Abstract
Surfactant, a lipoprotein complex, was originally described for its essential role in reducing surface tension at the air–liquid interface of the lung; however, it is now recognized as being a critical component in lung immune host defense. Surfactant proteins (SP)-A and -D are pattern recognition molecules of the collectin family of C-type lectins. SP-A and SP-D are part of the innate immune system and regulate the functions of other innate immune cells, such as macrophages. They also modulate the adaptive immune response by interacting with antigen-presenting cells and T cells, thereby linking innate and adaptive immunity. Emerging studies suggest that SP-A and SP-D function to modulate the immunologic environment of the lung so as to protect the host and, at the same time, modulate an overzealous inflammatory response that could potentially damage the lung and impair gas exchange. Numerous polymorphisms of SPs have been identified that may potentially possess differential functional abilities and may act via different receptors to ultimately alter the susceptibility to or severity of lung diseases.
Keywords: collectins, innate immunity, lung disease
The enormous surface area of the lung epithelium is constantly exposed to inhaled pathogens, particulates, allergens, and oxidant gases. Because its primary function is gas exchange, this portal of entry to the entire body is a thin, delicate barrier, highly susceptible to injury if an immune challenge is not contained and uncontrolled inflammation develops. Thus, the presence of a local host defense system in the lung is critical for maintenance of normal lung function and defense against infection. Although many factors contribute to lung immunity, pulmonary surfactant plays unique and important roles in lung host defense.
SURFACTANT COMPONENTS AND FUNCTIONS
Although surfactant was originally described for its essential role in reducing surface tension at the air–liquid interface of the lung, it is now recognized as a sentinel in lung immune host defense. Isolated by lung lavage, it is a collection of interrelated macromolecular lipoprotein complexes that vary in composition, structure, and function. Surfactant is comprised of phospholipids and proteins (10%), which predominantly include the hydrophobic proteins surfactant protein (SP)-B and SP-C and the hydrophilic proteins SP-A and SP-D. Surfactant is synthesized and packaged into the type II cell secretory organelle called the lamellar body. In response to the stimulus of a deep breath, the contents of the lamellar bodies are secreted into the thin, liquid hypophase that covers the alveolar epithelium. It was initially thought that all four proteins were important in facilitating the adsorption of phospholipids to the air–liquid interface, where they reduce surface tension; however, subsequent studies have shown that only SP-B is essential for this function. For instance, infants who have low levels of SP-B due to genetic mutations that impair processing and/or secretion suffer from respiratory distress syndrome (RDS) (1). Treatment of babies who are born before the maturation of surfactant biosynthesis machinery with exogenous preparations of surfactant that contain SP-B and SP-C revolutionized pediatric medical care by reducing morbidity and mortality due to RDS. SP-C has been shown to bind to LPS, a bacterial cell wall component, and genetic mutations are associated with interstitial lung disease. The host defense functions of surfactant are primarily mediated by SP-A and SP-D. Nonciliated airway Clara cells and submucosal cells also synthesize SP-A and SP-D, which are translated into 26- to 36-kD and 43-kD products, respectively (2). It is not known whether the function of airway-derived proteins are the same as those produced at the alveolar level, but the lack of phospholipid synthesis suggests that the function of these proteins in the proximal airways is not related to reducing surface tension.
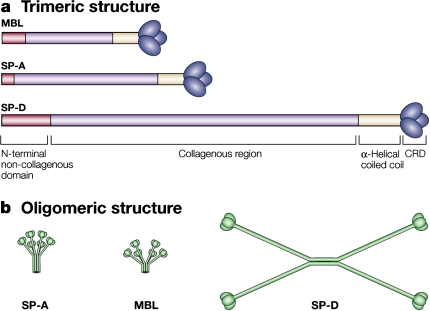
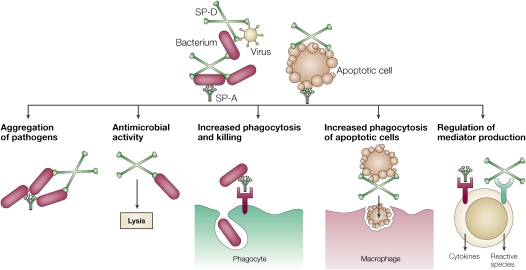
SP-A and SP-D are members of the collectin family of C-type lectins named for their aminoterminal collagen-like region and carboxyterminal lectin or carbohydrate recognition domain (CRD). Both are assembled as multimers: SP-A is an octodecamer resembling a “bouquet,” and SP-D is a dodecomeric cruciform (Figure 1). SP-A is structurally similar to C1q, a component of the complement system that does not possess a lectin domain, and mannose-binding lectin, a collectin found in serum. SP-A, SP-D, and mannose-binding lectin have all been mapped to the long arm of chromosome 10 in humans, and all have collectin domains (2). Collectins function as pattern recognition molecules, binding most frequently via their CRDs to oligosaccharides on the surface of microorganisms to promote phagocytosis. That collectins have a relatively high affinity for oligosaccharides is an important determinant of self/non–self recognition, because most carbohydrates in animals are monosaccharides. Therefore, as collectins, SP-A and SP-D enhance microbial phagocytosis by innate immune cells, such as macrophages and neutrophils, by opsonizing and aggregating bacteria and viruses, by acting as an activation ligand, and by upregulating the expression of immune cell surface receptors that recognize microbes. In addition, SP-A and SP-D also promote apoptotic cell uptake by innate immune cells and regulate cytokine and free radical production in a context-dependent manner. As an example, SP-A inhibits LPS-stimulated nitric oxide (NO) production by alveolar macrophages isolated from normal lungs, but promotes NO production in macrophages that have been activated by IFN-γ (3). Both proteins possess direct bactericidal activity against bacteria and fungi through currently unknown mechanisms (4) (Figure 2).
