.
Methodologies
Plethysmography, one of the first techniques used to assess lung function abnormalities in infants with CF (23), measures thoracic gas volume (TGV). TGV is typically measured at end-inspiration rather than end-expiration as in adults because it is less disturbing to the infant and avoids glottic closure as well as distal airway closure at low lung volumes (10, 24–27). Functional residual capacity (FRC) as measured by plethysmography (FRCpleth) is obtained by subtracting the lung volume above the end-expiratory level of the tidal breath from the TGV. FRC can also be measured with gas dilution techniques using inert gases such as nitrogen, helium, or sulfur hexafluoride (SF6). Unlike plethysmography, which measures the volume of gas in both the communicating and noncommunicating airways, gas dilution techniques only measure gas volumes in the communicating airways. See Table 1.
TABLE 1.
COMMON INFANT LUNG FUNCTION TECHNIQUES, CORRESPONDING MEASURES AND ADVANTAGES/DISADVANTAGES
| Technique | Measure | Advantages | Disadvantages |
|---|---|---|---|
| Partial flow–volume curves | V̇maxFRC | Commercial device | Limited to tidal volume range |
| Extensive publications using V̇maxFRC as endpoint | FRC is variable | ||
| Flow limitation may not be attained | |||
| Infants may not exhale to RV | |||
| RVRTC | FVC, FEV0.4 or FEV0.5, FEF25–75, FEF75 | Commercial device | Requires extensive training |
| Simulates adult-type flow–volume curves | Expensive equipment | ||
| Flow limitation possible | Limited reference data | ||
| Flows measured from a reproducible lung volume | |||
| Infants reliably exhale to RV | |||
| Plethysmography | FRCpleth | Commercial device Measures gas in communicating and noncommunicating airways | Expensive equipment |
| Limited reference data with commercial devices | |||
| Gas dilution | FRC | Commercial device | Only measures gas in communicating airways |
| Gas trapping may be underestimated | |||
| Measures of ventilation inhomogeneity | LCI, MR, SnIII | May be sensitive markers of early airway disease | No published guidelines |
| Minimal reference data | |||
| No data demonstrating that measures respond to treatment | |||
| No commercially available device (mass spectrometer) | |||
| Fractional lung volumes (requires both RVRTC and plethysmography) | RV, TLC, RV/TLC, FRC/TLC | May be sensitive markers of early airway disease | Limited reference data from a single center |
Definition of abbreviations: FEF25–75 = forced expiratory flows between 25 and 75% of FVC; FEF75 = forced expiratory flow at 75% of FVC; FEV0.4 or FEV0.5 = forced expiratory volume at 0.4 or 0.5 seconds; LCI (lung clearance index); MR = mixing ratio; RV = residual volume; RVRTC = raised volume rapid thoracoabdominal compression; SnIII = phase III slope; TLC = total lung capacity; V̇maxFRC = maximal flow referenced to FRC.
Recent data using breath-by-breath inert gas washout techniques suggest that these measures may be particularly sensitive to early CF lung disease. Using these techniques, indices of ventilation inhomogeneity are calculated, including the lung clearance index (LCI). The LCI is the ratio of cumulative exhaled volume required to decrease the inert gas to 1/40th of the initial concentration divided by the FRC. Other indices measured using the breath-by-breath washout techniques include mixing index and phase III slopes (SnIII) (28). Currently, there is no commercially available equipment for performing gas washout techniques that is in widespread use (see Table 1).
Forced expiratory flow–volume measurements may be assessed from a tidal breath or from a raised lung volume. When assessing flow–volume measures from tidal breathing, flow is referenced to FRC (maximal flow at functional residual capacity [V̇maxFRC]). Shortcomings of this technique are that measurements are limited to the tidal volume range, FRC is variable, and flow limitation may not be achieved (10). In contrast, the raised volume rapid thoracoabdominal compression (RVRTC) technique produces forced expiratory maneuvers in infants that simulate adult-type flow–volume curves (29) (Figure 1). Advantages of the raised volume technique are that flows are assessed from a reproducible lung volume (i.e., that associated with an airway pressure of 30 cm H2O), and flow limitation is achieved, thus measures are more likely to be effort-independent and to reflect underlying lung mechanics. Measurement variability is reduced because flows are not referenced to a variable lung volume such as FRC. Thus, the RVRTC technique has largely superseded tidal forced expiratory flows. Indices measured using the RVRTC technique are outlined in Table 1. By combining plethysmography and the RVRTC technique, fractional lung volume measurements are now possible (17, 30). Commercial equipment is internationally available with which to perform RVRTC and plethysmographic measurements in sedated infants. See Table 1.
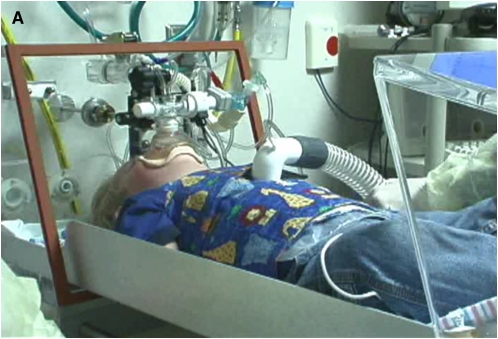
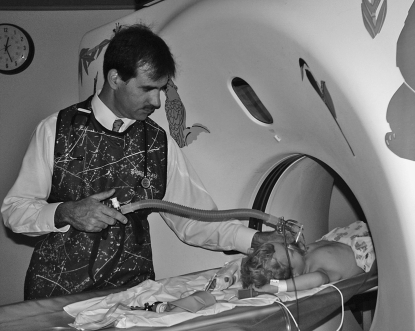
Figure 1.
(A) A sedated infant undergoing lung function testing with the raised volume rapid thoracoabdominal compression technique. (B) Schematic of the raised volume rapid thoracoabdominal compression technique. To initiate inflation, air is delivered from a compressed air source to the sedated infant through the inspiratory circuit. A pressure-relieve valve, located in the inspiratory circuit, is set at 30 cm H2O. During the inflation, the expiratory valve is closed, leading to inflation of the infant's lungs to 30 cm H2O. At the end of the inflation, the expiratory valve is then opened and the infant exhales passively. These inflation–passive exhalation maneuvers are repeated until a short respiratory pause is noted. Inflation is then repeated and the jacket is inflated (by opening a valve located between the jacket and the pressure reservoir) at end-inspiration to initiate the forced exhalation toward residual volume. The maneuver is repeated at increasing jacket pressures until flow limitation is achieved. (Reprinted by permission from Reference 48; and originally created by Marcus H. Jones, M.D., Ph.D.)
Clinical and Biologic Relevance
Several investigators have reported an increase in FRCpleth or FRC measured by gas dilution in infants with CF as compared with healthy control subjects, reflecting early hyperinflation (3, 17, 19, 23). Gappa and colleagues (31) and McCoy and colleagues (26) have previously demonstrated that normal infants have trapped gas (higher FRCpleth values compared with FRC measured using gas dilution), likely due to airway closure at low lung volumes. Due to an inability to measure air trapping in airways not communicating with the airway opening, FRC measured using gas dilution techniques in infants with CF may not be as sensitive to hyperinflation as plethysmography. Recently, LCI measurements were performed using multiple breath washout techniques and were found to be significantly elevated in 39 infants with CF compared with healthy control subjects (16). Very few studies using multiple breath washout in infants with CF have been performed (5, 32, 33); however, this technique is promising as a means to detect early CF lung disease (see Preschool Pulmonary Function Testing). See Table 2.
TABLE 2.
SELECTED STUDIES EVALUATING LUNG FUNCTION MEASURES IN INFANTS WITH CYSTIC FIBROSIS
| Technique | No. of Subjects with CF | Study |
|---|---|---|
| Partial flow–volume curve | ||
| Baseline V̇maxFRC as well as values 1 yr later more diminished in infants with respiratory symptoms at diagnosis compared with asymptomatic infants | 32 | Tepper and colleagues (4) |
| V̇maxFRC, measured 1 to 2 mo after treatment for an exacerbation leading to hospitalization, was significantly higher in infants randomized to steroids compared with placebo | 20 | Tepper and colleagues (7) |
| V̇maxFRC was decreased at baseline compared with controls and this decrease persisted after viral illness | 22 | Hiatt and colleagues (9) |
| V̇maxFRC significantly improved after treatment for exacerbation leading to hospitalization | 17 | Clayton and colleagues (6) |
| RVRTC | ||
| FEV0.5 and FEV0.75 significantly diminished compared with controls (V̇maxFRC unable to differentiate CF from controls) | 12 | Turner and colleagues (11) |
| RVRTC values were more frequently diminished compared with controls than V̇maxFRC | 47 | Ranganathan and colleagues (13) |
| RVRTC values significantly diminished compared with controls soon after diagnosis (even in those without respiratory symptoms) | 33 | Ranganathan and colleagues (15) |
| FEV0.5 significantly diminished soon after diagnosis as well as 6 mo later compared with controls | 34 | Ranganathan and colleagues (14) |
| RVRTC values decreased compared with controls | 29 | Castile and colleagues (17) |
| RVRTC values improved after intravenous antibiotic therapy | 44 | Sheikh and colleagues (21) |
| FRC measured via plethysmography | ||
| FRCpleth significantly increased compared with controls | 29 | Castile and colleagues (17) |
| FRCpleth improved after intravenous antibiotic therapy | 44 | Sheikh and colleagues (21) |
| FRC measured via gas dilution | ||
| Baseline FRC measured via helium dilution, as well as 1 yr later, not significantly different in infants with CF with respiratory symptoms at diagnosis compared with asymptomatic infants with CF | 32 | Tepper and colleagues (4) |
| FRC measured via nitrogen washout 1 to 2 mo after treatment for an exacerbation improved more in infants with CF treated with steroids compared with those who received placebo | 20 | Tepper and colleagues (7) |
| FRC measured via nitrogen washout increased compared with controls | 29 | Castile and colleagues (17) |
| Measures of ventilation inhomogeneity | ||
| LCI significantly increased; RVRTC values diminished compared with controls | 39 | Lum and colleagues (16) |
| Fractional lung volumes | ||
| RV, RV/TLC significantly increased compared with controls | 29 | Castile and colleagues (17) |
| RV/TLC improved after intravenous antibiotic therapy | 44 | Sheikh and colleagues (21) |
Definition of abbreviations: FEV0.5 = forced expiratory volume at 0.5 seconds; FEV0.75 = forced expiratory volume at 0.75 seconds; LCI = lung clearance index; RV = residual volume; RVRTC = raised volume rapid thoracoabdominal compression; TLC = total lung capacity; V̇maxFRC = maximal expiratory flow measured at FRC.
