Abstract
Many environmental factors and a large number of genetic polymorphisms have been reported to be associated with asthma risk in different locales and at different ages. It seems that what we call asthma is a heterogeneous set of conditions for which the only common feature is recurrent airway obstruction that is at least partially responsive to usual asthma therapy. Recent studies in which environmental factors and genetic variants were studied concomitantly have suggested a potential unifying concept for the disease. It seems that asthma is a genetically mediated development dysregulation of diverse immune and airway responses to a variety of specific and nonspecific exposures. It thus seems improbable that most genetic variants associated with asthma influence the disease regardless of which environmental factors trigger it and at which lifetime phase they are present. More likely, the most important gene variants for asthma are polymorphisms that exert their influence on the network system controlling biological responses to asthma-related exposures.
Keywords: asthma, genetics, environment, interaction
Understanding what causes complex diseases such as asthma is fraught with what often seem to be overwhelming difficulties. Cross-sectional studies of the phenotypic characteristics of subjects who have symptoms compatible with a diagnosis of asthma invariably underscore its heterogeneity to the point that the only feature that seems common to all patients with asthma are the symptoms themselves and their responsiveness to usual therapy. In the last decades, researchers have proposed hundreds of molecules and dozens of cells as crucially involved in asthma pathogenesis. These claims were sometimes based on findings in humans, but more often they relied exclusively on animal models that imitate the pathophysiology of asthma but that are seldom (if ever) the defining feature of the disease (i.e., its symptoms). Successive cells, genes, or molecules were touted as the central players in causing the disease to be replaced by more fashionable rivals shortly thereafter. Trials of products designed to counteract what seemed to be the most likely protagonists of the asthma drama (interleukin [IL]-4, IL-5, and eosinophils) have been unsuccessful. Exceptions are antileukotrienes and more recently anti-IgE antibodies, but the role and effectiveness of these medicines in asthma therapeutics remains to be determined. The two main kinds of medicines available for the treatment of asthma, glucocorticosteroids and β2-adrenergic agonists, are sophisticated variants of the extracts of adrenal glands that were already in use in the early 20th century.
This situation is by no means exclusive to asthma. A recent editorial in the Journal of Clinical Investigation lamented the status of our understanding of the pathogenesis of rheumatoid arthritis (1). After having described as a “great success” the way in which “researchers have dismantled the pathogenic subunits of rheumatoid arthritis, adding gene to gene, molecule to molecule, and pathway to pathway in an ever more complex scheme of dysfunction,” the authors acknowledge that “the complexity of the emerging disease model leaves us speechless.” Based on the idea that “true understanding of a natural phenomenon is reached when it can be expressed as a simple formula,” they “yearn for a theory that would fit it all together, under one formula, one idea, one mechanism.” Otherwise, they conclude, “autoimmune diseases may be just a mess, lacking a unifying disease concept and representing nothing more than an overwhelming conglomerate of defective cells, mediators, and pathways.”
THE CENTRALITY OF ENVIRONMENTAL EXPOSURES AS A UNIFYING THEORY FOR ASTHMA
There have been recent unifying theories to explain the majority of cases of asthma. In the 1980s, the theory that exposure to house dust mites and other allergens was an important worldwide cause of the disease was espoused by many scientists and experts (2, 3). This theory was based on strong evidence suggesting that, in regions where indoor exposure to one of the main house dust mite allergens (Der p I) was high, more children were sensitized to house dust mites, and these children had significantly more airway hyperresponsiveness and recent wheeze (4). It was also reported that the risk of house dust mite–sensitized children having current asthma doubled with every doubling of Der p I level (4). Strong evidence against this theory has emerged in recent years, in part generated by the same scientist who initially supported it. For example, and contrary to what should have been expected based on the theory, the prevalence of asthma in geographic regions with high levels of exposure was reported to be similar to that observed in regions of low or very low exposure to house dust mites in Sweden and the United States (5, 6). In the latter regions, other allergens (e.g., molds) seem to take the place of mites (7, 8). Perhaps the most decisive evidence against the theory that the level of exposure to allergens is causally related to asthma comes from interventional studies. In these studies, measures were taken to decisively decrease exposure to house dust mites during the first years of life in areas of the United Kingdom (9) and Australia (10) where the association between mites and asthma is the strongest. These prospective, randomized studies have shown that decreasing exposure to mites from birth has no effect on asthma incidence, that is, on the development of new cases of asthma during the first years of life.
The idea that exposure to specific allergens causes asthma had a great impact on the type of experimental animal models used to study the potential role of different molecular mechanisms in the pathogenesis of the disease. Indeed, most of these models are based on timed exposures to allergens (mostly ovalbumin) (11). However, in the light of the recent negative findings of randomized avoidance trials described previously, the relevance of these models for human asthma remains to be determined.
