Abstract
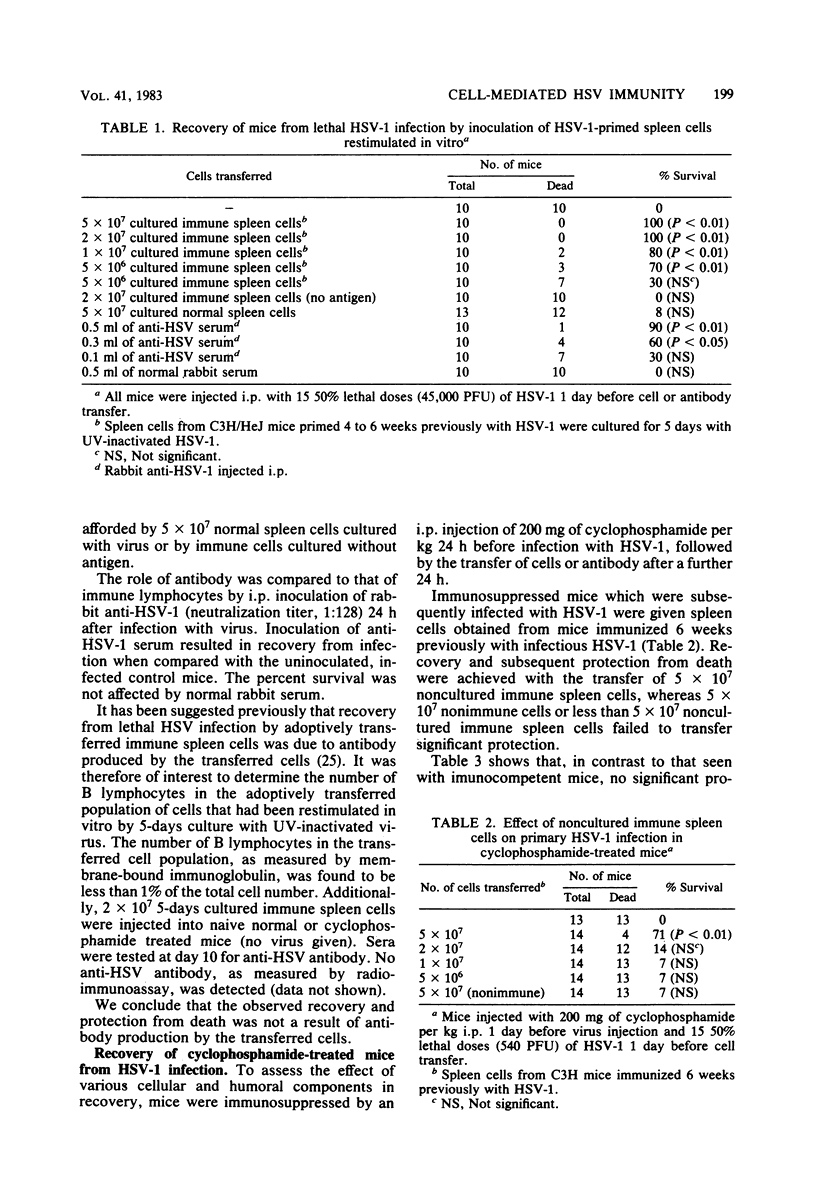
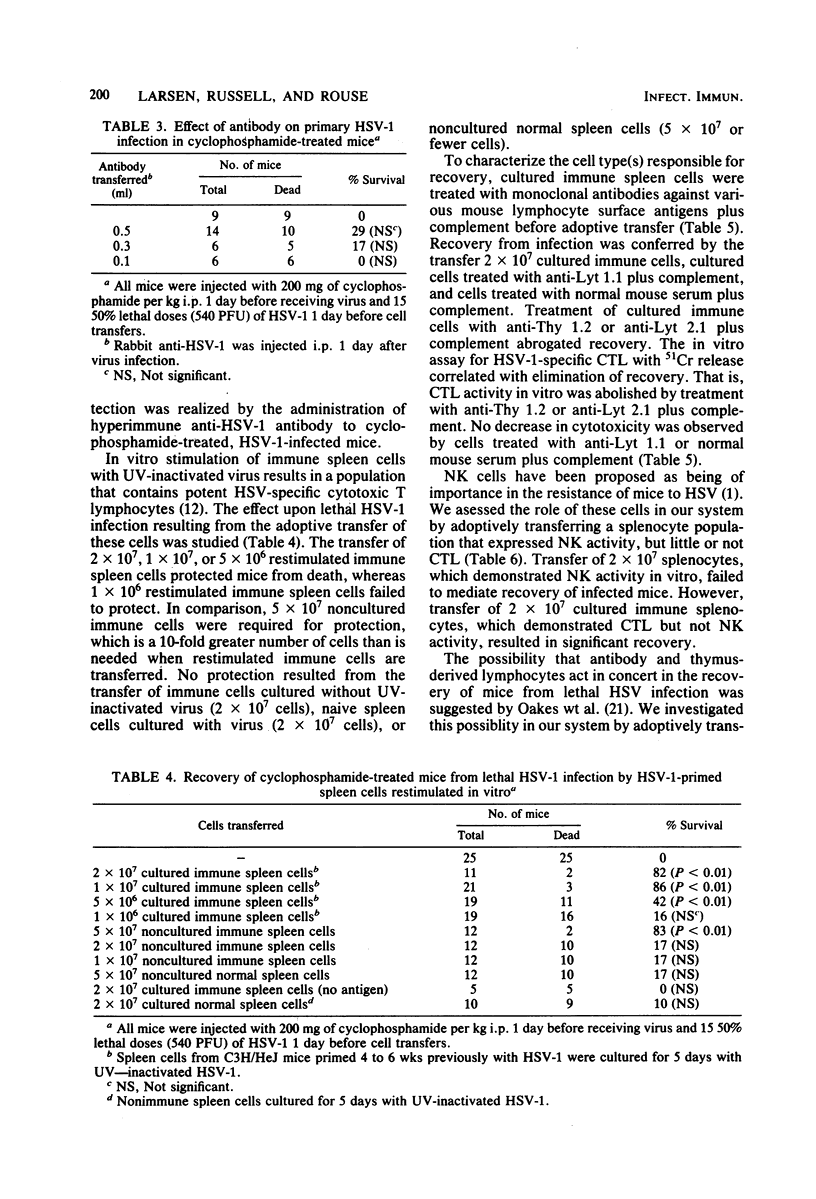
The ability of herpes simplex virus (HSV)-specific, cytotoxic T lymphocytes to mediate recovery of mice lethally infected with HSV was examined. Adoptive transfer of splenocytes from mice that had been primed in vivo with HSV and restimulated with HSV in vitro protected lethally infected normal and cyclophosphamide-immunosuppressed mice from death. In contrast, equal numbers of normal splenocytes or immune splenocytes cultured without antigen failed to mediate recovery. Recovery was also transferred by noncultured, primary immune splenocytes, although the protective efficacy of these cells was 10-fold less than when immune splenocytes restimulated in vitro were used. Treatment of the cells with anti-Thy 1 or anti-Lyt 2.1 plus complement before adoptive transfer abrogated recovery. No decrease in protection was seen when in vitro-restimulated splenocytes were treated with anti-Lyt 1.1 plus complement. Splenocytes expression natural killer activity also failed to effect recovery. The administration of hyperimmune anti-HSV antibody to normal, immunocompetent mice resulted in recovery, whereas no significant protection of immunosuppressed mice by anti-HSV was observed. When antibody was given concurrently with cultured immune splenocytes, the percentage of mice that recovered from infection was greater than that seen with either antibody or cultured immune splenocytes alone. These experiments demonstrate that Lyt 2-positive cells with cytotoxic activity generated by in vitro immunization can mediate recovery from lethal HSV infection, whereas, under the conditions chosen, Lyt 1-positive cells were unable to mediate recovery.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armerding D., Rossiter H. Induction of natural killer cells by herpes-simplex virus type 2 in resistant and sensitive inbred mouse strains. Immunobiology. 1981;158(4):369–379. doi: 10.1016/S0171-2985(81)80007-1. [DOI] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone D. R., Courtney R. J. A temperature-sensitive mutant of herpes simplex virus type 1 defective in the synthesis of the major capsid polypeptide. J Gen Virol. 1974 Jul;24(1):17–27. doi: 10.1099/0022-1317-24-1-17. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. Regulation of the immune response by T-cell subclasses. Contemp Top Immunobiol. 1977;7:47–67. doi: 10.1007/978-1-4684-3054-7_2. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H., Zawatzky R., Goldbach A., Schröder C. H., Weyand C., Hämmerling G. J., Kirchner H. Experimental infection of inbred mice with herpes simplex virus. II. Interferon production and activation of natural killer cells in the peritoneal exudate. J Gen Virol. 1981 Jul;55(Pt 1):25–30. doi: 10.1099/0022-1317-55-1-25. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Kirchner H., Hirt H. M., Becker H., Munk K. Production of an antiviral factor by murine spleen cells after treatment with Corynebacterium parvum. Cell Immunol. 1977 Jun 1;31(1):172–176. doi: 10.1016/0008-8749(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Kohl S., Loo L. S. Protection of neonatal mice against herpes simplex virus infection: probable in vivo antibody-dependent cellular cytotoxicity. J Immunol. 1982 Jul;129(1):370–376. [PubMed] [Google Scholar]
- McKendall R. R., Klassen T., Baringer J. R. Host defenses in herpes simplex infections of the nervous system: effect of antibody on disease and viral spread. Infect Immun. 1979 Feb;23(2):305–311. doi: 10.1128/iai.23.2.305-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Role of activated macrophages in resistance of congenitally athymic nude mice to hepatitis induced by herpes simplex virus type 2. Infect Immun. 1978 Mar;19(3):792–798. doi: 10.1128/iai.19.3.792-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Kern E. R., Glasgow L. A. Immunomodulator-induced resistance against herpes simplex virus. Proc Soc Exp Biol Med. 1977 Apr;154(4):615–620. doi: 10.3181/00379727-154-39730. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Thomson T. A., Kohl S., Murray B. K. Immune responses to labial infection of BALB/c mice with herpes simplex virus type 1. Infect Immun. 1981 Apr;32(1):180–187. doi: 10.1128/iai.32.1.180-187.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. G., Lin Y. L., Askonas B. A. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982 Jan 14;295(5845):150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Hayashida I., Higa K., Wada T., Mori R. Role of Lyt-1 positive immune T cells in recovery from herpes simplex virus infection in mice. Microbiol Immunol. 