Forced expiratory flows measured during tidal breathing (4–9, 22) and with the raised volume technique (11, 12, 14, 15, 17) have been shown to be significantly reduced in infants with CF as compared with healthy control subjects. In addition, Ranganathan and colleagues demonstrated that airway function was reduced even without a history of prior respiratory illness (15) and that diminished lung function persisted over a 6-month period (14). Forced expiratory volume in 0.5 seconds (FEV0.5) has been reported to be lower in subjects with infection noted in BAL fluid compared with those without infection (20). Fractional lung volumes (residual volume [RV] and the ratio of RV to total lung capacity [RV/TLC]) have been reported to be significantly increased in infants with CF compared with healthy control subjects, reflecting distal air trapping and hyperinflation (17). The raised volume technique detected lung function abnormalities in the same percentage of subjects as the multiple breath washout technique (16). These findings differ from results reported in preschoolers, in which LCI was more likely to be abnormal than forced expiratory flows (Preschool Pulmonary Function Testing) (34). Based on the work of the London Cystic Fibrosis Collaboration, RVRTC and LCI may prove to be complementary measures when assessing the presence of lung disease in infants (16). See Table 2.
Sensitivity and Specificity to Treatment Effect
Infant lung function measures have been shown to improve after a therapeutic intervention in a number of published studies. Significant improvement in V̇maxFRC has been demonstrated after treatment of a pulmonary exacerbation with antibiotics (6, 21) and with intravenous hydrocortisone (7), and after receiving a combined therapy of salbutamol, aerosolized N-acetylcysteine, and chest physiotherapy (8). Forced expiratory flows assessed with the raised volume technique, FRCpleth, and fractional lung volumes significantly improved after intravenous antibiotic therapy (21). Currently, there are no data assessing the sensitivity to treatment of the multiple breath washout technique. See Table 2.
Reference Data
To identify the presence of disease in infants with CF, appropriate reference data are critical. Reference data from healthy control subjects have been published for the partial flow–volume technique, FRC, and the raised volume technique (30, 35–39). In 1995, reference values for FRC were published by an American Thoracic Society (ATS)/European Respiratory Society (ERS) working group (38). Since the 1995 publication, Hulskamp and colleagues (40) reported that FRCpleth measures obtained with commercial equipment were significantly lower compared with reference data reported by this taskforce. This disparity may be due to equipment or protocol differences. Jones and coworkers (37) published reference values for the raised volume technique in 153 healthy infants 3 to 149 weeks of age at two centers, and these values were recently shown to be similar to reference data from London and Brazil (29). There is only one publication reporting reference data for fractional lung volumes (RV, RV/TLC, TLC, expiratory reserve volume), generated from 33 measurements from 22 infants without respiratory disease who were 3 to 120 weeks of age (30). There are no reference data for ventilation inhomogeneity in healthy infants (28). Ideally, the investigator should perform the desired technique on a cohort of healthy control subjects to assess if he or she is using the appropriate reference values. This testing may be difficult and deemed ethically inappropriate at certain institutions due to the need to sedate for infant lung function testing.
Variability
For healthy control subjects, the mean within-occasion (same testing session), mean coefficients of variation (CV) for FRCpleth, V̇maxFRC, FVC, FEV0.5, and forced expiratory flows between 25 and 75% of FVC (FEF25–75) have been reported to be 4 to 6%, 6 to 15%, 3 to 6%, 4 to 5%, and 5 to 8%, respectively (10, 30, 39, 41, 42). There are very few data evaluating between-occasion repeatability measurements for both healthy control subjects (37) and infants with CF due to the need for repeated sedation. Ranganathan and associates (14) did report that there was no significant change in z scores for FVC and forced expiratory flows at 75% of FVC (FEF75) when lung function was assessed 6 months apart in infants with CF (Spearman correlation coefficient: 0.55 and 0.80, respectively) (14). Currently, there is a paucity of data evaluating the concordance of infant lung function measurements collected by different laboratories, physiciansm and/or technicians. However, a recently completed 10-center U.S. Cystic Fibrosis Foundation–sponsored study should help address some of the questions surrounding reproducibility and intrasite variability (18).
Feasibility and Risks
The ATS/ERS taskforce for infant lung function testing has published guidelines for performing plethysmography (43), gas dilution (44), forced expiratory flows from tidal breathing (45), and the raised volume technique (29). These standards papers provide guidelines for equipment setup, performing the technique, and data analysis. The recent availability of standardized commercial equipment will greatly facilitate the use of infant pulmonary function tests as endpoints in multicenter clinical trials.
On the basis of the large number of published studies and thousands of studies performed, infant lung function testing is safe in a controlled environment. The main risk is that of sedation. Minimal adverse events have been reported with this technique; however, two well-trained persons should be performing the technique and monitoring the child. Careful screening of the child for exclusion criteria related to sedation must be conducted (29, 46).
Future Studies
A recently completed U.S. multicenter study of infant pulmonary function testing was designed to assess the utility of infant lung function testing as potential outcome measures for future clinical trials. Results of this study will provide information, complementary to that published from specialized single-center labs, regarding the safety and feasibility of infant lung function testing in the multicenter setting, the ability of various measurements to distinguish infants with CF from historical healthy control subjects, and measurement variability over time periods relevant to future clinical trials. The next step in the development of this endpoint will be to use infant lung function testing in an intervention trial. Investigators in the Cystic Fibrosis Foundation Therapeutics Development Network are currently developing a trial evaluating the efficacy of hypertonic saline in infants with CF using infant lung function testing as the primary endpoint. This type of intervention trial will better define the sensitivity and specificity of infant lung function measurements to a treatment effect.
PRESCHOOL PULMONARY FUNCTION TESTING
Background
In the past, assessing preschool respiratory physiology was considered a “black box” due to the inability both to sedate for infant lung function testing and of the child to cooperate with voluntary maneuvers. This myth has been debunked by recent publications demonstrating the utility of various tidal breathing techniques in this age group together with the realization that many preschoolers actually can perform acceptable spirometry. Performing lung function testing in this age group is important due to the considerable lung growth that occurs between the ages of 3 and 6 years and to aid in the longitudinal assessment of physiologic measures of the lung from infancy to adulthood. Current maneuvers that have been evaluated in the preschooler with CF include the following: (1) spirometry, (2) the interrupter resistance technique, (3) forced oscillation (FO), (4) specific airway resistance, and (5) gas washout techniques (47, 48).
Methodologies
Acceptable, reproducible spirometry is now feasible in preschoolers, with the proportion able to perform acceptable maneuvers increasing with increasing age (47–57). ATS/ERS statements on spirometry were written for older children and adults and investigators have recently demonstrated that these guidelines are inappropriate for preschoolers (56). Common challenges in assessing the preschooler include the inability for the child to inhale to TLC and reach a distinct peak flow. The child often has difficulty forcefully exhaling to RV and/or maintaining exhalation for a full second. Due to physiologic and maturity differences between the preschooler and adult, guidelines for performing spirometry have recently been modified for the child between the ages of 3 and 6 years by the ATS/ERS Working Group on Infant and Preschool Lung Function Testing (47). On the basis of these guidelines, the following indices should be obtained from forced exhalation curves in preschoolers: FVC, FEV0.5, FEV at 0.75 seconds (FEV0.75), FEV1, FEF25–75, volume of back extrapolation, and the point at which flow ceases as a proportion of peak expiratory flow (PEF). Other recommendations are that termination is considered premature if it occurs at greater than 10% of PEF, and if possible, the child should produce at least two reproducible curves. Clinicians evaluating preschoolers with CF should introduce the child to spirometry as early as 3 years of age to encourage “practice” and technicians should adhere to the modified spirometry guidelines for this age group. See Table 3.
TABLE 3.
COMMONS PRESCHOOL LUNG FUNCTION TECHNIQUES, CORRESPONDING MEASURES, AND ADVANTAGES/DISADVANTAGES OF PRESCHOOL LUNG FUNCTION TECHNIQUES
| Technique | Measures | Advantages | Disadvantages |
|---|---|---|---|
| Spirometry | FVC, FEV0.5, FEV0.75, FEF25–75 | Published guidelines | May be difficult to obtain technically acceptable data, particularly in youngest children |
| Allows longitudinal assessment from infancy to adulthood | |||
| Minimal between-occasion reproducibility data available | |||
| Reference data | |||
| Interrupter resistance | Rint Rrs Xrs | Published guidelines | Assumes respiratory system a single compartment |
| Forced oscillation (FO) | Simple, noninvasive | May not be sensitive to small airway disease | |
| Quick Reference data available | |||
| Specific airway resistance | sRaw | Simple, quick | Expensive equipment |
| Minimal reference data | |||
| Gas washout | LCI, MR | Simple, noninvasive | Minimal reference data |
| May be sensitive to early airway disease | No published guidelines | ||
| Expensive equipment | |||
| No published data as to whether responsive to intervention |
Definition of abbreviations: FEV0.75 = forced expiratory volume at 0.75 seconds; FEF25–75 = forced expiratory flows between 25 and 75% of FVC; FEV0.5 = forced expiratory volume at 0.5 seconds; ; LCI = lung clearance index; MR = mixing ratio); Rint = interrupter resistance measure; Rrs = respiratory system resistance measured using forced oscillation; sRaw = plethysmographic specific airway resistance; Xrs = respiratory system reactance measured using forced oscillation.