The house dust mite theory is one example of the many unifying environmental causes of asthma that have been proposed in recent years. Others include the possibility that cockroaches may be the cause of the high prevalence of asthma in inner cities in the United States (12), that viral infections may trigger the asthma process in early life (13), and that air pollution may be an important cause of asthma in the modern world (14). Not all of these theories have been scrutinized or tested as thoroughly or as rigorously as the mite theory, but it is unlikely that any of them may be the basis for that “single formula” that will “fit it all together.”
THE CENTRALITY OF GENES AS A UNIFYING THEORY FOR ASTHMA
That genetic factors play a major role in determining susceptibility to asthma is undisputed. There is strong evidence suggesting higher concordance for asthma among monozygotic than among dizygotic twins (15), and the study of the familiar aggregation and segregation of asthma is clearly suggestive of a heritable component (16). One of the most remarkable successes in genetic and genomic sciences since the description of the structure of DNA in the 1950s has been the identification of thousands of mutations in genes that cause monogenic disease. Prompted by these successes and by the recent development of reliable new technologies to screen the whole human genome for genetic variants, many geneticists proposed a next frontier for genetic research: the discovery of the genetic variants that cause complex diseases. Two mains approaches were undertaken: linkage and association studies. A few studies reported on genome-wide linkage scans using sib pairs or other family-based approaches (17). These studies resulted in many suggestive and a few highly suggestive linkage signals, and in four such cases, genetic variants in genes located close to the linkage signals were identified as potentially explaining them by positional cloning (18). On the other hand, hundreds of studies of the association of asthma, variably defined, and asthma-related traits to polymorphisms in tens of candidate genes have been published (19). A recent, thorough review of the results of these studies and of attempts to replicate them provides a sobering scenario: In the great majority of cases, including those derived from genome-wide searches, the association was not replicated, and when it was replicated, it was often with a different allele for the same polymorphism, with a different polymorphism within the same gene, or with an asthma-related phenotype that was different from the one reported in the original study that was being replicated (19). The most consistent feature of the studies of the genetics of asthma is their remarkable inconsistency.
Not all genetic (or environmental) studies are of similar, high quality, and the possibility that these inconsistencies may be due to methodologic flaws should be seriously considered. There is little doubt that, when first reporting their results, researchers tend to stress their positive findings and often fail to report their concomitant negative ones, thus increasing the possibility of a type I error (20). It is also possible that studies attempting replication may be too small or may define the phenotype in less restrictive ways than that of the original report, thus falling generally into type II errors. For example, the original publication of the discovery of ADAM33 as a gene for asthma (21) was based on a subset of the original families in which asthma was defined as having symptoms of the disease and bronchial hyperresponsiveness (BHR). Replication of this finding among subjects with asthma, defined regardless of BHR, would require a much larger number of subjects because those without BHR would dilute the signal. Thus, it is possible that the difficulty in reproducing results of the genetics of asthma may be in part attributable to the kind of methodologic flaws that can plague these types of studies (22).
Results of a recent report using a large general population sample from the United Kingdom have provided new insights into this issue. Maier and coworkers (23) chose an easily definable phenotype strongly associated with asthma and repeatedly shown in the past to have a strong genetic component: total serum IgE levels. They genotyped several single nucleotide polymorphisms previously reported to be associated with total serum IgE in over 4,000 subjects. For only one of these polymorphisms (IL-13/−1112, in the promoter region of IL-13) was the association replicated, and the polymorphism explained less than 1% of total serum IgE variance in the population. Maier and coworkers embraced one of the potential conclusions that could be derived from these results: Genetic studies should be limited to very large samples; otherwise, false-positive results will be obtained. The implication was that the findings of most of the previous studies (with the exception perhaps of those for the IL-13 gene) were spurious.
Before such a conclusion can be accepted at face value, the undeclared assumption on which association studies, such as that by Maier and coworkers, are based needs to be thoroughly discussed. Engaging in such a discussion is not a useless “philosophical” diversion but is a necessary step that allows us to develop more potent strategies to identity asthma-related genes.
ASSUMPTIONS OF DIRECT STUDIES OF THE GENETIC ASSOCIATIONS IN ASTHMA
The most important assumption of studies of marginal genetic effects (i.e., of direct effects of genetic variants, independent of context) in asthma is what can be called the theory of the low-hanging fruits: the complexity of the determination of the disease was acknowledged, but the costs and difficulties implicit in assessing the contexts and the ready accessibility of modern genotyping technologies prompted researchers (the author included) to surmise that there are genetic variants in the human genome that show strong, unidirectional, and consistent relations with asthma (or related traits) regardless of the phenotypic or environmental background in which they are present. In almost all cases, we are referring to common variants. There are few (if any) available datasets that can reasonably expect to detect even strong and nonrecessive associations with variants of frequencies of less than 5%.