Figure 1.
Collectin structure. (a) Surfactant protein (SP)–A and SP-D are members of the collectin family that function as pattern recognition molecules. Collectins are composed of an N-terminal noncollagenous domain and of a C-type (calcium-dependent) carbohydrate recognition domain (CRD; lectin domain). (b) Structurally, collectins are trimeric polypeptide chains that are assembled into oligomers; SP-A and mannose-binding lectin (MBL) are octodecamers, consisting of six trimeric subunits, and SP-D is a dodecomer, consisting of four trimeric subunits. Models are not drawn to scale. Reprinted by permission from Reference 2.
Figure 2.
Functions of SP-A and SP-D as collectins. SP-A and SP-D bind to and opsonize viruses, bacteria, allergens, and apoptotic cells. SP-A and SP-D enhance microbial phagocytosis by aggregating bacteria and viruses. SP-A and SP-D also possess direct bactericidal effects and potentially bind to a variety of receptors to modulate immune cell cytokine and inflammatory mediator expression. Reprinted by permission from Reference 2.
SP-A and SP-D gene–null mice have been invaluable in deciphering the immunomodulatory roles of SPs. In the absence of SP-A, the structure of pulmonary surfactant large aggregates is altered, tubular myelin is absent, and SP-A is no longer available to contribute to the formation of the typical lattice-like structure. However, the surface tension–reducing function of surfactant appears unaffected, suggesting that SP-A does not appear to play a primary role in surfactant homeostasis. Although the mice are able to survive with no apparent pathology in a sterile environment, and respond similarly to wild-type (WT) mice in exercised or hyperoxic conditions (5), their pulmonary immune responses are insufficient during immune challenge. SP-D–null mice have a more complex phenotype. Even in the absence of pathogens, the SP-D–null mice display advancing alveolar proteinosis and increased lipid pools, indicating that SP-D has a role in surfactant homeostasis. Metalloproteinases are also elevated in their lungs, which develop an emphysema-like phenotype. SP-A– and SP-D–null mice are more susceptible to bacterial and viral infections and LPS-mediated inflammation, confirming roles for SP-A and SP-D in modulating immune responses in the lung (2).
SURFACTANT LINKS INNATE AND ADAPTIVE IMMUNITY
Increasing evidence shows that SP-A and SP-D modulate the functions of adaptive immune system cells, dendritic cells (DCs) and T cells. DCs form a tightly meshed network within the upper airways, parenchyma, and alveolar airspace, and are ideally positioned to sample inhaled antigens (6). DC density increases upon inflammatory stimuli, particularly in the lower airways as a result of recruited myeloid DC precursors (7). DCs function varies with their state of maturity, and immature DCs are primarily phagocytic. Upon exposure to microbial products or inflammatory signals, DCs differentiate, or “mature,” and exhibit increased surface expression of major histocompatibility complex II and costimulatory molecules, such as CD80, CD86, and CD40, which bind to receptors expressed on T cells. The result of these cognate interactions is antigen presentation to and stimulation of T cells in regional lymph nodes and, locally, in the tissue. The expression of CC-chemokine receptor-7, the receptor for secondary lymphoid tissue chemokine and macrophage inflammatory protein-3β, is also up-regulated on mature DCs. Secondary lymphoid tissue chemokine and macrophage inflammatory protein-3β are constitutively expressed in afferent lymph endothelium and in the T-cell area of lymph nodes, respectively, explaining why DCs migrate to the draining lymph nodes (8). By modulating the pattern of costimulatory molecule expression, the triggering of the T-cell receptor, and the production of polarizing cytokines, DCs determine the outcome of the primary T-cell response of tolerance, unpolarized Th (T helper cell) 0, or polarized Th1 or Th2 (8). However, the ability of DCs to initiate an immune response is highly dependent on signals present in the local environment.