Two tidal breathing techniques, the interrupter resistance and FO measurements, are quick, noninvasive measures that may be more easily performed in the preschooler than spirometry (47, 48, 58–72). During the interrupter resistance technique, the airway opening of the preschooler is briefly occluded (∼ 100 ms). Resistance (Rint) is calculated by measuring the ratio of pressure change to flow assessed at the airway opening during the occlusion. Two important assumptions during this technique are that the valve closes quickly and that the pressure change at the airway opening equilibrates with pressure changes within the alveoli (47, 48). The recent ATS/ERS guidelines recommend that occlusions for Rint occur during expiration (47). To perform FO, the child also tidal breathes through a mouthpiece as pressure oscillations are transmitted to the airway opening. The respiratory system impedance (Zrs) is measured from the resultant flow and pressure changes. Zrs represents both the real or in-phase element (resistance [Rrs]) and the out-of-phase or imaginary element (reactance [Xrs]) of this flow–pressure relationship. Reactance represents inertial forces and respiratory system compliance. Single-frequency sine waves or multiple frequencies may transmit these pressure oscillations, typically through a loudspeaker (47, 48, 64–66). Rrs and Xrs are reported at different frequencies, from 4 to 48 Hz. The simplicity of these measures has led to their widespread use in preschoolers (47, 48, 58–72). However, FO and the interrupter resistance techniques assume that the respiratory system is a single compartment; therefore, in a multicompartment system, such as CF small airway disease, abnormality may be difficult to detect. Commercially available equipment exists for measurement of preschool spirometry, Rint, and FO. See Table 3.
Plethysmographic specific airway resistance (sRaw) has been adapted for preschoolers (34, 47, 73–75). To perform sRaw measures, flow at the airway and pressure changes within a plethysmograph are measured while the child tidal breathes through a mouthpiece or modified facemask. Because pressure changes within the plethysmograph reflect volume changes, one is able to calculate sRaw by simultaneously measuring airway flow and volume changes within the box. sRaw is then calculated from the product of the thoracic gas pressure and the ratio of the volume changes within the box divided by measured changes in flow within the airways (75). See Table 3.
Recently, gas washout measures have become more popular in the preschool age group. To perform the multiple-breath washout technique, the preschooler tidal breathes an inert gas (tracer gas) through a modified facemask. This gas (helium, nitrogen, argon, or SF6) is first “washed in” then “washed out.” From this maneuver, indices such as LCI, mixing ratio, and moment ratios may be calculated (28, 34, 47, 76). See Table 3.
Clinical and Biologic Relevance
In 33 preschoolers with CF, FVC, FEV1, FEV1/FVC, and FEF25–75 values were significantly diminished compared with historical normal control subjects (77). Brasfield chest radiograph scores significantly correlated with FVC and FEV1. In a more recent five-center study (78), children with CF between the ages of 2.5 and 6.9 years were found to have significantly lower flows and FEV0.5 values compared with historical normal subjects. Older children in this cohort had lower FEV0.5 and FEF25–75 z scores compared with the younger children; however, clinical parameters did not correlate with the lung function variables. There is a paucity of published longitudinal spirometry data in preschoolers with CF. Recently, in 30 preschoolers with CF, LCI and sRaw were significantly increased and FEV0.5 was significantly decreased compared with healthy control subjects. These investigators reported that abnormal lung function was detected in 73, 47, and 13% of children as assessed by the multiple-breath washout technique, sRaw, and spirometry, respectively (34). These results indicate that the multiple-breath washout technique may be more sensitive at detecting early abnormalities in lung function in the preschooler with CF compared with sRaw and spirometry. See Table 4.
TABLE 4.
SELECTED STUDIES EVALUATING LUNG FUNCTION IN PRESCHOOL CHILDREN WITH CYSTIC FIBROSIS
| Study | Results |
|---|---|
| Vilozni and colleagues (78), n = 66 | FVC, FEV0.5, FEF25–75 diminished compared with control subjects |
| Marostica and colleagues (77), n = 33 | FVC, FEV1, FEF25–75 diminished compared with control subjects |
| Nielsen and colleagues (79), n = 30 | sRaw abnormal in CF; Rint/oscillometry not significantly different in subjects with CF compared with control subjects |
| Aurora and colleagues (34), n = 30 | LCI discriminated subjects with CF from control subjects more often than spirometry and sRaw |
| Beydon and colleagues (62), n =39 | Rint increased in subjects with CF compared with control subjects |
Definition of abbreviations: CF = cystic fibrosis; FEF25–75 = forced expiratory flows between 25 and 75% of FVC; FEV0.5 = forced expiratory volume at 0.5 seconds; ; LCI = lung clearance index; Rint = interrupter resistance measure; sRaw = plethysmographic specific airway resistance.
Nielsen and colleagues (79) reported that sRaw in 30 preschool and early school-aged children with CF was significantly increased compared with control subjects; however, there was no significant difference in oscillometry and Rint measurements between the two groups. In contrast, Rint has been reported to be significantly increased in 39 preschoolers with CF compared with healthy control subjects (62) (Table 4). There are very few data evaluating FO in preschoolers with CF; however, investigators recently demonstrated a significant correlation between low-frequency oscillation measurements in toddlers (1–3 yr) and measures of inflammation in BAL fluid (80). Because resistance values may reflect the upper airway more than the peripheral airways (48), more studies are needed to better define the role of these outcome measures in CF.
Sensitivity and Specificity to Treatment Effect
There is currently a lack of data evaluating the responsiveness of these various preschool techniques to an intervention. Studies are needed using a variety of lung function techniques to validate the utility of these measures for future intervention studies in CF.
Reference Data and Measurement Feasibility
Reference data have been published for spirometry for children between the ages of 3 and 6 years (49–51, 54, 55). Reference values are also available for FO (67–71), interrupter resistance (58–61, 70), and sRaw (70, 81) in children as young as 2 years of age. For spirometry, investigators report that approximately 60 to 80% (49, 55) of children between the ages of 3 and 6 years can perform acceptable spirometry (51, 56). Approximately 62, 69, and 75% of preschoolers with a mean age of 3, 4 and 5 years, respectively, have been reported to produce acceptable spirometry (51). In a different study (54), 51, 69, 76, and 78% of 3-, 4-, 5-, and 6-year-old children had three reproducible flow–volume curves, respectively. A recently completed multicenter trial (72) evaluated both spirometry and oscillometry as outcome measures in children between the ages of 2 and 3 years with recurrent wheezing. Results of this study indicated that oscillometry was feasible in this age group in a multicenter setting, but only 56% of the participants completed acceptable spirometry.
Standardization is needed for multicenter trials using preschool lung function techniques as an outcome measure. Recently, the ATS/ERS Working Group on Infant and Young Children Pulmonary Function Testing developed guidelines for the following preschool lung function techniques: (1) spirometry, (2) tidal breathing measures, (3) the interrupter technique, (4) the FO technique, (5) gas washout techniques, and (6) bronchial challenge tests (47). These guidelines will facilitate multicenter collaboration for future studies in this age group.
Variability
During spirometry in healthy 3- to 6-year-old children, the measured CVs within a single testing session (within-occasion repeatability) have been reported to be 2.7, 2.5, and 8.3% for FEV1, FVC, and FEF25–75, respectively (49), and the mean difference between the two best maneuvers has been reported to be approximately 3.5% for FVC and FEF75 in children with a mean age of 4 years (56).
For the interrupter technique, the within-occasion CV for healthy subjects (age, 2–7 yr) has been reported to be 8 to 12% (59, 61, 70), and one study reported a CV of 11% for preschoolers with CF (62). In healthy 2- to 7-year-old subjects, Klug and Bisgaard (70) reported a within-occasion CV of 11% for sRaw that was independent of age. Finally, healthy subjects 2 to 7 years of age performing FO have been reported to have a within-occasion CV of 6 to 10% for resistance values at 5 Hz (70, 71).
A few investigators have reported between-occasion reproducibility data for the interrupter technique in both healthy subjects and those with respiratory disease (58, 82). There are limited data for between-occasion reproducibility in the CF population for preschool lung function techniques (47). For intervention studies, reproducibility data are critical and further studies are urgently needed.
Future Studies
Currently, a U.S. Cystic Fibrosis Foundation–sponsored multicenter trial is being conducted in 3- to 5-year-old preschoolers to evaluate spirometry, FO, and inductance plethysmography as potential outcome measures. Results from this study will better define the utility of these measures in a multicenter setting.
Due to a lack of data regarding feasibility, reproducibility over time periods relevant to clinical trials, and responsiveness to interventions, preschool lung function measures are not as ready for utilization as endpoints in clinical trials as are infant lung function measures. In addition, which of the many measures available for preschoolers might be “the best” remains unclear. Indeed, combinations of these tests may ultimately provide complementary information. Incorporating several of these measures as secondary or exploratory endpoints in an intervention trial would be an appropriate next step in developing these measurements as clinical trial endpoints.
COMPUTED TOMOGRAPHY OF THE CHEST
Background
Potential imaging endpoints for young children with CF include chest radiographs, computed tomography (CT), magnetic resonance imaging, and positron emission tomography. Each may have a role as a clinical trial endpoint, and each has advantages and disadvantages. Chest radiographs are inexpensive and nearly universally available. They may be useful in long-term longitudinal studies (83). Magnetic resonance imaging provides cross-sectional images without using ionizing radiation, but resolution is limited compared with CT scanning (84). Hyperpolarized helium allows functional imaging with magnetic resonance imaging, but this technique has very limited availability (85). Positron emission tomography scanning evaluates metabolic activity and has been shown to correlate with inflammation in the CF lung (86), but is limited by cost and radiation dose. Currently, CT scanning is the furthest developed technique as an outcome measure.
CT images reflect the morphologic changes of CF lung disease. CT scanning forms cross-sectional images of the lung using the same X-rays used for chest radiographs, although CT scans require a significantly higher radiation dose (87). These images provide a level of detail similar to gross pathological specimens (88). CT scanning allows direct observation and measurement of bronchiectasis, bronchial wall thickening, mucus plugging, and parenchymal infiltrates (Figure 2). Airways as small as approximately 1 mm can be measured directly (89). Air flow limitation in smaller airways can be inferred from air trapping observed on expiratory images.