The assumption that there are common genetic variants that increase susceptibility to asthma independent of other influences is legitimate and plausible. This assumption would require that, along human evolution, a genetic variant would have been selected (or at least would not have been selected against) that makes people have recurrent airway obstruction and hunger for air independent of any other genetic variants and of the environmental context in which the individual was raised. This seems unlikely, unless the variant protected against some other outcome that markedly decreased the individual's fitness. For example, it is possible that a polymorphism that consistently increases the likelihood of having asthma may improve immune responses against an infection or infestation. This may be the case of IL-13/−1112, which has been extensively replicated as a determinant of higher total serum IgE levels and has been shown to protect against parasitic infections (24). There is also evidence of selective pressure in and around the IL-13 locus (25). Other polymorphisms in genes involved in T-helper 2–like responses, such as IL-13, may show similar, highly replicable associations with asthma-related traits. An example could be the gene for the IL-4α receptor chain. Polymorphisms in this gene have been found to be associated with asthma-related traits in isolation (26) and through interactive (“epistatic”) effects with IL-13/−1112 (27).
Thus, the search for polymorphisms that may consistently increase the likelihood of having asthma or asthma-related traits is legitimate and is supported by replicated findings. What is implausible is to surmise that all, or even most, polymorphisms associated with complex traits invariably behave in this manner.
OPPOSITE EFFECTS OF THE SAME POLYMORPHISMS IN DIFFERENT POPULATIONS
A good example of a situation in which this may not be the case is our recent report of the association between polymorphisms in the defensin-β1 (DEFB1) gene and asthma (28). DEFB1 encodes for a protein belonging to a group of antimicrobial peptides with broad spectrum of activity against gram-positive and gram-negative bacteria and fungal species (29). DEFB1 is constitutively expressed in airway epithelium (30) and is believed to play an important role in mucosal immunity in the lung (31). Thus, it is a potential candidate gene for asthma, given the importance of innate immunity in the development of the disease. We therefore screened the gene for common polymorphisms and reported on results obtained by genotyping four of these polymorphisms (i.e., those that correctly classified 80% of the total haplotype diversity among European Americans) in two samples: a nested case-control study of over 1,000 participants in the Nurses' Health Study (32) and a family-based study of over 400 participants in the Childhood Asthma Management Program and their nuclear families (33). We found that a haplotype based on the different alleles (A, C, T, and A) for the four polymorphisms studied (at cDNA positions −1,905, −1,816, −390, and +692, respectively) was associated with increase likelihood of having asthma in the Nurses' Health Study sample (all females) but with decreased risk for asthma in girls, but not in boys, enrolled in CAMP. Thus, there were two sources of heterogeneity in these studies: (1) adult female nurses were protected against asthma by the same alleles that increased this risk in girls of the same ethnic group and (2) boys showed no association between asthma and DEFB1.
What causes these evident discrepancies? It is possible that what is called asthma in childhood is not the same disease that is called asthma in adults and that DEFB1 may play opposite roles in both illnesses. In order for DEFB1 to do so, genetic, developmental, and environmental factors need to influence the functional effects that the polymorphisms under study have on the causal pathway that leads to recurrent airway obstruction. In other words, the expression of the genotype is strictly dependent on the context in which it acts and this context may even have an antagonistic interaction with the genotype: the same allele that increases risk in one context may decrease it in a different one. The fact that the sex of the child determines if the polymorphism will increase the risk of asthma suggests that sex may act as a kind of “internal environment” (34) that modulates the association between genes and phenotypes.
GENETICS OF RESPONSES TO ENVIRONMENTAL EXPOSURES: THE REACTION NORM
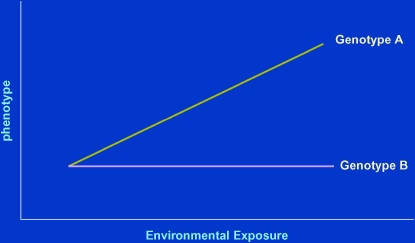
Important insights into the manner in which genetic polymorphisms and environmental influences may jointly determine susceptibility to complex phenotypes such as asthma is offered by a commonplace observation: an almost invariable feature of responses to any exposure by a biological system is the presence of reproducible interindividual variability. This can be expressed graphically in the so-called reaction norms: measured phenotypic responses of different groups of individuals (e.g., those with different genotypes for a certain gene locus) are plotted against the extent (or dose) of an environmental exposure (Figure 1). The group identified as “genotype A” shows a clear dose–response relationship between the exposure and the phenotype (this responsiveness is called phenotypic plasticity), whereas “genotype B” is unresponsive. In this situation, if the association between the genotype and the phenotype were to be studied without knowledge of the exposure, completely different conclusions would be reached by investigators studying this association at the two extremes of the exposure spectrum: those studying it at high exposure would conclude that the locus is strongly related to the outcome, whereas those studying at low levels of exposure would conclude that it is not. It is evident from Figure 1 that both would be right. Similarly, if investigators interested in the biological effects of exposure studied this phenomenon in a population in which most subjects were of genotype A, they would reach a very different conclusion from another set of investigators studying it in persons of predominant genotype B.