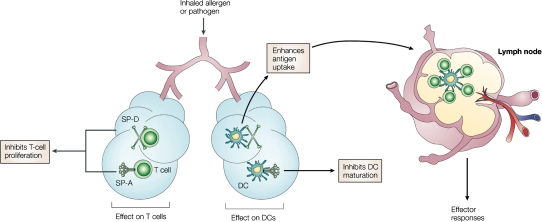
We have shown that, although SP-A and SP-D both bind to DCs in a calcium-dependent manner, they have differential effects on DC function. For example, SP-D enhances antigen uptake and presentation by bone marrow–derived DCs, but SP-A inhibits DC maturation and phagocytic and chemotactic function (9, 10) in vitro. Also, SP-A and SP-D inhibit T-cell proliferation via two mechanisms: an IL-2–dependent mechanism observed with accessory cell–dependent T cell mitogens and specific Ag, as well as an IL-2–independent mechanism of suppression that potentially involves attenuation of calcium signaling (11, 12). A recent study suggested that SP-A–mediated inhibition of T cell proliferation might partially result from transforming growth factor-β present in the SP-A preparations (13). In contrast to the results we obtained using bone marrow–derived DCs, we recently illustrated that SP-D decreases antigen presentation by DCs isolated from the mouse lung during both resting and inflammatory conditions (14). A role for SP-D in regulating T-cell responses in vivo is demonstrated in a study by Fisher and colleagues (15) showing that SP-D–null mice have an accumulation of CD4+ and CD8+ T cells expressing activation markers CD69 and CD25 in the perivascular and peribronchial regions of the lung. Collectively, these studies suggest that surfactant may be a critical regulator of organ-specific immune regulation in the lung, and that the hyporesponsive immunologic environment of the lung is, in part, facilitated by the actions of SP-A and SP-D in an effort to thwart inflammatory cascades that could potentially damage the lung and impair gas exchange (Figure 3).
Figure 3.
SP-A and SP-D link innate and adaptive immunity to regulate host defense. Although both SP-A and SP-D can bind to T cells and directly inhibit proliferation, SP-A can also indirectly inhibit T-cell proliferation via suppression of dendritic cell (DC) maturation. SP-D has been shown to enhance antigen uptake and presentation. Taken together, these in vitro results suggest that the combined role of SP-A and SP-D is to modulate the immunologic environment of the lung so as to protect the host, yet thwart an overzealous inflammatory response that could potentially damage the lung and impair gas exchange. Reprinted by permission from Reference 2.
SP RECEPTORS
The mechanism of both antiinflammatory and inflammatory functions of SP-A and SP-D are thought to involve binding of SPs to microorganisms through their globular heads, which contribute to phagocytosis and the initiation of an inflammatory response. A number of mammalian cell surface receptors have been identified on the surface of both endothelial and hematopoietic cells. Although the molecular identity of most of these receptors has been determined, there are currently three that have not been fully characterized. We briefly discuss here the known receptors and their proposed functions, as greater detail can be found in reviews that focus on SP receptor recognition.
The C1q receptor for phagocytosis (C1qRp or CD93) is a 126-kD type I CRD containing membrane protein, which is expressed on the surface of hematopoietic and endothelial cells (16). Different from SP function, C1q has the ability to activate the complement cascade to enhance pathogen clearance. In concert with SP-A, C1q can interact with the cell surface of pathogens and enhance the uptake of particles suboptimally opsonized with IgG. Both C1q and SP have been suggested to enhance the clearance of apoptotic cells in vivo while inhibiting an immune response. Recent studies have suggested that C1q and SP, under conditions of enhanced phagocytosis, can inhibit inflammatory gene induction by innate immune cells through the receptor, CD93. This observed function is thought to require both C1q and SP, which is evident by the observation that SP-null mice have heightened activation of inflammatory mediators. More recent studies have suggested that CD93 can be shed from the cell surface of monocytes induced by multiple inflammatory mediators (17), although the functional significance of this shedding is as yet unknown.
Pattern recognition receptors, including the Toll-like receptor (TLR) family, are thought to mediate phagocytosis and inflammatory gene induction, thereby promoting pathogen clearance. The association of SPs with TLRs and/or the TLR-associated molecule, CD14, may be one mechanism by which SP functions as an inflammatory mediator (18). Studies have suggested that SP-A directly associates with the TLR2 signaling molecule and thereby inhibits downstream gene activation (47). More recently, Ohya and colleagues have suggested that human SP-D binds to the extracellular domains of TLR2 and TLR4 through its CRD by a mechanism different from its binding to phosphatidylinositol and LPS, although the functional outcome in relationship to downstream TLR signaling was not assessed (19). Through association with rough LPS, SP-A decreases the ability of LPS to bind to CD14, but not to the LPS-binding protein (20).