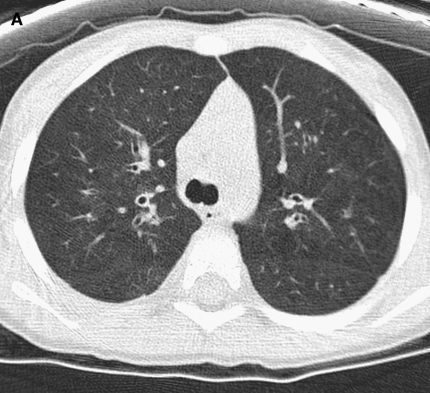
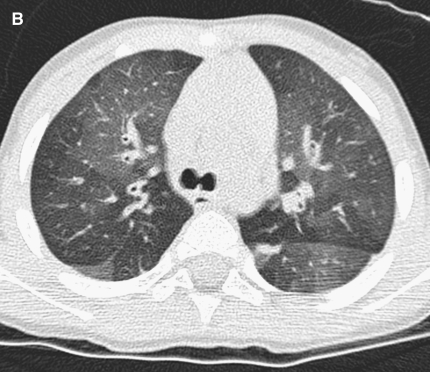
Figure 2.
(A) Image shows mild bronchial wall thickening associated with bronchiectasis in the right upper lobe in a 2-year-old female with cystic fibrosis (CF). (B) Image displays multiple areas of air trapping (seen as dark areas), greatest in the right upper lobe in the same 2-year-old female with CF.
Methodology
CT scanners in current clinical use can provide high-quality lung images in people of any age. Multislice CT scanners with 16 or more channels allow either contiguous (volumetric or spiral) imaging, where the entire lung is imaged, or so-called high-resolution CT (HRCT) imaging where the lung is sampled with thin sections obtained at intervals (Figure 3). With current technology, both methods can produce high-quality images. HRCT uses less radiation, whereas contiguous imaging provides more data and allows three-dimensional imaging. HRCT is adequate to provide an overall assessment of the presence and severity of CF lung disease, but is limited when serial studies are compared [see the other symposium in this issue] (90). No single CT technique has been widely accepted for use in young children with CF.
Figure 3.
(A) High-resolution computed tomography (CT) scanning is a sampling technique that uses thin sections at intervals. Only the portion of the lungs indicated by the white lines is imaged. (B) Volumetric CT uses a helical technique to image a volume of tissue. The shaded square shows the area imaged using a volumetric technique.
Young children have difficulty lying still and cannot reliably sustain lung volumes near TLC and FRC, which are needed for high-quality CT images. Lung volume can be controlled with the use of sedation and mask ventilation (the controlled ventilation method; Figure 4) (91) in children younger than 3 or 4 years, or with general anesthesia at any age. In children older than 3 or 4 years, careful training or spirometer control (92) may allow imaging without sedation or anesthesia. Although studies of young children have been performed without lung volume control (see below), the improved image quality obtained with this control suggests that sensitivity and reproducibility will be markedly increased by the use of these methods.
Figure 4.
Controlled ventilation computed tomography scanning. The respiratory circuit includes a pop-off valve that is set to 25 cm H2O pressure. The operator closes the circuit with his right thumb to administer positive pressure and augment the patient's inspiration. Several augmented inspirations cause a respiratory pause during which imaging is performed.
CT images are graphic data that must be converted to numerical data for use in research trials. This can be performed by expert observers, direct measurement, or computer analysis. Expert observer scores that assess the presence and severity of the different findings of CF lung disease have been developed and have been shown to be reproducible (93, 94). Airway size measurement has been used to show differences between CF and normal airways (89) and progression of CF airway dilation and airway wall thickening (95). Computer analysis of air trapping has been used to assess CF lung disease and response to intervention (96–98).
The risks of CT scanning are the risks of cancer from low-dose ionizing radiation and the risk of sedation or anesthesia for younger children. The risk of cancer from CT scanning is an area of active debate. The risk is too small to measure directly, and so assumptions must be made to assign a risk level. It is important to recognize that young children are more radiosensitive than older children and adults (87), and that lower dose techniques can be used in small children, unlike larger children or adults (99). In a study of over 6,000 children who received sedation in a radiology department, there were four overnight hospitalizations and no deaths (100).
Most studies of CT in CF have been performed on older children or adults. A review of the use of CT scanning as an imaging endpoint in this older age group has been recently published (90). Thus, only studies on children younger than 6 years are discussed below.
Clinical and Biologic Relevance
CT measurements of airway lumen diameter and airway wall thickness can detect subtle, early abnormalities in infants with CF and demonstrate progression with advancing age. Thirty-four children with CF, who were between the ages of 10 weeks to 5.5 years, were compared with children with no known lung disease (89). Mean airway lumen diameter and airway wall thickness were significantly greater in the children with CF (p < 0.001). The ratio of airway lumen diameter to vessel diameter, a measure of relative lumen size, increased with age in the children with CF and remained stable in children without CF (p = 0.026). An additional study found that detection of bronchiectasis and air trapping was improved by the controlled ventilation technique compared with quiet breathing (101).
Structural damage identified by CT also appears to correlate with airway inflammation detected by BAL. HRCT scanning using controlled ventilation was performed in 17 children younger than 4 years old experiencing a pulmonary exacerbation (102). The radiologist identified the lobe with greatest disease and the lobe with least disease and these lobes were subsequently lavaged. Interleukin (IL)-8 levels and percentage of neutrophils were significantly higher in the lobe with greatest disease (p = 0.01 and 0.04, respectively).
Sensitivity and Specificity to Treatment Effects
In this same study by Davis and colleagues, CT scans were repeated at the end of therapy in 13 of the 17 subjects (102). Total CT score, hyperinflation, and bronchial dilation improved on the repeat CT scan (p = 0.01 for all). This study used the Brody CT scoring system (94), suggesting that this scoring system is responsive to treatment in young children as well as in older subjects.
A 100-day placebo controlled study of DNase was performed in 12 children younger than 5 years old to assess the ability of CT scanning to detect changes after a therapeutic intervention (103). HRCT scanning was performed during quiet breathing. CT scans improved in the treatment group and worsened in control subjects (p = 0.02). Chest radiograph scores did not change.
Reference Data
Airway measurements in children from infancy to 5 years old have been published in children with CF and in control subjects as described above (89). This study used the controlled ventilation technique with a distending pressure of 25 cm H2O. Consistent use of this pressure should minimize airway size changes due to differences in pressure. CT technique has been shown to affect airway measurement, with greater variability when lower radiation techniques are used (104). The authors note that measuring more airways can compensate for increased variability. Lung density at inspiration and expiration have been studied in normal children and reference values have been published (105). These values may be useful in detecting subtle air trapping in young children with CF.
Future Studies
To use CT scanning as an outcome measure in young children with CF, standard protocols for CT scanning should be developed. For research trials, volumetric CT scanning provides far more data and allows image reconstruction, improving airway assessment and localization of abnormalities. Control of lung volume also improves the detection of abnormalities (101) and should be used when possible. Multicenter investigation will be needed to assess the reproducibility of CT scanning between centers. CT scoring systems should continue to be developed to best capture the information provided by the CT images. Optimizing the combination of CT subscores (94) would obtain a weighted outcome measure with the greatest statistical power for detecting differences in morphology between groups (106). A second area of study is to assess potential differences in sensitivity between scoring scales that use a discrete scale of 0, 1, 2, or 3 versus a continuous scale, either by expert scores or via computer-assisted voxel counting of the volume involved in a particular morphologic change (107).
The natural history of CF lung disease detected by CT should be assessed to understand the expected progression of disease, and to identify findings that may respond to interventions. The lack of a “gold standard” is a major challenge in developing CT scanning as an outcome measure. In children aged 6 to 10 years old, CT scanning has been shown to be more sensitive to the presence of pulmonary abnormalities than are pulmonary function tests (108). This will very likely be true in younger patients, and indicates that pulmonary function tests cannot be used to validate CT studies. An additional complication is that the changes seen on CT scanning in response to intervention would be expected to differ depending on the specific intervention. A mucolytic agent would be expected to have its greatest effect on mucus plugging and air trapping, whereas an antiinflammatory agent would be expected to decrease peribronchial thickening. Two possible trial designs for the assessment of CT scanning can be suggested. First, CT scanning could be performed before and after a known effective intervention. The response of CT scanning to this intervention could then be assessed. Second, CT scanning could be included in the early phases of a drug development trial. In this case, the CT methodology could be optimized in the initial phases and then used to assess the effect of the intervention in a subset of subjects in a phase 3 trial.
BAL
Clinical and Biologic Relevance
In expectorating patients with CF, sputum cultures accurately reflect lower respiratory organisms (109–111). In contrast, although oropharyngeal cultures are widely used as a noninvasive surrogate for lower airway cultures in nonexpectorating patients with CF, they have poor sensitivity and positive predictive value for lower airway infection (112, 113), and oropharyngeal isolates of Pseudomonas aeruginosa can have different genotypes than lower airway isolates from the same patient (110, 114). Thus, in preexpectorating infants and young children with CF too young to successfully perform sputum induction with hypertonic saline, flexible bronchoscopy with BAL is the only means to directly sample the lower respiratory tract for detection of bacteria and inflammation.
Studies using BAL have been instrumental in demonstrating that lower airway infection and neutrophilic inflammation begin early in CF, often before the onset of symptoms (20, 22, 112, 114–117). BAL is being used increasingly to identify lower airway pathogens in infants identified through newborn screening (118). The role of serial bronchoscopic cultures in guiding early intervention in CF is currently under investigation in Australia (119). The concentrations of pro- and antiinflammatory cytokines and neutrophil products, such as neutrophil elastase (20, 22, 116, 117), as well as biomarkers of oxidative stress, such as 3-chlorotyrosine (120), have been assayed by BAL in infants with CF. The reader is referred to the article in this symposium by Sagel and colleagues (pp. 406–417) for further review of lower airway inflammation. BAL has also been used to measure lower airway concentrations of inhaled drugs, including tobramycin solution for inhalation (121) and DNase (122).
In terms of validation of BAL cultures, the presence of lower airway pathogens at concentrations consistent with infection (>105 cfu/ml [112]) has been associated with a number of measures of disease severity, including greater lower airway inflammation (neutrophil count and concentration of IL-8) (20, 22, 116, 117); worse pulmonary function (FRC [22], ratio of FRC to TLC [20], specific respiratory system compliance [20], raised volume forced expiratory volumes [116]); prior or current respiratory symptoms (20); and worse chest radiograph scores (22). More recently, Davis and coworkers (102) have shown an association between findings on HRCT scan of the chest and inflammatory markers in BAL fluid. In longitudinal studies, both the prevalence and density of pathogens in BAL fluid has been shown to increase with age (22).