Figure 1.
Schematic representation of the “reaction norms” for two genotypes. Genotype A shows a strong dose–response effect between an environmental exposure and the phenotype. Genotype B shows no phenotypic response to the same exposure.
GENE–ENVIRONMENT INTERACTIONS IN ASTHMA AND ATOPY
There are several reported cases of this type of gene–environment interaction in asthma. McIntire and coworkers first observed, using an animal model of allergy, that the gene that encodes for the receptor for the hepatitis A virus (HAV), TIM-1, is a regulator of T-helper cell differentiation (35). They next identified three coding polymorphisms in the TIM-1 gene in humans, one of which resulted in a 6–amino-acid insertion at residue 157, dubbed 157insMTTTVP (36). In over 300 individuals, they tested sera for evidence of past infection with HAV and allergic sensitization (atopy). They found that subjects with a previous HAV infection were less likely to be sensitized but only if they had the 157insMTTTVP variant (36). They concluded that, for the protective effect of the variant or HAV to occur, both needed to be present. Several other groups have studied the association between this same variant and atopy but without assessing concomitantly evidence for previous infection with HAV. Noguchi and colleagues (37) found no association between polymorphisms in TIM1 and atopic asthma and suggested that this discrepancy may be due to a low frequency of HAV in the young generation in Japan. In a Korean case-control study, Chae and colleagues (38) reported a decreased risk for atopic dermatitis but not for asthma with the insertion. In our own work using children enrolled in the Tucson Children's Respiratory Study (39), we found that the insertion was significantly but positively related to atopy (relative risk [RR], 1.24; 95% confidence interval [CI], 1.07–1.45) and borderline significantly with eczema (RR, 1.43; 95% CI 1.01–2.01) but not with asthma (RR, 0.95; 95% CI, 0.68–1.33). Taken as whole, these apparently contradictory results suggest that, much as it was true for DEFB1, the nature and even the direction of the association between the TIM1 insertion variant and atopy may vary depending on the genetic and environmental background in which the association is studied.
A similar type of gene–environment interaction was observed by Eder and colleagues (40) in studies of children living in central Europe on farms or in the same rural communities but away from farms. These researchers had previously reported that the children of farmers were significantly less likely to have atopic asthma, allergic rhinitis, and atopy than their nonfarming counterparts (41). Eder and colleagues hypothesized that these protective effects could be modulated by genetic variants in the Toll-like receptors (TLRs) and genotyped these populations for known polymorphisms in the genes for TLR2 and TLR4. They found that a farming environment was associated with less asthma and atopy but only among carriers of one of the two alleles of a single nucleotide polymorphism (SNP) in the TLR2 gene. Much like was the case for HAV and TIM1, the protective TLR2 allele and the farming environment needed to be present for the children to be at less risk of having asthma and related traits.
Similarly, Hoffjan and colleagues (42) studied several SNPs in genes that had been previously reported to be associated with asthma and atopy and assessed the relation between these SNPs and early-life immune phenotypes (ascertained in peripheral blood cells and also suspected to be associated with asthma risk) in infants who were taken to day care and in those who were not. Day care attendance in infancy has been reported to protect against the development of asthma and atopy during the school years (43), and the authors reasoned that this could happen because day care attendance influenced the development of immune responses in early life in susceptible children. They found that, for several of these SNPs (i.e., those in IL-4R and in nitric oxide synthase 3), strong antagonistic interactions were observed with day care attendance as determinants of the immune phenotypes under study. This means that carriers of certain alleles for these genes showed increases in the likelihood of having higher values of these phenotypes if taken to day care and decreases in the same phenotypes if not taken to day care.
CD14, ASTHMA, ATOPY, AND ENDOTOXIN EXPOSURE
The examples provided previously are indicative of how complex the genotype–phenotype mapping functions may be and of the need to better understand the biological mechanisms that underlie the interactions described. However, many of these interactions have not been replicated in studies done in different environments and at different levels of exposure for the same environmental factor.