The immunostimulatory and immunomodulatory effects of SPs have long been observed; however, it was not until elegant experiments by Gardai and colleagues that the ability of the proteins to mediate these effects could be explained (21). The investigators proposed that it was the orientation of the surfactant molecule and its interaction with receptors on the cell surface that augmented or modulated inflammatory gene induction. Specifically, in the absence of pathogen, SP binds via its lectin domain to signal regulating protein-α on the surface of resident cells, resulting in the activation of the tyrosine phosphatase, SHP-1. SHP-1 mediates suppression of proinflammatory genes by blocking the downstream signaling through Src-family kinases and p38 mitogen-activated protein kinase. SPs mediate immunostimulatory effects through a different orientation resulting from the binding of the CRD domain to the pathogen, LPS, or apoptotic cells, and results in aggregation via the collagen region. The aggregated molecule in reverse orientation then interacts with the CRT–CD91 receptor complex on the cell surface, resulting in increased phagocytosis and proinflammatory gene induction. These data provide one mechanism by which SP molecules can function to enhance or inhibit inflammatory responses.
ALLELIC VARIATIONS AND SIGNIFICANCE IN RELATION TO DISEASE
The importance of SPs is underscored by the association of qualitative and quantitative differences in SP-A, SP-B, SP-C, and SP-D, and their correlation with disease. Polymorphic alleles that alter the level of protein expression and functionality are associated with a number of different pulmonary diseases, including RDS, bronchopulmonary dysplasia, alveolar proteinosis, respiratory syncytial virus (RSV) bronchiolitis, chronic obstructive pulmonary disease (COPD), familial cases of interstitial pneumonia, as well as increasing the risk of pulmonary fungal infections and the susceptibility to high-altitude pulmonary edema. Although more research is required, we are beginning to understand the molecular mechanisms by which these polymorphisms affect SP function. A comprehensive documentation of the allelic variations of all SPs and their effect on function is beyond the scope of this review, but we will focus on the most common genetic polymorphisms that occur in SP-A and SP-D and, where possible, provide the mechanism by which the polymorphism affects the function.
The human SP-A locus consists of one pseudogene and two functional genes, SP-A1 and SP-A2, whereas all other organisms encode only one functional SP-A gene. Human SP-A1 and SP-A2 have a 96% degree of similarity at the protein level, suggesting that the critical functions of SP-A require protein conservation. Important research has discerned the functional differences between these two genes. For example, SP-A2 exhibits a higher level of activity than SP-A1 in its ability to enhance inflammatory gene induction and pathogen clearance, potentially due to the increased stability of the protein (22, 23). Another method of regulation exists in the observed splice variations between the two genes leading to differences in mRNA and protein levels. Although there are known differences in functionality between the two genes, more than four alleles of SP-A1 (6A, 6A2–4) and six alleles of SP-A2 (1A, 1A0–5) are frequently observed in the general population (24). Amino acid changes occur in the mature secreted SP-A1 alleles at positions 50 (L,V) and 219 (R, W), and in the SP-A2 alleles at amino acids 91 (P, A) and 223 (Q, K). SP-A human bronchial lavage consists of both SP-A1 and SP-A2. The major SP-A1/SP-A2 allele, 1A0/6A2 (50V, 219R/91A, 223Q), represents 55–57% of all SP-A alleles, is associated with RDS in independent populations (25–28), and is suggested to result in a decrease in SP-A mRNA levels, which correlate with disease (29). Genetic variation in SP-A has been clearly linked to susceptibility to RSV infection. Specifically, SP-A2 Gln223Lys allele is overrepresented in infants with severe RSV infection, whereas the SP-A2 Ala91Pro allele is underrepresented in severe RSV (30). It is suspected that the SP-A2 Gln223Lys allele may modify the ability of SP-A2 to bind to RSV antigen, whereas the Ala93Pro mutation influences the rigidity of the collagenous region of SP-A2, thereby affecting the innate immune functions of SP-A. An allele of SP-A2 1A1, in which there is a substitution of glutamine with lysine at residue 223 in the CRD, increases susceptibility to meningococcal disease after infection with Neisseria meningitidis, resulting in an increased risk of death (31). In a recent study of risk factors in susceptibility to high-altitude pulmonary edema, associations with nucleotide polymorphisms in SP-A1 (C1101T, T3192C, and T3234C) and SP-A2 (A3265C) were found (32). The finding that two SP-A2 alleles result in greater sensitivity supported the observation that mutations in SP-A2 play a role in susceptibility to Aspergillus-mediated allergies (33). The synonymous nucleotide polymorphism at position A1660G showed a significant association with allergic bronchopulmonary aspergillosis, and this linkage was enhanced when associated with the nonsynonymous allele, G1649C. Together, these alleles were reported to result in a marked increase in total IgE, peripheral eosinophilia, and a decrease in lung performance (% FEV1). It is interesting to note that both of these mutations (A1660G and G1649C) showed association with susceptibility to Mycobacterium tuberculosis infection (34). Analysis of a nucleotide polymorphism that resulted in a synonymous allele at amino acid 62 of SP-A revealed a correlation with COPD (35). One SP-A1 (6A4) allele and the single-nucleotide polymorphisms (SNPs) that characterize the 6A4 allele were found with higher frequency in idiopathic pulmonary fibrosis (36). Because the majority of these SNPs are synonymous, it is possible to speculate that these SNPs alter translation and, potentially, mRNA stability that, in turn, affect protein expression levels.