Sensitivity to Treatment Effects
One clinical trial in young children with CF has used BAL cultures as the primary endpoint, and demonstrated a profound treatment effect. Gibson and colleagues (123) conducted a randomized, double-blind, placebo-controlled, multicenter trial of tobramycin solution for inhalation in patients with CF aged 6 months to 6 years. Although 100 patients were to be randomized, the trial was stopped after enrollment of 21 subjects because of evidence of a significant microbiological treatment effect. Although the groups had comparable densities of P. aeruginosa in BAL fluid at enrollment, no P. aeruginosa was detected on Day 28 in 8 of 8 active group patients compared with 1 of 13 placebo group patients. Thus, BAL cultures have clearly been shown to change in response to treatment.
Variability
Although considered the gold standard for sampling lower respiratory secretions, BAL may produce variable results due to regional heterogeneity of infection (124) and inflammation (125). Gutierrez and coworkers (124) demonstrated discordance in bacterial load between the right middle lobe and lingula among infants undergoing BAL, with bacterial load generally being highest in the right middle lobe. Meyer and Sharma (125) found that neutrophil count and neutrophil elastase activity was generally greater in upper lobe BAL fluid. These findings have implications for the design of clinical trials using BAL, suggesting that lavage of multiple lobes may be appropriate in some studies, and that, in studies involving serial lavages over time, these lavages should be performed in the same lobe. In addition, BAL techniques may vary to some degree between centers. Sources of variability include type of anesthesia/sedation, introduction of bronchoscope via nose versus laryngeal mask airway, lavage aliquot size and number, and lobe(s) lavaged. In multicenter studies, efforts should be made to standardize BAL technique (22, 119, 123).
Feasibility
Although BAL has been demonstrated to be feasible in multicenter trials, it is invasive and not without risks. Wainwright and colleagues (126) are rigorously documenting adverse events associated with bronchoscopy in the ongoing Australian multicenter BAL study. When complete, their documentation will serve as the most comprehensive description of the safety of BAL in infants and young children with CF.
Because of its invasive nature and expense, BAL is best suited for phase 1 and 2 clinical trials. BAL cultures have been shown to be an effective primary endpoint for trials of antimicrobial therapies (123). Lower airway measures of inflammation (neutrophil count, IL-8, other neutrophil products, and cytokines) may serve as endpoints for antiinflammatory therapies, although this approach has not been validated.
PULMONARY EXACERBATIONS
Pulmonary exacerbations as a clinical endpoint in older patients is discussed in the articles by Mayer-Hamblett and colleagues (pp. 370–377) and Goss and Quittner (pp. 378–386) in this symposium. In very young patients, pulmonary exacerbation is the only clinical endpoint currently available. Unfortunately, no standardized definition of a pulmonary exacerbation exists, particularly in very young patients. Rabin and colleagues (127), in a large cohort of patients with CF followed in the Epidemiologic Study of CF, a large, prospective, multicenter database, identified the following characteristics as predictive of a pulmonary exacerbation in children younger than 6 years: new crackles on lung exam, increased cough, decreased weight percentile, and increased sputum production. This definition has never been used in a clinical trial. A trial-specific definition was developed for the Early Pseudomonal Infection Control (EPIC) clinical trial, an ongoing multicenter, randomized trial of tobramycin solution for inhalation and oral ciprofloxacin for eradication of newly isolated P. aeruginosa in young children with CF. Results of this study may inform the definition of a pulmonary exacerbation for future trials in young patients with CF. In very young patients, the substantial overlap between acute viral respiratory infections and pulmonary exacerbations may render this endpoint difficult to interpret. Currently, no data exist on the sensitivity and specificity to treatment effects or reproducibility of a pulmonary exacerbation endpoint in children younger than 6 years.
More recently, there has been increased interest in developing CF-specific patient-reported outcomes, such as respiratory symptom scores. To date, research in this area has not extended to patients younger than 2 years. Clearly, there is an urgent need for the development of a standardized pulmonary exacerbation definition and symptom score in very young patients with CF.
CONCLUSIONS AND NEXT STEPS
Over the past 10 years, much progress has been made in developing infant and preschool lung function tests, chest CT scans, and BAL as clinical trial endpoints for the youngest patients with CF. For infant pulmonary function tests, measurements using the RVRTC technique, possibly in conjunction with plethysmography, appear the most promising clinical trial endpoints. These measurements have been shown to discriminate patients with CF from healthy control subjects, and improve with an intervention. Commercial devices are available and standardized guidelines for the conduct and interpretation of the measurements have been published. The time seems appropriate for using these measurements as a clinical trial endpoint, with careful attention to rigorous performance of the measurements through extensive training and central oversight and quality control. For preschool lung function measures, commercial equipment is also available for many of the measures, and standardized guidelines for the conduct of the measurements have been recently published. Which of the measures is the most promising for use as a clinical trial endpoint is unclear, so the next step would be to incorporate several measures as secondary or exploratory endpoints in a clinical trial. CT scans appear promising because of their ability to detect early structural changes. Next steps would include the development of standardized operating procedures for multicenter trials, agreement on the most appropriate scoring systems for children younger than 6 years of age, and a better understanding of the sensitivity and specificity to treatment effects. CT also seems poised to be used as a secondary or exploratory endpoint in a clinical trial. BAL has been used successfully in two multicenter trials, and is an appropriate endpoint for trials in which direct evaluation of lower airway infection or inflammation is critical. A standardized pulmonary exacerbation definition in children younger than 6 is lacking and deserves development. During the next 10 years, intervention trials in the youngest CF population using physiologic, anatomic, bronchoscopic, and clinical endpoints will likely lead to improved outcomes due to the identification and treatment of early lung disease (see Table 5).
TABLE 5.
FUTURE DIRECTIONS OF OUTCOME MEASURES IN YOUNG CHILDREN WITH CYSTIC FIBROSIS
| Outcome Measure | Future Directions |
|---|---|
| Infant lung function testing | 1. Use as endpoint in multicenter trial (RVRTC technique, plethysmography) |
| 2. Define measures that are most responsive to different types of interventions | |
| Preschool lung function testing | 1. Standardization of techniques, equipment, and software |
| 2. Use several measures, potentially simultaneously, as secondary endpoints in a clinical trial | |
| 3. Evaluate response of lung clearance index to an intervention | |
| Computed tomography of the chest | 1. Standardize scoring system for use in infants and young children |
| 2. Standardization of the controlled breathing technique | |
| 3. Standard guidelines for minimizing radiation exposure | |
| 4. Better define natural history of the disease | |
| 5. Standardized operating procedures for multicenter trials | |
| 6. Use as exploratory endpoint in clinical trial | |
| Bronchoalveolar lavage | 1. Standardize methods for multicenter trials |
| Pulmonary exacerbations | 1. Development of a standardized definition |
| 2. Use as a clinical trial endpoint |
Definition of abbreviation: RVRTC = raised volume rapid thoracoabdominal compression.
Acknowledgments
The authors thank the following participants in the Therapeutic Development Network's Outcome Measures Workshop entitled “Outcome for Young Children”: Charlene Hallmark (Baylor College of Medicine), Summer Adams (Boston Children's Hospital), Bobbi Ksenich (Case Western Reserve University), Peter Mogayzel (Johns Hopkins University), Jane Solomon (Massachusetts General Hospital), Terri Johnson (Ohio State University), Colleen Dunn (Stanford University), Terry Robinson (Stanford University), Sarah Holland (University of California San Diego), Diana Kardous (University of Cincinnati), Churee Pardee (University of Colorado), Richard Ahrens (University of Iowa), Jackie Zirbes (University of Minnesota), Barbara Chatfield (University of Utah), Kelly Worrell (University of Washington), Mary Boyle (Washington University), Amanda Bailey (Therapeutic Development Network [TDN] Coordinating Center), Barbara Mathewson (TDN Coordinating Center), George Strang (TDN Coordinating Center), Peter Cornelisse (TDN Coordinating Center), Steve Knutzen (TDN Coordinating Center).
Supported by National Institutes of Health/NCRR M01 RR00037 (Bonnie W. Ramsey, University of Washington, Seattle, WA).
Conflict of Interest Statement: S.D.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.J.E. has a pending grant with Novartis. L.C.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hammer J, Eber E, editors. Paediatric pulmonary function testing. Basel: Karger; 2005.