The exception to this last caveat is provided by CD14. Polymorphisms in this gene are among the most widely studied, not only in relation to asthma and atopy but also to many other diseases and conditions known or suspected to be associated with exposure to endotoxin or to its main biologically active component, lipopolysaccharide (LPS). CD14 is indeed a crucial component of the innate immune response, acting as a coadjuvant that allows activation of TLR4, the receptor for LPS, at fentomolar concentrations of LPS, thus enhancing the subsequent triggering of the intracellular signaling mechanism. Because the protective effect of the farming environment was attributed to exposure to environmental endotoxin (44), it was natural to surmise that SNPs in CD14 could regulate this protective influence. Our group first described several SNPs in CD14 (45, 46), and we reported that the T allele for one such SNP, a C-to-T conversion at position −159 from the transcription start site (−260 from the translation start site), was associated with elevated serum sCD14 levels and decreased number of positive skin tests in atopic subjects raised in Tucson, Arizona (45). These findings suggested that, in an environment of generally low exposure to endotoxin (surmised but not directly measured), the T allele of CD14/−159, by increasing the availability of CD14 receptor, made children more sensitive to the purported protective influence of endotoxin. Two subsequent reports seemed to support this hypothesis: functional studies revealed that the T allele was associated with increased CD14 transcription rates (47), and investigators studying the same SNP in the Netherlands replicated our finding of a protective effect of the T allele with respect to allergic sensitization (48). However, as has happened for many other polymorphisms, other researchers were unable to reproduce our findings (49), and, among the Hutterites living in rural communities in the midwestern United States, the T allele was found to increase the risk for the development of allergic sensitization (50). Although endotoxin exposure was not measured among the Hutterites, the assumption was made that they could be high and therefore that the direction of the association could depend on the level of exposure to the CD14 ligand.
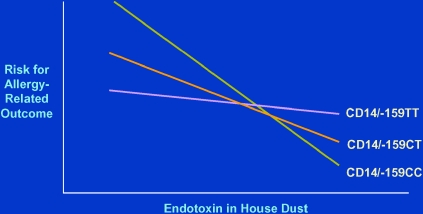
Important contributions to the understanding of these issues have been made by more recent studies in which the concentrations of endotoxin in dust from homes were measured, and, concomitantly, subjects living in those homes were genotyped for CD14/−159 (51–53). The results of these studies have been consistent and convincing (Figure 2): among subjects exposed to low levels of endotoxin, the T allele for CD14/−159 was protective against asthma or atopy; at high levels of exposure, the opposite was observed, whereby the C allele was protective as compared with the T allele. At intermediate levels, no clear trends were observed.
Figure 2.
Schematic representation of the association between allergy-related phenotypes and exposure to endotoxin for three genotypes of CD14/−159 (51–53).
The biological bases for this pattern are not well understood. However, some indications have been given by studies of healthy human subjects exposed to aerosolized endotoxin (Le Van and colleagues, unpublished data). These studies suggest that, although at baseline carriers of the CD14/−159 TT genotype have higher sCD14 circulating levels, carriers of the CC genotype show increased sCD14 levels when exposed to endotoxin, whereas carriers of the T allele show no such responses. This observation is compatible with the reaction norms depicted in Figure 2: carriers of the C allele show the greatest degree of phenotypic plasticity when exposed to different doses of endotoxin, whereas the dose–response curve seems to be much flatter among carriers of the T allele.
A UNIFYING DISEASE CONCEPT FOR ASTHMA: GENE–ENVIRONMENT INTERACTIONS
In contrast to other complex diseases, the heterogeneous phenotypic expressions of asthma have one, almost invariable common feature: environmental factors can be identified (or at least suspected) that may initiate the disease process and that can trigger asthma exacerbations and periods of loss of asthma control. The latter (e.g., viral infection, allergens, aspirin, cold air, pollution, hormones, chemicals) can vary from patient to patient and within patients at different times or ages, but it is rare to find a patient with the disease who cannot identify any such triggers. Equally, environmental factors (e.g., exposure to a farm [41] or to day care [43]) may be able to determine or protect against the inception of the disease, especially during the first years of life. Because asthma is also known to have a strong heritable component, a unifying disease concept seems to emerge from all these observations: asthma is a genetically mediated, developmental dysregulation of diverse immune and airway responses to a variety of specific and nonspecific (usually airborne) exposures, all resulting in the final common pathway of recurrent, partially reversible bronchial obstruction. Given the multiplicity of environmental factors for which asthma symptoms are the final common pathway, it seems improbable that most genetic variants associated with asthma influence the disease regardless of which those environmental factors may be and at which lifetime phase they are present. More likely, the most important gene variants for asthma are polymorphisms that exert their influence on the network systems controlling biological responses to asthma-related exposures.