1982;26(4):359–362. doi: 10.1111/j.1348-0421.1982.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Oda H., Mori R., Taniguchi T. Mechanism of acquired resistance to herpes simplex virus infection as studied in nude mice. J Gen Virol. 1979 Sep;44(3):715–723. doi: 10.1099/0022-1317-44-3-715. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Field H. J., Quartey-Papafio R. Cell-mediated immunity in herpes simplex virus-infected mice: induction, characterization and antiviral effects of delayed type hypersensitivity. J Gen Virol. 1980 Jun;48(Pt 2):351–357. doi: 10.1099/0022-1317-48-2-351. [DOI] [PubMed] [Google Scholar]
- Oakes J. E., Davis W. B., Taylor J. A., Weppner W. A. Lymphocyte reactivity contributes to protection conferred by specific antibody passively transferred to herpes simplex virus-infected mice. Infect Immun. 1980 Aug;29(2):642–649. doi: 10.1128/iai.29.2.642-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Rosemond-Hornbeak H. Antibody-mediated recovery from subcutaneous herpes simplex virus type 2 infection. Infect Immun. 1978 Aug;21(2):489–495. doi: 10.1128/iai.21.2.489-495.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- Sethi K. K., Omata Y., Schneweis K. E. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983 Feb;64(Pt 2):443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- Starr S. E., Visintine A. M., Tomeh M. O., Nahmias A. J. Effects of immunostimulants on resistance of newborn mice to herpes simplex type 2 infection. Proc Soc Exp Biol Med. 1976 May;152(1):57–60. doi: 10.3181/00379727-152-39327. [DOI] [PubMed] [Google Scholar]
- Worthington M., Conliffe M. A., Baron S. Mechanism of recovery from systemic herpes simplex virus infection II. Effectiveness of antibody reconstitution of nude and neonatally thymectomized mice. Proc Soc Exp Biol Med. 1980 Dec;165(3):462–468. doi: 10.3181/00379727-165-41005. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Engler H., Kirchner H. Experimental infection of inbred mice with herpes simplex virus. III. Comparison between newborn and adult C57BL/6 mice. J Gen Virol. 1982 May;60(Pt 1):25–29. doi: 10.1099/0022-1317-60-1-25. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]