- 2.Gappa M, Ranganathan S, Stocks J. Lung function testing in infants with cystic fibrosis: lessons from the past and future directions. Pediatr Pulmonol 2001;32:228–245. [DOI] [PubMed] [Google Scholar]
- 3.Beardsmore CS, Bar-Yishay E, Maayan C, Yahav Y, Katznelson D, Godfrey S. Lung function in infants with cystic fibrosis. Thorax 1988;43:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tepper RS, Montgomery GL, Ackerman V, Eigen H. Longitudinal evaluation of pulmonary function in infants and very young children with cystic fibrosis. Pediatr Pulmonol 1993;16:96–100. [DOI] [PubMed] [Google Scholar]
- 5.Tepper RS, Hiatt P, Eigen H, Scott P, Grosfeld J, Cohen M. Infants with cystic fibrosis: pulmonary function at diagnosis. Pediatr Pulmonol 1988;5:15–18. [DOI] [PubMed] [Google Scholar]
- 6.Clayton RG Sr, Diaz CE, Bashir NS, Panitch HW, Schidlow DV, Allen JL. Pulmonary function in hospitalized infants and toddlers with cystic fibrosis. J Pediatr 1998;132:405–408. [DOI] [PubMed] [Google Scholar]
- 7.Tepper RS, Eigen H, Stevens J, Angelicchio C, Kisling J, Ambrosius W, Heilman D. Lower respiratory illness in infants and young children with cystic fibrosis: evaluation of treatment with intravenous hydrocortisone. Pediatr Pulmonol 1997;24:48–51. [DOI] [PubMed] [Google Scholar]
- 8.Maayan C, Bar-Yishay E, Yaacobi T, Marcus Y, Katznelson D, Yahav Y, Godfrey S. Immediate effect of various treatments on lung function in infants with cystic fibrosis. Respiration (Herrlisheim) 1989;55:144–151. [DOI] [PubMed] [Google Scholar]
- 9.Hiatt PW, Grace SC, Kozinetz CA, Raboudi SH, Treece DG, Taber LH, Piedra PA. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics 1999;103:619–626. [DOI] [PubMed] [Google Scholar]
- 10.Allen JL, Bar-Yishay E, Bryan AC, Budd J, Castile RG, Coates AL, Davis GM, England S, Gaultier C, Godfrey S, et al. Respiratory mechanics in infants: physiologic evaluation in health and disease. Am Rev Respir Dis 1993;147:474–496. [DOI] [PubMed] [Google Scholar]
- 11.Turner DJ, Lanteri CJ, LeSouef PN, Sly PD. Improved detection of abnormal respiratory function using forced expiration from raised lung volume in infants with cystic fibrosis. Eur Respir J 1994;7:1995–1999. [PubMed] [Google Scholar]
- 12.Davis S, Jones M, Kisling J, Howard J, Tepper R. Comparison of normal infants and infants with cystic fibrosis using forced expiratory flows breathing air and heliox. Pediatr Pulmonol 2001;31:17–23. [DOI] [PubMed] [Google Scholar]
- 13.Ranganathan S, Bush A, Dezateux C, Carr S, Hoo A, Lum S, Madge S, Price J, Stroobant J, Wade A, et al.; London Collaborative Cystic Fibrosis Group. Relative ability of full and partial forced expiratory maneuvers to identify diminished airway function in infants with cystic fibrosis. Am J Respir Crit Care Med 2002;166:1350–1357. [DOI] [PubMed] [Google Scholar]
- 14.Ranganathan SC, Stocks J, Dezateux C, Bush A, Wade A, Carr S, Castle R, Dinwiddie R, Hoo AF, Lum S, et al.; London Collaborative Cystic Fibrosis Group. The evolution of airway function in early childhood following clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med 2004;169:928–933. [DOI] [PubMed] [Google Scholar]
- 15.Ranganathan SC, Dezateux C, Bush A, Carr SB, Castle RA, Madge S, Price J, Stroobant J, Wade A, Wallis C, et al.; London Collaborative Cystic Fibrosis Group. Airway function in infants newly diagnosed with cystic fibrosis. Lancet 2001;358:1964–1965. [DOI] [PubMed] [Google Scholar]
- 16.Lum, S, Gustafsson P, Ljungberg H, Hulskmap G, Bush A, Siobhan BC, Castle R, Hoo A-F, Price J, Ranganathan S, et al. Early detection of cystic fibrosis lung disease: multiple-breath washout vs. raised-volume tests. Thorax 2007;62:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castile RG, Durdana I, McCoy K. Gas trapping in normal infants and in infants with cystic fibrosis. Pediatr Pulmonol 2004;37:461–469. [DOI] [PubMed] [Google Scholar]
- 18.Davis S, Kerby G, Acton J, Castile R, Colin A, Conrad C, Hart M, Hiatt P, Mogayzel P, Johnson R, et al. Feasibility, sensitivity and variability of adult-type pulmonary function tests in infants with CF in a multicenter, longitudinal trial. Pediatr Pulmonol 2006;(Suppl 29):A360.
- 19.Phelan PD, Gracey M, Williams HE, Anderson CM. Ventilatory function in infants with cystic fibrosis: physiological assessment of inhalation therapy. Arch Dis Child 1969;44:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dakin CJ, Numa AH, Wang HE, Morton JR, Vertzysa CC, Henry RL. Inflammation, infection and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 2002;165:904–910. [DOI] [PubMed] [Google Scholar]
- 21.Sheikh, Flucke R, McCoy K, Castile R. Changes in lung function during treatment for pulmonary exacerbations in infants with cystic fibrosis. Pediatr Pulmonol 2000;(Suppl 20):A429.
- 22.Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, Hiatt P, McCoy K, Wilson CB, Inglis A, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32:356–366. [DOI] [PubMed] [Google Scholar]
- 23.Godfrey S, Mearns M, Howlett G. Serial lung function studies in cystic fibrosis in the first 5 years of life. Arch Dis Child 1978;53:83–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanteri CJ, Raven JM, Sly PD. Should TGV be measured from end-inspiratory occlusions rather than end-expiratory occlusions in wheezy infants? Pediatr Pulmonol 1990;9:214–219. [DOI] [PubMed] [Google Scholar]
- 25.Beardsmore CS, Stocks J, Silverman M. Problems in measurement of thoracic gas volume in infancy. J Appl Physiol 1982;52:995–999. [DOI] [PubMed] [Google Scholar]
- 26.McCoy KS, Castile RG, Allen ED, Filbrun DA, Flucke RL, Bar-Yishay E. Functional residual capacity (FRC) measurements by plethysmography and helium dilution in normal infants. Pediatr Pulmonol 1995;19:282–290. [DOI] [PubMed] [Google Scholar]
- 27.Helms P. Problems with plethysmographic estimation of lung volume in infants and young children. J Appl Physiol 1982;53:698–702. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson PM, Ljungberg H. Measurement of functional residual capacity and ventilation inhomogeneity by gas dilution techniques: pediatric pulmonary function testing. Prog Respir Res 2005;33:54–65. [Google Scholar]
- 29.American Thoracic Society; European Respiratory Society. The ATS/ERS Working Group on Infant and Young Children Pulmonary Function Testing Consensus Statement. Raised volume forced expirations in infants: guidelines for current practice. Am J Respir Crit Care Med 2005;172:1463–1471. [DOI] [PubMed] [Google Scholar]
- 30.Castile RG, Filbrun D, Flucke R, Franklin W, McCoy K. Adult-type pulmonary function tests in infants without respiratory disease. Pediatr Pulmonol 2000;30:215–227. [DOI] [PubMed] [Google Scholar]
- 31.Gappa M, Fletcher M, Dezateux C, Stocks J. Comparison of nitrogen washout and plethysmographic measurements of lung volume in healthy infants. Am Rev Respir Dis 1993;148:1496–1501. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson PM, Kallmann S, Ljungberg H, Lindblad A. Method for assessment of volume of trapped gas in infants during multiple-breath inert gas washout. Pediatr Pulmonol 2003;35:42–49. [DOI] [PubMed] [Google Scholar]
- 33.Schibler A, Schneider M, Frey U, Kraemer R. Moment ratio analysis of multiple breath nitrogen washout in infants with lung disease. Eur Respir J 2000;15:1094–1101. [DOI] [PubMed] [Google Scholar]
- 34.Aurora P, Bush A, Gustafsson P, Oliver C, Wallis C, Price J, Stroonbant J, Carr S, Stocks J. Multiple-breath washout as a marker of lung disease in preschool children with cystic fibrosis. Am J Respir Crit Care Med 2005;171:249–256. [DOI] [PubMed] [Google Scholar]
- 35.Hanrahan JP, Tager IB, Castile RG, Segal MR, Weiss ST, Speizer FE. Pulmonary function measures in healthy infants: variability and size correction. Am Rev Respir Dis 1990;141:1127–1135. [DOI] [PubMed] [Google Scholar]
- 36.Hoo AF, Dezateux C, Hanrahan JP, Cole TJ, Tepper RS, Stocks J. Sex-specific prediction equations for Vmax(FRC) in infancy: a multicenter collaborative study. Am J Respir Crit Care Med 2002;165:1084–1092. [DOI] [PubMed] [Google Scholar]
- 37.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper RS. Forced expiratory flows and volumes in infants normative data and lung growth. Am J Respir Crit Care Med 2000;161:353–359. [DOI] [PubMed] [Google Scholar]
- 38.Stocks J, Quanjer PH. Reference values for residual volume functional residual capacity and total lung capacity. Eur Respir J 1995;8:492–506. [DOI] [PubMed] [Google Scholar]
- 39.Stocks J, Marchal F, Kraemer R, Gutkowski P, Bar-Yishay E, Godfrey S. Plethysmographic assessment of functional residual capacity and airway resistance. In: Stocks J, Sly PD, Tepper RS, Morgan WJ, editors. Infant respiratory function testing. New York: John Wiley & Sons; 1996. pp. 190–240.
- 40.Hulskamp G, Hoo A, Ljungberg H, Lum S, Pillow J, Stocks J. Progressive decline in plethysmographic lung volumes in infants. Am J Respir Crit Care Med 2003;168:1003–1009. [DOI] [PubMed] [Google Scholar]
- 41.Ranganathan S, Hoo A, Lum S, Goetz I, Castle R, Stocks J. Exploring the relationship between forced maximal flow at functional residual capacity and parameters of forced expiration from raised lung volume in healthy infants. Pediatr Pulmonol 2002;33:419–428. [DOI] [PubMed] [Google Scholar]
- 42.Turner DJ, Stick SM, Lesouef KL, Sly PD, Lesouef PN. A new technique to generate and assess forced expiration from raised lung volume in infants. Am J Respir Crit Care Med 1995;151:1441–1450. [DOI] [PubMed] [Google Scholar]
- 43.Stocks J, Godfrey S, Beardsmore C, Bar-Yishay E, Castile R, for the ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. Plethsymographic measurements of lung volume and air resistance. Eur Respir J 2001;17:302–312. [DOI] [PubMed] [Google Scholar]
- 44.Morris MG, Gustafsson P, Tepper R, Gappa M, Stocks J, for the ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. The bias flow nitrogen washout technique for measuring the functional residual capacity in infants. Eur Respir J 2001;17:529–536. [DOI] [PubMed] [Google Scholar]
- 45.Sly PD, Tepper R, Henschen M, Gappa M, Stocks J, for the ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. Tidal forced expirations. Eur Respir J 2000;16:741–748. [DOI] [PubMed] [Google Scholar]
- 46.Gaultier C, Fletcher M, Beardsmore C, Motoyama E, Stocks J. Measurement conditions. In: Stocks J, Sly PD, Tepper RS, Morgan WJ, editors. Infant respiratory function testing. New York: Wiley; 1996. pp. 29–44.