Thanks to major recent advances in evolutionary biology, we are beginning to understand the fundamental nature of these response systems (54), but given their complexity, the discussion of these advances goes beyond the scope of this article. Nevertheless, a central, emerging feature of these response systems is that they arise from weak linkages between a small number of highly conserved core regulatory processes (55). This essentially means that, apart from these core processes—the genes for which are highly conserved along evolution (e.g., those for metabolism, gene expression, signaling between cells)—all other systems are highly flexible and establish indirect, undemanding, low-information regulatory connections between them and with the core processes. As a consequence, a specific protein may exert opposite effects when participating in coordinated responses to different external stimuli, and therefore, a genetic variant that increases transcription of that protein may enhance an “asthmatic” response to one exposure and hinder an “asthmatic” response to a different exposure. The specific role of any element of the response system is thus determined not only by its intrinsic characteristics but also by the biological context in which it is expressed.
This novel approach to the etiology of asthma necessarily implies that a better understanding of its causes will come from unraveling the heterogeneous response systems that are involved in its pathogenesis. Therefore, and contrary to monogenic conditions in which what is diseased is directly the gene responsible for the disease, in asthma what is altered are the airway/immune response systems that are activated against the different external stimuli that may initiate or trigger the disease. Whether a genetic variation contributes to the development of asthma depends on the role that the gene product influenced by the variation plays in each response system. For example, in the case of DEFB1, Levy and colleagues speculated that DEFB1 may play opposite roles in asthma triggered by viral infection, which is predominant in children, with respect to asthma triggered by other factors in adults (28). In that same case, the fact that the association was observed in female subjects but not in male subjects suggests that different response systems may be influenced differentially by sexual hormones and other sex-related factors.
This view of asthma is much more complex and is thus perhaps less parsimonious and attractive (in Ockham's razor sense that the simplest explanation is likely to be the correct one) than the original hope that genetic tests would allow us to identify who is at risk of which complex disease, regardless of any other influences. On the other hand, this view seems more in tone with the degree of heterogeneity and unpredictability of the expression of the disease that is evident in any asthma clinic.
Supported by NIH grants HL56177, HL64307, HL67672, and HL80083.
Conflict of Interest Statement: F.D.M. has in the last 3 yr served on a Merck advisory board. He has acted as a consultant for Genentech and Pfizer. He has received symposium reimbursement and honoraria from Merck.
References
- 1.Weyand CM, Goronzy JJ. Pathomechanisms in rheumatoid arthritis: time for a string theory? J Clin Invest 2006;116:869–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sporik R, Platts-Mills TA. Epidemiology of dust-mite-related disease. Exp Appl Acarol 1992;16:141–151. [DOI] [PubMed] [Google Scholar]
- 3.Sporik R, Chapman MD, Platts-Mills TA. House dust mite exposure as a cause of asthma. Clin Exp Allergy 1992;22:897–906. [DOI] [PubMed] [Google Scholar]
- 4.Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, Woolcock AJ. House dust mite allergens: a major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med 1996;153:141–146. [DOI] [PubMed] [Google Scholar]
- 5.Ronmark E, Lundback B, Jonsson E, Platts-Mills T. Asthma, type-1 allergy and related conditions in 7- and 8-year-old children in northern Sweden: prevalence rates and risk factor pattern. Respir Med 1998;92:316–324. [DOI] [PubMed] [Google Scholar]
- 6.Sporik R, Ingram JM, Price W, Sussman JH, Honsinger RW, Platts-Mills TA. Association of asthma with serum IgE and skin test reactivity to allergens among children living at high altitude: tickling the dragon's breath. Am J Respir Crit Care Med 1995;151:1388–1392. [DOI] [PubMed] [Google Scholar]
- 7.Perzanowski MS, Sporik R, Squillace SP, Gelber LE, Call R, Carter M, Platts-Mills TA. Association of sensitization to Alternaria allergens with asthma among school-age children. J Allergy Clin Immunol 1998;101:626–632. [DOI] [PubMed] [Google Scholar]