SP-D is mainly synthesized in alveolar type II cells of the lung, and it is released into the blood during certain types of lung injury. Four polymorphisms of SP-D have been identified within the protein, including Met11Thr, Ala160Thr, Ser270Thr, and nonsynomymous Ala286Ala mutation, although, to date, only amino acid 11 has been associated with disease. The Met11Thr and Ala160Thr polymorphisms in the amino terminal and collagen domain have a frequency exceeding 20%, whereas the Ser270Thr and Ala286Ala polymorphisms are relatively rare. There is a strong linkage of the Met11Thr allele within SP-D being associated with protection from severe RSV infection in infants; whereas the Met11Met allele is associated with RSV bronchiolitis, the Thr11-coding allele is associated with susceptibility to M. tuberculosis infection (37). Studies have suggested that alleles of codon 11 influence the ability of SP-D to oligomerize, which results in significantly different SP-D serum levels (38). The Met11Met allele produces SP-D of both low- and high-molecular-weight structures, whereas the Thr11 produces mainly low-molecular-weight structures. The importance of this finding was underscored by the observation that the high-molecular-weight form of SP-D results in an increased binding affinity to complex microorganisms, and the low-molecular-weight SP-D preferentially binds simpler ligands, such as LPS. The physiologic functions of high versus low-molecular-weight SP-D oligomers is not yet known, although it is easy to speculate that they may have alternative outcomes.
SPs IN CHRONIC LUNG DISEASE
Although acute infectious disease models show that SP-A and SP-D inhibit inflammation and enhance pathogen clearance, relatively little is known about the in vivo role of these proteins in chronic lung diseases. However, in recent studies of nonallergic lung disease and lung injury, a consistent theme that appears to be emerging is that SP-D levels correlate with susceptibility or severity of disease/injury. A relative collectin deficiency has been identified in cystic fibrosis (CF) airways, and this deficiency appears to be inversely related to inflammation (39). Upon oxidation, the quaternary structure of SP-D changes and its ability agglutinate bacteria is impaired. This could potentially contribute to decreases in host defense and suppurative lung diseases, like CF (40). Smokers and patients with COPD, characterized by mucus hypersecretion and chronic bronchitis that impair ventilatory capacity and gas exchange, had higher serum SP-D but not SP-A and SP-B concentrations compared with normal control subjects (41). In addition, children (nonsymptomatic) who have a tracheostomy had low levels of SP-D that correlated with bacterial counts and neutrophilic inflammation (42).
Mouse models of lung injury have shown that the susceptibility to ozone (O3)-induced inflammatory changes varies between different mouse strains and appears to be associated with different levels of SP-D (43). For instance, C57BL/6 mice that express high levels of SP-D also produced high levels of IL-10 and of IL-6. In contrast, BALB/c mice released significantly more keratinocyte-derived chemokine and IL-12 p70. Elevated levels of SP-D were associated with the resolution of O3-induced inflammation, and low levels or lack of SP-D predisposed mice to a severe inflammatory response. Intratracheal instillation of bleomycin in rodents, in a model of subacute lung injury and fibrosis, resulted in an upregulation of SP-D protein content (44). In a follow-up study, Casey and colleagues (45) demonstrated that intratracheal instillation of bleomycin administration to SP-D–null mice results in increased mortality, enhanced lung inflammation and tissue injury, and alterations in NO metabolism. Furthermore, mice constitutively overexpressing recombinant SP-D were protected from morbidity and mortality. Fujita and colleagues (46) used a bleomycin model to study acute inflammation and a transgenic mouse that overexpresses tumor necrosis factor-α under the control of the SP-C promoter to study chronic lung inflammation. Results showed that mRNA for all SPs was reduced early in the acute model, but serum SP-D increased at later time points. In the chronic model, expression of SP-A, SP-B, and SP-C was reduced, but both serum and lung SP-D concentrations were increased. These latter investigations support a role for SP-D in the local modulation of the pulmonary inflammatory response to noninfectious lung injury. Taken together, these studies suggest that SP-D may be a potential biomarker of lung inflammation.