- 47.Beydon N, Davis S, Lombardi E, Allen JL, Aurora P, Bisgaard H, Davis M, Ducharme F, Eigen H, Gappa M, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 2007;175:1304–1345. [DOI] [PubMed] [Google Scholar]
- 48.Davis SD. Neonatal and pediatric respiratory diagnostics. Respir Care 2003;8:367–384. [PubMed] [Google Scholar]
- 49.Eigen H, Bieler H, Grant D, Christoph K, Terrill D, Heilman DK, Ambrosius WT, Tepper RS. Spirometric pulmonary function in healthy preschool children. Am J Respir Crit Care Med 2001;163:619–623. [DOI] [PubMed] [Google Scholar]
- 50.Vilozni D, Barker M, Jellouschek H, Heimann G, Blau H. An interactive computer-animated system (SpiroGame) facilitates spirometry in preschool children. Am J Respir Crit Care Med 2001;164:2200–2205. [DOI] [PubMed] [Google Scholar]
- 51.Vilozni D, Barak A, Efrati O, Augarten A, Springer C, Yacov Y, Bentur L. The role of computer games in measuring spirometry in healthy and “asthmatic” preschool children. Chest 2005;128:1146–1155. [DOI] [PubMed] [Google Scholar]
- 52.Kanengiser S, Dozer A. Forced expiratory maneuvers in children ages 3 to 5 years. Pediatr Pulmonol 1994;18:144–149. [DOI] [PubMed] [Google Scholar]
- 53.Crenesse D, Berlioz M, Bourrier T, Albertini M. Spirometry in children aged 3–5 years: reliability of forced expiratory maneuvers. Pediatr Pulmonol 2002;32:56–61. [DOI] [PubMed] [Google Scholar]
- 54.Nystad W, Samuelsen SO, Nafstad P, Edvardsen E, Stensrud T, Jaakkola JJK. Feasibility of measuring lung function in preschool children. Thorax 2002;57:1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zapletal A, Chalupova J. Forced expiratory parameters in healthy preschool children (3–6 years of age). Pediatr Pulmonol 2003;35:200–207. [DOI] [PubMed] [Google Scholar]
- 56.Aurora P, Stocks J, Oliver C, Saunders C, Castle R, Chaziparasidis G, Bush A. Quality control for spirometry in preschool children with and without lung disease. Am J Respir Crit Care Med 2004;169:1152–1159. [DOI] [PubMed] [Google Scholar]
- 57.Davis S. Preschool spirometry. Pediatr Respir Rev 2006;7:S11–S13. [DOI] [PubMed] [Google Scholar]
- 58.Lombardi E, Sly PD, Concutelli G, Novembre E, Veneruso G, Frongia G, Bernardini R, Vierucci A. Reference values of interrupter respiratory resistance in healthy preschool white children. Thorax 2001;56:691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merkus PJFM, Mijnsbergen JY, Hop WCJ, de Jongste JC. Interrupter resistance in preschool children: measurement characteristics and reference values. Am J Respir Crit Care Med 2001;163:1350–1355. [DOI] [PubMed] [Google Scholar]
- 60.Bridge PD, Ranganathan S, McKenzie SA. Measurement of airway resistance using the interrupter technique in preschool children in the ambulatory setting. Eur Respir J 1999;3:792–796. [DOI] [PubMed] [Google Scholar]
- 61.Beydon N, Amsallem F, Bellet M, Boule M, Chaussain M, Denjean A, Matran R, Wuyam B, Alberti C, Gaultier C; French Pediatric Programme Hospitalier de Recherche Clinique Group. Pre/postbronchodilator interrupter resistance values in healthy young children. Am J Respir Crit Care Med 2002;165:1388–1394. [DOI] [PubMed] [Google Scholar]
- 62.Beydon N, Amsallem F, Bellet M, Boule M, Chaussain M, Denjean A, Matran R, Pin I, Alberti C, Gaultier C. Pulmonary function tests in preschool children with cystic fibrosis. Am J Respir Crit Care Med 2002;166:1099–1104. [DOI] [PubMed] [Google Scholar]
- 63.Beydon N, Pin I, Matran R, Chaussain M, Boule M, Alain B, Bellet M, Amasallem F, Alberti C, Denjean A, et al. Pulmonary function tests in preschool children with asthma. Am J Respir Crit Care Med 2003;168:640–644. [DOI] [PubMed] [Google Scholar]
- 64.Desager K, Marchal F, Van de Woestijne K. Forced oscillation technique. In: Stocks J, Sly PD, Tepper RS, Morgan WJ, editors. Infant respiratory function testing. New York: John Wiley and Sons; 1996. pp. 355–378.
- 65.Johnson B, Beck K, Zeballos J, Weisman I. Global theme issue: emerging technology in clinical medicine: advances in pulmonary laboratory testing. Chest 1999;116:1377–1386. [DOI] [PubMed] [Google Scholar]
- 66.Navajas D, Farre R. Forced oscillation technique: from theory to clinical applications. Monaldi Arch Chest Dis 2001;56:555–562. [PubMed] [Google Scholar]
- 67.Ducharme FM, Davis GM, Ducharme GR. Pediatric reference values for respiratory resistance measured by forced oscillation. Chest 1998;113:1322–1328. [DOI] [PubMed] [Google Scholar]
- 68.Duiverman EJ, Clement J, van de Woestijne KP, Neijens HJ, van den Bergh ACM, Kerrebijn KF. Forced oscillation technique: reference values for resistance and reactance over a frequency spectrum of 2–26 Hz in healthy children aged 2.3–12.5 years. Bull Eur Physiopathol Respir 1985;21:171–178. [PubMed] [Google Scholar]
- 69.Mazurek H, Willim G, Marchal FHJ, Tomalak W. Input respiratory impedance measured by head generator in preschool children. Pediatr Pulmonol 2000;30:47–55. [DOI] [PubMed] [Google Scholar]
- 70.Klug B, Bisgaard H. Specific airway resistance, interrupter resistance, and respiratory impedance in healthy children aged 2–7 years. Pediatr Pulmonol 1998;25:322–331. [DOI] [PubMed] [Google Scholar]
- 71.Malmberg LP, Pelkonen A, Poussa T, Pohianpalo A, Haahtela T, Turpeinen M. Determinants of respiratory system input impedance and bronchodilator response in healthy Finnish preschool children. Clin Physiol Funct Imaging 2002;22:64–71. [PubMed] [Google Scholar]
- 72.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF Jr, Strunk RC, Allen DB, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006;354:1985–1997. [DOI] [PubMed] [Google Scholar]
- 73.Bisgaard H, Nielsen KG. Plethysmographic measurements of specific airway resistance in young children. Chest 2005;128:355–362. [DOI] [PubMed] [Google Scholar]
- 74.Nielsen KG, Bisgaard H. Cold air challenge and specific airway resistance in preschool children. Paediatr Respir Rev 2005;6:255–266. [DOI] [PubMed] [Google Scholar]
- 75.Nielsen KG. Plethysmographic specific airway resistance. Paediatr Respir Rev 2006;7S:S17–S19. [DOI] [PubMed] [Google Scholar]
- 76.Aurora P. Multiple-breath washout in preschool children: FRC and ventilation inhomogeneity. Paediatr Respir Rev 2006;7S:S14–S16. [DOI] [PubMed] [Google Scholar]
- 77.Marostica PJ, Weist AD, Eigen H, Angelicchio C, Christoph K, Savage J, Grant D, Tepper RS. Spirometry in 3- to 6-year-old children with cystic fibrosis. Am J Respir Crit Care Med 2002;166:67–71. [DOI] [PubMed] [Google Scholar]
- 78.Vilozni D, Bentur L, Efrati O, Minuskin T, Barak A, Szeinberg A, Blau H, Picard E, Kerem E, Yahav Y, et al. Spirometry in early childhood in cystic fibrosis patients. Chest 2007;131:356–361. [DOI] [PubMed] [Google Scholar]
- 79.Nielsen KG, Pressler T, Klug B, Koch C, Bisgaard H. Serial lung function and responsiveness in cystic fibrosis during early childhood. Am J Respir Crit Care Med 2004;169:1209–1216. [DOI] [PubMed] [Google Scholar]
- 80.Brennan S, Hall GL, Horak F, Moeller A, Pitrez PM, Franzmann A, Turner S, de Klerk N, Franklin P, Winfield KR, et al. Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax 2005;60:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lowe L, Murray CS, Custovic A, Simpson BM, Kissen PM, Woodcock A. Specific airway resistance in 3-year-old children: a prospective cohort study. Lancet 2002;359:1904–1908. [DOI] [PubMed] [Google Scholar]
- 82.Chan EY, Bridge PD, Dundas I, Pao CS, Healy MJ, McKenzie SA. Repeatability of airway resistance measurements made using the interrupter technique. Thorax 2003;58:344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slattery DM, Zurakowski D, Colin AA, Cleveland RHCF. An X-ray database to assess effect of aerosolized tobramycin. Pediatr Pulmonol 2004;38:23–30. [DOI] [PubMed] [Google Scholar]
- 84.Puderbach, M, Eichinger, M, Gahr, J, Ley, S, Tuengerthal, S, Schmahl, A, Fink, C, Plathow, C, Wiebel, M, Muller, FM, Kauczor, HU. Proton MRI appearance of cystic fibrosis: comparison to CT. Eur Radiol 2007;17:716–724. [DOI] [PubMed] [Google Scholar]
- 85.van Beek EJ, Hill C, Woodhouse N, Fichele S, Fleming S, Howe B, Bott S, Wild JM, Taylor CJ. Assessment of lung disease in children with cystic fibrosis using hyperpolarized 3-helium MRI: comparison with Shwachman score, Chrispin-Norman score and spirometry. Eur Radiol 2007;17:1018–1024. [DOI] [PubMed] [Google Scholar]
- 86.Chen DL, Ferkol TW, Mintun MA, Pittman JE, Rosenbluth DB, Schuster DP. Quantifying pulmonary inflammation in cystic fibrosis with positron emission tomography. Am J Respir Crit Care Med 2006;173:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frush DP. Review of radiation issues for computed tomography. Semin Ultrasound CT MR 2004;25:17–24. [DOI] [PubMed] [Google Scholar]