- 8.Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med 1997;155:1356–1361. [DOI] [PubMed] [Google Scholar]
- 9.Woodcock A, Lowe LA, Murray CS, Simpson BM, Pipis SD, Kissen P, Simpson A, Custovic A. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med 2004;170:433–439. [DOI] [PubMed] [Google Scholar]
- 10.Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, Ampon RD, Crisafulli D, Belousova EG, Mellis CM, et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol 2006;118:53–61. [DOI] [PubMed] [Google Scholar]
- 11.Pauluhn J, Mohr U. Experimental approaches to evaluate respiratory allergy in animal models. Exp Toxicol Pathol 2005;56:203–234. [DOI] [PubMed] [Google Scholar]
- 12.Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomes A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol 2001;107: 419–428. [DOI] [PubMed] [Google Scholar]
- 13.Dakhama A, Lee YM, Gelfand EW. Virus-induced airway dysfunction: pathogenesis and biomechanisms. Pediatr Infect Dis J 2005;24:S159–S169. [Discussion: S166–S167.] [DOI] [PubMed] [Google Scholar]
- 14.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet 2002;359:386–391. [DOI] [PubMed] [Google Scholar]
- 15.Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis 1990;142:1351–1358. [DOI] [PubMed] [Google Scholar]
- 16.Holberg CJ, Elston RC, Halonen M, Wright AL, Taussig LM, Morgan WJ, Martinez FD. Segregation analysis of physician-diagnosed asthma in Hispanic and non-Hispanic white families: a recessive component? Am J Respir Crit Care Med 1996;154:144–150. [DOI] [PubMed] [Google Scholar]
- 17.Los H, Koppelman GH, Postma DS. The importance of genetic influences in asthma. Eur Respir J 1999;14:1210–1227. [DOI] [PubMed] [Google Scholar]
- 18.Kere J, Laitinen T. Positionally cloned susceptibility genes in allergy and asthma. Curr Opin Immunol 2004;16:689–694. [DOI] [PubMed] [Google Scholar]
- 19.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun 2006;7:95–100. [DOI] [PubMed] [Google Scholar]
- 20.Ott J. Association of genetic loci: replication or not, that is the question. Neurology 2004;63:955–958. [DOI] [PubMed] [Google Scholar]
- 21.Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, Torrey D, Pandit S, McKenny J, Braunschweiger K, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature 2002;418:426–430. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DC, Clayton DG. Betting odds and genetic associations. J Natl Cancer Inst 2004;96:421–423. [DOI] [PubMed] [Google Scholar]
- 23.Maier LM, Howson JM, Walker N, Spickett GP, Jones RW, Ring SM, McArdle WL, Lowe CE, Bailey R, Payne F, et al. Association of IL13 with total IgE: evidence against an inverse association of atopy and diabetes. J Allergy Clin Immunol 2006;117:1306–1313. [DOI] [PubMed] [Google Scholar]
- 24.Kouriba B, Chevillard C, Bream JH, Argiro L, Dessein H, Arnaud V, Sangare L, Dabo A, Beavogui AH, Arama C, et al. Analysis of the 5q31-q33 locus shows an association between IL13–1055C/T IL-13–591A/G polymorphisms and Schistosoma haematobium infections. J Immunol 2005;174:6274–6281. [DOI] [PubMed] [Google Scholar]
- 25.Tarazona-Santos E, Tishkoff SA. Divergent patterns of linkage disequilibrium and haplotype structure across global populations at the interleukin-13 (IL13) locus. Genes Immun 2005;6:53–65. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuyasu H, Izuhara K, Mao XQ, Gao PS, Arinobu Y, Enomoto T, Kawai M, Sasaki S, Dake Y, Hamasaki N, et al. Ile50Val variant of IL4R alpha upregulates IgE synthesis and associates with atopic asthma. Nat Genet 1998;19:119–120. [DOI] [PubMed] [Google Scholar]
- 27.Howard TD, Koppelman GH, Xu J, Zheng SL, Postma DS, Meyers DA, Bleecker ER. Gene-gene interaction in asthma: IL4RA and IL13 in a Dutch population with asthma. Am J Hum Genet 2002;70:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy H, Raby BA, Lake S, Tantisira KG, Kwiatkowski D, Lazarus R, Silverman EK, Richter B, Klimecki WT, Vercelli D, et al. Association of defensin beta-1 gene polymorphisms with asthma. J Allergy Clin Immunol 2005;115:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganz T. Defensins and host defense. Science 1999;286:420–421. [DOI] [PubMed] [Google Scholar]
- 30.Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, Conway BA, Greenberg EP, Valore EV, Welsh MJ, Ganz T, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA 1998;95:14961–14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond G, Bevins CL. beta-Defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol 1998;88: 221–225. [DOI] [PubMed] [Google Scholar]
- 32.Colditz GA, Stampfer MJ, Willett WC, Rosner B, Speizer FE, Hennekens CH. A prospective study of parental history of myocardial infarction and coronary heart disease in women. Am J Epidemiol 1986;123:48–58. [DOI] [PubMed] [Google Scholar]