Although substantial progress has been made in understanding the role of the surfactant collectins in lung host defense, to the best of our knowledge there are currently no clinical trials in humans investigating the efficacy of surfactant preparations containing SP-A and SP-D for the treatment of lung injury or disease. A greater understanding of the impact of allelic variation on disease, and the identification of surfactant receptors, will invaluably assist in the quest to create SP therapeutics aimed at specific diseases.
CONCLUSIONS
The lipoprotein complex surfactant is essential for reducing surface tension at the air–liquid interface of the lung and for lung immune host defense. Two of the SPs, SP-A and SP-D, specifically play a critical role in lung host defense. They not only regulate the function of innate immune cells, but also interact with antigen-presenting cells and T cells, thereby linking innate and adaptive immunity. Acute infectious disease models show that SP-A and SP-D inhibit inflammation and enhance pathogen clearance; however, relatively little is known about the in vivo role of these proteins in chronic lung diseases. Emerging studies in noninfectious lung disease and lung injury suggest that SP-D levels in bronchoalveolar lavage fluid and serum can vary, and may potentially serve as a biomarker of disease or injury. The numerous polymorphisms of SPs may have altered functions and may act via different receptors to ultimately alter the susceptibility to or severity of lung diseases. Taken together, SP-A and SP-D appear to modulate the immunologic environment of the lung to aid in host defense and, at the same time, thwart an overzealous inflammatory response that could potentially damage the lung and impair gas exchange.
Supported by National Institiutes of Health grants HL-30923, HL-51134, HL-68072, HL-84917, and ES-011961 (J.R.W.).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Nogee LM. Genetic mechanisms of surfactant deficiency. Biol Neonate 2004;85:314–318. [DOI] [PubMed] [Google Scholar]
- 2.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 2005;5:58–68. [DOI] [PubMed] [Google Scholar]
- 3.Stamme C, Walsh E, Wright JR. Surfactant protein A differentially regulates IFN-γ– and LPS-induced nitrite production by rat alveolar macrophages. Am J Respir Cell Mol Biol 2000;23:772–779. [DOI] [PubMed] [Google Scholar]
- 4.Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, Kim KS, McCormack FX. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest 2003;111:1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikegami M, Jobe AH, Whitsett J, Korfhagen T. Tolerance of SP-A–deficient mice to hyperoxia or exercise. J Appl Physiol 2000;89:644–648. [DOI] [PubMed] [Google Scholar]
- 6.Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev 2002;82:97–130. [DOI] [PubMed] [Google Scholar]
- 7.Jahnsen FL, Moloney ED, Hogan T, Upham JW, Burke CM, Holt PG. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax 2001;56:823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs (comment). J Exp Med 1999;189:447–450.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinker KG, Martin E, Borron P, Mostaghel E, Doyle C, Harding CV, Wright JR. Surfactant protein D enhances bacterial antigen presentation by bone marrow–derived dendritic cells. Am J Physiol Lung Cell Mol Physiol 2001;281:L1453–L1463. [DOI] [PubMed] [Google Scholar]
- 10.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow–derived dendritic cells. Am J Physiol Lung Cell Mol Physiol 2003;284:L232–L241. [DOI] [PubMed] [Google Scholar]
- 11.Borron P, Veldhuizen RA, Lewis JF, Possmayer F, Caveney A, Inchley K, McFadden RG, Fraher LJ. Surfactant associated protein-A inhibits human lymphocyte proliferation and IL-2 production. Am J Respir Cell Mol Biol 1996;15:115–121. [DOI] [PubMed] [Google Scholar]
- 12.Borron PJ, Crouch EC, Lewis JF, Wright JR, Possmayer F, Fraher LJ. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol 1998; 161:4599–4603. [PubMed]
- 13.Kunzmann S, Wright JR, Steinhilber W, Kramer BW, Blaser K, Speer CP, Schmidt-Weber C. TGF-β1 in SP-A preparations influence immune suppressive properties of SP-A on human CD4+ T lymphocytes. Am J Physiol Lung Cell Mol Physiol 2006;291:L747–L756. [DOI] [PubMed] [Google Scholar]