- 88.Coddington R, Mera SL, Goddard PR, Bradfield JW. Pathological evaluation of computed tomography images of lungs. J Clin Pathol 1982;35:536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Long FR, Williams RS, Castile RG. Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr 2004;144:154–161. [DOI] [PubMed] [Google Scholar]
- 90.Tiddens HAWM, Rosenfeld M, Brody AS, editors. Symposium: Imaging endpoints for cystic fibrosis clinical trials. Proc Am Thorac Soc 2007;4:295–363. [DOI] [PubMed] [Google Scholar]
- 91.Long FR, Castile RG, Brody AS, Hogan MJ, Flucke RL, Filbrun DA, McCoy KS. Lungs in infants and young children: improved thin-section CT with a noninvasive controlled-ventilation technique–initial experience. Radiology 1999;212:588–593. [DOI] [PubMed] [Google Scholar]
- 92.Robinson TE, Leung AN, Moss RB, Blankenberg FG, al-Dabbagh H, Northway WH. Standardized high-resolution CT of the lung using a spirometer-triggered electron beam CT scanner. AJR Am J Roentgenol 1999;172:1636–1638. [DOI] [PubMed] [Google Scholar]
- 93.de Jong PA, Ottink MD, Robben SG, Lequin MH, Hop WC, Hendriks JJ, Pare PD, Tiddens HA. Pulmonary disease assessment in cystic fibrosis: comparison of CT scoring systems and value of bronchial and arterial dimension measurements. Radiology 2004;231:434–439. [DOI] [PubMed] [Google Scholar]
- 94.Brody AS, Kosorok MR, Li Z, Broderick LS, Foster JL, Laxova A, Bandla H, Farrell PM. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging 2006;21:14–21. [DOI] [PubMed] [Google Scholar]
- 95.de Jong PA, Nakano Y, Hop WC, Long FR, Coxson HO, Pare PD, Tiddens HA. Changes in airway dimensions on computed tomography scans of children with cystic fibrosis. Am J Respir Crit Care Med 2005;172:218–224. [DOI] [PubMed] [Google Scholar]
- 96.Robinson TE, Leung AN, Northway WH, Blankenberg FG, Bloch DA, Oehlert JW, Al-Dabbagh H, Hubli S, Moss RB. Spirometer-triggered high-resolution computed tomography and pulmonary function measurements during an acute exacerbation in patients with cystic fibrosis. J Pediatr 2001;138:553–559. [DOI] [PubMed] [Google Scholar]
- 97.Bonnel AS, Song SM, Kesavarju K, Newaskar M, Paxton CJ, Bloch DA, Moss RB, Robinson TE. Quantitative air-trapping analysis in children with mild cystic fibrosis lung disease. Pediatr Pulmonol 2004;38:396–405. [DOI] [PubMed] [Google Scholar]
- 98.Robinson TE, Goris ML, Zhu HJ, Chen X, Bhise P, Sheikh F, Moss RB. Dornase alfa reduces air trapping in children with mild cystic fibrosis lung disease: a quantitative analysis. Chest 2005;128:2327–2335. [DOI] [PubMed] [Google Scholar]
- 99.Donnelly LF, Frush DP. Pediatric multidetector body CT. Radiol Clin North Am 2003;41:637–655. [DOI] [PubMed] [Google Scholar]
- 100.Egelhoff JC, Ball WS Jr, Koch BL, Parks TD. Safety and efficacy of sedation in children using a structured sedation program. AJR Am J Roentgenol 1997;168:1259–1262. [DOI] [PubMed] [Google Scholar]
- 101.Long FR, Williams RS, Adler BH, Castile RG. Comparison of quiet breathing and controlled ventilation in the high-resolution CT assessment of airway disease in infants with cystic fibrosis. Pediatr Radiol 2005;35:1075–1080. [DOI] [PubMed] [Google Scholar]
- 102.Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, Yankaskas BC, Johnson RC, Leigh MW. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med 2007;175:943–950. [DOI] [PubMed] [Google Scholar]
- 103.Nasr SZ, Kuhns LR, Brown RW, Hurwitz ME, Sanders GM, Strouse PJ. Use of computerized tomography and chest X-rays in evaluating efficacy of aerosolized recombinant human DNase in cystic fibrosis patients younger than age 5 years: a preliminary study. Pediatr Pulmonol 2001;31:377–382. [DOI] [PubMed] [Google Scholar]
- 104.de Jong PA, Long FR, Nakano Y. Computed tomography dose and variability of airway dimension measurements: how low can we go? Pediatr Radiol 2006;36:1043–1047. [DOI] [PubMed] [Google Scholar]
- 105.Long FR, Williams RS, Castile RG. Inspiratory and expiratory CT lung density in infants and young children. Pediatr Radiol 2005;35:677–683. [DOI] [PubMed] [Google Scholar]
- 106.Pocock SJ, Geller NL, Tsiatis AA. The analysis of multiple endpoints in clinical trials. Biometrics 1987;43:487–498. [PubMed] [Google Scholar]
- 107.Heitjan DF. Ignorability and coarse data: some biomedical examples. Biometrics 1993;49:1099–1109. [PubMed] [Google Scholar]
- 108.Brody AS, Klein JS, Molina PL, Quan J, Bean JA, Wilmott RW. High-resolution computed tomography in young patients with cystic fibrosis: distribution of abnormalities and correlation with pulmonary function tests. J Pediatr 2004;145:32–38. [DOI] [PubMed] [Google Scholar]
- 109.Thomassen MJ, Klinger JD, Badger SJ, van Heeckeren DW, Stern RC. Cultures of thoracotomy specimens confirm usefulness of sputum cultures in cystic fibrosis. J Pediatr 1984;104:352–356. [DOI] [PubMed] [Google Scholar]
- 110.Jung A, Kleinau I, Schonian G, Bauernfeind A, Chen C, Griese M, Doring G, Gobel U, Wahn U, Paul K. Sequential genotyping of Pseudomonas aeruginosa from upper and lower airways of cystic fibrosis patients. Eur Respir J 2002;20:1457–1463. [DOI] [PubMed] [Google Scholar]
- 111.Aaron SD, Kottachchi D, Ferris WJ, Vandemheen KL, St Denis ML, Plouffe A, Doucette SP, Saginur R, Chan FT, Ramotar K. Sputum versus bronchoscopy for diagnosis of pseudomonas aeruginosa biofilms in cystic fibrosis. Eur Respir J 2004;24:631–637. [DOI] [PubMed] [Google Scholar]
- 112.Armstrong DS, Grimwood K, Carlin JB, Carzino R, Olinsky A, Phelan PD. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatr Pulmonol 1996;21:267–275. [DOI] [PubMed] [Google Scholar]
- 113.Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castile R, Grimwood K, Hiatt P, McCoy K, McNamara S, Ramsey B, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol 1999;28:321–328. [DOI] [PubMed] [Google Scholar]
- 114.Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, Hiatt P, McCoy K, Castile R, Smith AL, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis 2001;183:444–452. [DOI] [PubMed] [Google Scholar]
- 115.Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, Grimwood K. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 2001;138:699–704. [DOI] [PubMed] [Google Scholar]
- 116.Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, Grimwood K, Wainwright C. Early airway infection, inflammation, and lung function in cystic fibrosis. Arch Dis Child 2002;87:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Armstrong DS, Hook SM, Jamesen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol 2005;40:500–510. [DOI] [PubMed] [Google Scholar]
- 118.Hilliard TN, Sukhani S, Francis J, Madden N, Rosenthal M, Balfour-Lynn I, Bush A, Davies J. Bronchoscopy following diagnosis with cystic fibrosis. Arch Dis Child 2006. [Epub ahead of print, Nov 6] [DOI] [PMC free article] [PubMed]
- 119.Wainwright C, Carline J, Cooper P, Byrnes C, Dakin C, Whitehead B, Carter R, Vidmar S, Cheney J, Tiddens H, et al. Australian cystic fibrosis BAL study interim analysis. Pediatr Pulmonol 2006;(Suppl 29):A313.
- 120.Kettle AJ, Chan T, Osberg I, Senthilmohan R, Chapman AL, Mocatta TJ, Wagener JS. Myeloperoxidase and protein oxidation in the airways of young children with cystic fibrosis. Am J Respir Crit Care Med 2004;170:1317–1323. [DOI] [PubMed] [Google Scholar]
- 121.Rosenfeld M, Gibson R, McNamara S, Emerson J, McCoy KS, Shell R, Borowitz D, Konstan MW, Retsch-Bogart G, Wilmott RW, et al. Serum and lower respiratory tract drug concentrations after tobramycin inhalation in young children with cystic fibrosis. J Pediatr 2001;139:572–577. [DOI] [PubMed] [Google Scholar]
- 122.Wagener JS, Rock MJ, McCubbin MM, Hamilton SD, Johnson CA, Ahrens RC. Aerosol delivery and safety of recombinant human deoxyribonuclease in young children with cystic fibrosis: a bronchoscopic study. Pulmozyme Pediatric Bronchoscopy Study Group. J Pediatr 1998;133:486–491. [DOI] [PubMed] [Google Scholar]
- 123.Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, Hamblett N, Accurso F, Dovey M, Hiatt P, et al.; Cystic Fibrosis Therapeutic Development Network Study Group. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med 2003;167:841–849. [DOI] [PubMed] [Google Scholar]
- 124.Gutierrez JP, Grimwood K, Armstrong DS, Carlin JB, Carzino R, Olinsky A, Robertson CF, Phelan PD. Interlobar differences in bronchoalveolar lavage fluid from children with cystic fibrosis. Eur Respir J 2001;17:281–286. [DOI] [PubMed] [Google Scholar]
- 125.Meyer KC, Sharma A. Regional variability of lung inflammation in cystic fibrosis. Am J Respir Crit Care Med 1997;156:1536–1540. [DOI] [PubMed] [Google Scholar]
- 126.Wainwright C. Bronchoalveolar lavage to guide management of lung disease in children with cystic fibrosis. Pediatr Pulmonol 2006;(Suppl 29):S18.3.
- 127.Rabin HR, Butler SM, Wohl ME, Geller DE, Colin AA, Schidlow DV, Johnson CA, Konstan MW, Regelmann WE; Epidemiologic Study of Cystic Fibrosis. Pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol 2004;37:400–406. [DOI] [PubMed] [Google Scholar]