- 33.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054–1063. [DOI] [PubMed] [Google Scholar]
- 34.Weiss LA, Pan L, Abney M, Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nat Genet 2006;38:218–222. [DOI] [PubMed] [Google Scholar]
- 35.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol 2001;2:1109–1116. [DOI] [PubMed] [Google Scholar]
- 36.McIntire JJ, Umetsu SE, Macaubas C, Hoyte EG, Cinnioglu C, Cavalli-Sforza LL, Barsh GS, Hallmayer JF, Underhill PA, Risch NJ, et al. Immunology: hepatitis A virus link to atopic disease. Nature 2003;425:576. [DOI] [PubMed] [Google Scholar]
- 37.Noguchi E, Nakayama J, Kamioka M, Ichikawa K, Shibasaki M, Arinami T. Insertion/deletion coding polymorphisms in hHAVcr-1 are not associated with atopic asthma in the Japanese population. Genes Immun 2003;4:170–173. [DOI] [PubMed] [Google Scholar]
- 38.Chae SC, Song JH, Lee YC, Kim JW, Chung HT. The association of the exon 4 variations of Tim-1 gene with allergic diseases in a Korean population. Biochem Biophys Res Commun 2003;312:346–350. [DOI] [PubMed] [Google Scholar]
- 39.Graves PE, Siroux V, Guerra S, Klimecki WT, Martinez FD. Association of atopy and eczema with polymorphisms in T-cell immunoglobulin domain and mucin domain-IL-2-inducible T-cell kinase gene cluster in chromosome 5q33. J Allergy Clin Immunol 2005;116:650–656. [DOI] [PubMed] [Google Scholar]
- 40.Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrlander C, Nowak D, Martinez FD. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol 2004;113:482–488. [DOI] [PubMed] [Google Scholar]
- 41.Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 2001;358:1129–1133. [DOI] [PubMed] [Google Scholar]
- 42.Hoffjan S, Nicolae D, Ostrovnaya I, Roberg K, Evans M, Mirel DB, Steiner L, Walker K, Shult P, Gangnon RE, et al. Gene-environment interaction effects on the development of immune responses in the 1st year of life. Am J Hum Genet 2005;76:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FM, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med 2000;343:538–543. [DOI] [PubMed] [Google Scholar]
- 44.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 2002;347:869–877. [DOI] [PubMed] [Google Scholar]
- 45.Baldini M, Lohman IC, Halonen M, Erickson RP, Holt P, Martinez FD. A polymorphism in the 5′-flanking region of the CD 14 gene is associated with circulating soluble CD14 levels and with total serum IgE. Am J Respir Cell Mol Biol 1999;20:976–983. [DOI] [PubMed] [Google Scholar]
- 46.Baldini M, Vercelli D, Martinez FD. CD14: an example of gene by environment interaction in allergic disease. Allergy 2002;57:188–192. [DOI] [PubMed] [Google Scholar]
- 47.LeVan TD, Bloom JW, Bailey TJ, Karp CL, Halonen M, Martinez FD, Vercelli D. A common single nucleotide polymorphism in the CD14 promoter decreases the affinity of Sp protein binding and enhances transcriptional activity. J Immunol 2001;167:5838–5844. [DOI] [PubMed] [Google Scholar]
- 48.Koppelman GH, Reijmerink NE, Colin Stine O, Howard TD, Whittaker PA, Meyers DA, Postma DS, Bleecker ER. Association of a promoter polymorphism of the CD14 gene and atopy. Am J Respir Crit Care Med 2001;163:965–969. [DOI] [PubMed] [Google Scholar]
- 49.Kabesch M, Hasemann K, Schickinger V, Tzotcheva I, Bohnert A, Carr D, Baldini M, Hackstein H, Leupold W, Weiland SK, et al. A promoter polymorphism in the CD14 gene is associated with elevated levels of soluble CD14 but not with IgE or atopic diseases. Allergy 2004;59:520–525. [DOI] [PubMed] [Google Scholar]
- 50.Ober C, Tsalenko A, Willadsen S, Newman D, Daniel R, Wu X, Andal J, Hoki D, Schneider D, True K, et al. Genome-wide screen for atopy susceptibility alleles in the Hutterites. Clin Exp Allergy 1999;29:11–15. [PubMed] [Google Scholar]
- 51.Zambelli-Weiner A, Ehrlich E, Stockton ML, Grant AV, Zhang S, Levett PN, Beaty TH, Barnes KC. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol 2005;115:1203–1209. [DOI] [PubMed] [Google Scholar]
- 52.Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrlander C, Nowak D, Martinez FD, nAllergy and Endotoxin Alex Study Team. Opposite effects of CD14/-260 on serum IgE levels in children raised in different environments. J Allergy Clin Immunol 2005;116:601–607. [DOI] [PubMed] [Google Scholar]
- 53.Simpson A, John SL, Jury F, Niven R, Woodcock A, Ollier WE, Custovic A. Endotoxin exposure, CD14 and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med 2006;174:386–392. [DOI] [PubMed] [Google Scholar]
- 54.Kirschner M, Gerhart J. The plausibility of life: resolving Darwin's dilemma. New Haven, CT: Yale University Press; 2005. pp. 109–142.
- 55.Conrad M. The geometry of evolution. Biosystems 1990;24:61–81. [DOI] [PubMed] [Google Scholar]