- 14.Hansen S, Lo B, Evans K, Neophytou P, Holmskov U, Wright JR. Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens. Am J Respir Cell Mol Biol 2007;36:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher JH, Larson J, Cool C, Dow SW. Lymphocyte activation in the lungs of SP-D null mice. Am J Respir Cell Mol Biol 2002;27:24–33. [DOI] [PubMed] [Google Scholar]
- 16.Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity 1997;6:119–129. [DOI] [PubMed] [Google Scholar]
- 17.Bohlson SS, Silva R, Fonseca MI, Tenner AJ. CD93 is rapidly shed from the surface of human myeloid cells and the soluble form is detected in human plasma. J Immunol 2005;175:1239–1247. [DOI] [PubMed] [Google Scholar]
- 18.Sano H, Sohma H, Muta T, Nomura S, Voelker DR, Kuroki Y. Pulmonary surfactant protein A modulates the cellular response to smooth and rough lipopolysaccharides by interaction with CD14. J Immunol 1999;163:387–395. [PubMed] [Google Scholar]
- 19.Ohya M, Nishitani C, Sano H, Yamada C, Mitsuzawa H, Shimizu T, Saito T, Smith K, Crouch E, Kuroki Y. Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry 2006;45:8657–8664. [DOI] [PubMed] [Google Scholar]
- 20.Stamme C, Muller M, Hamann L, Gutsmann T, Seydel U. Surfactant protein a inhibits lipopolysaccharide-induced immune cell activation by preventing the interaction of lipopolysaccharide with lipopolysaccharide-binding protein. Am J Respir Cell Mol Biol 2002;27:353–360. [DOI] [PubMed] [Google Scholar]
- 21.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003;115:13–23. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Phelps DS, Umstead TM, Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-α production in the THP-1 cell line. Am J Physiol Lung Cell Mol Physiol 2000;278:L946–L954. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Umstead TM, Phelps DS, Al-Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein A variants to stimulate cytokine production. Environ Health Perspect 2002;110:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagaram HR, Wang G, Umstead TM, Mikerov AN, Thomas NJ, Graff GR, Hess JC, Thomassen MJ, Kavuru MS, Phelps DS, et al. Characterization of a human surfactant protein A1 (SP-A1) gene–specific antibody: SP-A1 content variation among individuals of varying age and pulmonary health. Am J Physiol Lung Cell Mol Physiol [online ahead of print] Dec 22, 2006; DOI: 10.1152/aplung.00249.2006v1. Most recent version available from: http://ajplung.physiology.org/cgi/reprint/00249.2006.1. [DOI] [PubMed]
- 25.Kala P, Ten Have T, Nielsen H, Dunn M, Floros J. Association of pulmonary surfactant protein A (SP-A) gene and respiratory distress syndrome: interaction with SP-B. Pediatr Res 1998;43:169–177. [DOI] [PubMed] [Google Scholar]
- 26.Ramet M, Haataja R, Marttila R, Floros J, Hallman M. Association between the surfactant protein A (SP-A) gene locus and respiratory-distress syndrome in the Finnish population. Am J Hum Genet 2000;66:1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haataja R, Ramet M, Marttila R, Hallman M. Surfactant proteins A and B as interactive genetic determinants of neonatal respiratory distress syndrome. Hum Mol Genet 2000;9:2751–2760. [DOI] [PubMed] [Google Scholar]
- 28.Marttila R, Haataja R, Ramet M, Pokela ML, Tammela O, Hallman M. Surfactant protein A gene locus and respiratory distress syndrome in Finnish premature twin pairs. Ann Med 2003;35:344–352. [DOI] [PubMed] [Google Scholar]
- 29.Wang G, Guo X, Floros J. Human SP-A 3′-UTR variants mediate differential gene expression in basal levels and in response to dexamethasone. Am J Physiol Lung Cell Mol Physiol 2003;284:L738–L748. [DOI] [PubMed] [Google Scholar]
- 30.Lofgren J, Ramet M, Renko M, Marttila R, Hallman M. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. J Infect Dis 2002;185:283–289. [DOI] [PubMed] [Google Scholar]
- 31.Jack DL, Cole J, Naylor SC, Borrow R, Kaczmarski EB, Klein NJ, Read RC. Genetic polymorphism of the binding domain of surfactant protein-A2 increases susceptibility to meningococcal disease. Clin Infect Dis 2006;43:1426–1433. [DOI] [PubMed] [Google Scholar]
- 32.Saxena S, Kumar R, Madan T, Gupta V, Muralidhar K, Sarma PU. Association of polymorphisms in pulmonary surfactant protein A1 and A2 genes with high-altitude pulmonary edema. Chest 2005;128:1611–1619. [DOI] [PubMed] [Google Scholar]
- 33.Saxena S, Madan T, Shah A, Muralidhar K, Sarma PU. Association of polymorphisms in the collagen region of SP-A2 with increased levels of total IgE antibodies and eosinophilia in patients with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 2003;111:1001–1007. [DOI] [PubMed] [Google Scholar]
- 34.Madan T, Saxena S, Murthy KJ, Muralidhar K, Sarma PU. Association of polymorphisms in the collagen region of human SP-A1 and SP-A2 genes with pulmonary tuberculosis in Indian population. Clin Chem Lab Med 2002;40:1002–1008. [DOI] [PubMed] [Google Scholar]
- 35.Guo X, Lin HM, Lin Z, Montano M, Sansores R, Wang G, DiAngelo S, Pardo A, Selman M, Floros J. Polymorphisms of surfactant protein gene A, B, D, and of SP-B–linked microsatellite markers in COPD of a Mexican population. Chest 2000;117(Suppl 1)249S–250S. [DOI] [PubMed] [Google Scholar]
- 36.Selman M, Lin HM, Montano M, Jenkins AL, Estrada A, Lin Z, Wang G, DiAngelo SL, Guo X, Umstead TM, et al. Surfactant protein A and B genetic variants predispose to idiopathic pulmonary fibrosis. Hum Genet 2003;113:542–550. [DOI] [PubMed] [Google Scholar]
- 37.Floros J, Lin HM, Garcia A, Salazar MA, Guo X, DiAngelo S, Montano M, Luo J, Pardo A, Selman M. Surfactant protein genetic marker alleles identify a subgroup of tuberculosis in a Mexican population. J Infect Dis 2000;182:1473–1478. [DOI] [PubMed] [Google Scholar]
- 38.Leth-Larsen R, Garred P, Jensenius H, Meschi J, Hartshorn K, Madsen J, Tornoe I, Madsen HO, Sorensen G, Crouch E, et al. A common polymorphism in the SFTPD gene influences assembly, function, and concentration of surfactant protein D. J Immunol 2005;174:1532–1538. [DOI] [PubMed] [Google Scholar]
- 39.Noah TL, Murphy PC, Alink JJ, Leigh MW, Hull WM, Stahlman MT, Whitsett JA. Bronchoalveolar lavage fluid surfactant protein-A and surfactant protein-D are inversely related to inflammation in early cystic fibrosis. Am J Respir Crit Care Med 2003;168:685–691. [DOI] [PubMed] [Google Scholar]
- 40.Starosta V, Griese M. Oxidative damage to surfactant protein D in pulmonary diseases. Free Radic Res 2006;40:419–425. [DOI] [PubMed] [Google Scholar]
- 41.Mutti A, Corradi M, Goldoni M, Vettori MV, Bernard A, Apostoli P. Exhaled metallic elements and serum pneumoproteins in asymptomatic smokers and patients with COPD or asthma. Chest 2006;129:1288–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griese M, Felber J, Reiter K, Strong P, Reid K, Belohradsky BH, Jager G, Nicolai T. Airway inflammation in children with tracheostomy. Pediatr Pulmonol 2004;37:356–361. [DOI] [PubMed] [Google Scholar]
- 43.Kierstein S, Poulain FR, Cao Y, Grous M, Mathias R, Kierstein G, Beers MF, Salmon M, Panettieri RA Jr, Haczku A. Susceptibility to ozone-induced airway inflammation is associated with decreased levels of surfactant protein D. Respir Res 2006;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savani RC, Godinez RI, Godinez MH, Wentz E, Zaman A, Cui Z, Pooler PM, Guttentag SH, Beers MF, Gonzales LW, et al. Respiratory distress after intratracheal bleomycin: selective deficiency of surfactant proteins B and C. Am J Physiol Lung Cell Mol Physiol 2001;281:L685–L696. [DOI] [PubMed] [Google Scholar]
- 45.Casey J, Kaplan J, Atochina-Vasserman EN, Gow AJ, Kadire H, Tomer Y, Fisher JH, Hawgood S, Savani RC, Beers MF. Alveolar surfactant protein D content modulates bleomycin-induced lung injury. Am J Respir Crit Care Med 2005;172:869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita M, Shannon JM, Morikawa O, Gauldie J, Hara N, Mason RJ. Overexpression of tumor necrosis factor-α diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-β. Am J Respir Cell Mol Biol 2003;29:669–676. [DOI] [PubMed] [Google Scholar]
- 47.Murakami S, Iwaki D, Mitsuzawa H, Sano H, Takahashi H, Voelker DR, Akino T, Kuroki Y. Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-alpha secretion in U937 cells and alveolar macrophages by direct interaction with toll-like receptor 2. J Biol Chem 2002;277:6830–6837. [DOI] [PubMed] [Google Scholar]