Abstract
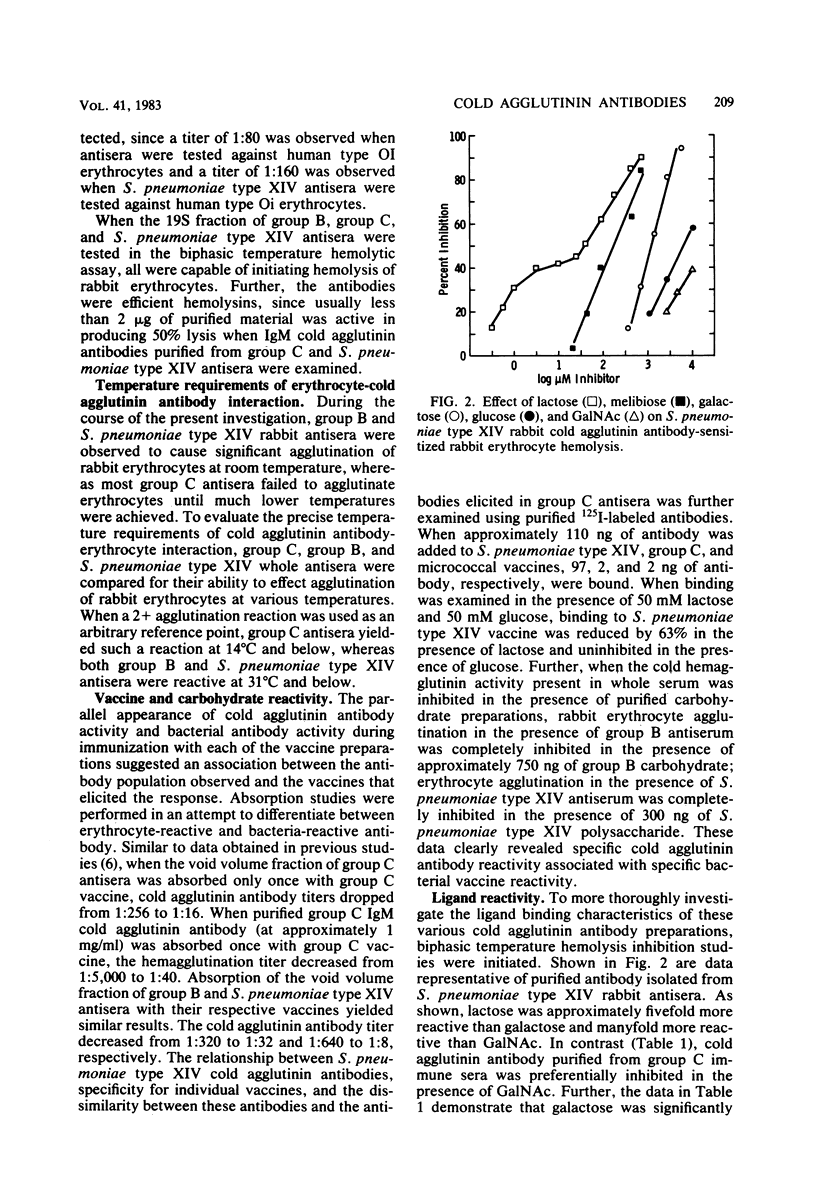
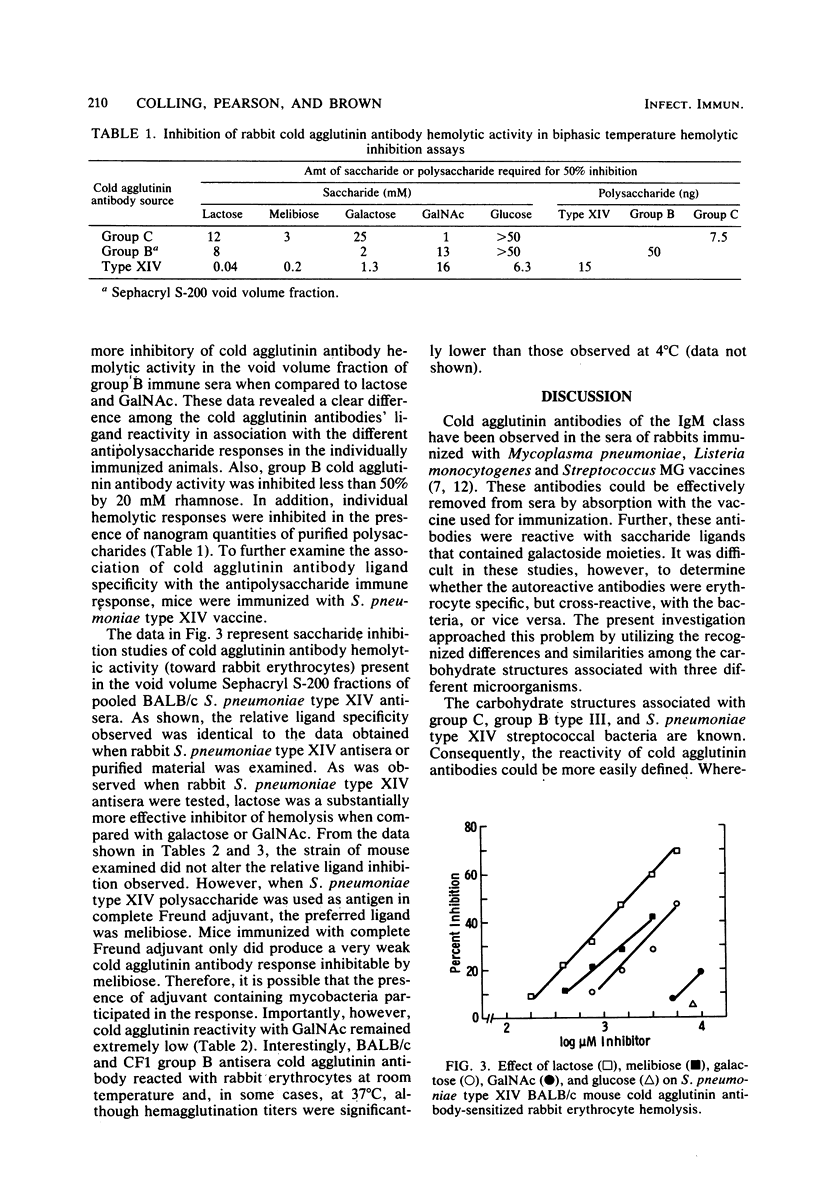
Rabbits immunized with group B type III, group C, and Streptococcus pneumoniae type XIV streptococcal vaccines developed autoantibodies reactive with autologous and isologous erythrocytes and human O-positive erythrocytes at reduced temperatures. The cold agglutinin antibodies were present in both the immunoglobulin M (IgM) and IgG fractions of group C streptococcal antiserum and in the IgM fraction of group B type III and S. pneumoniae type XIV antisera. BALB/c, CF1, and local strains of mice immunized with group B type III and S. pneumoniae type XIV streptococcal vaccines also produced a cold agglutinin antibody reactive with rabbit and human erythrocytes. The cold agglutinin antibodies were reactive with saccharide compounds representative of the determinants present on the individual bacterial carbohydrate structures, individual vaccine preparations, and isolated polysaccharides. The group C antibodies in rabbits were reactive with sugar ligands in the following order: N-acetylgalactosamine greater than melibiose greater than lactose greater than galactose greater than glucose. Group B type III and S. pneumoniae type XIV cold agglutinin antibodies in rabbit antisera, however, displayed reactivities different from group C antibodies and from each other. Group B type III antibodies reacted with galactose greater than lactose greater than N-acetylgalactosamine greater than glucose greater than rhamnose; S. pneumoniae type XIV antibodies reacted with lactose greater than melibiose greater than galactose greater than glucose greater than N-acetylgalactosamine. The same relative ligand specificity was observed for the cold agglutinin antibodies in S. pneumoniae type XIV mouse antisera. The cold agglutinin antibodies in group B type III and S. pneumoniae type XIV antiserum reacted with erythrocytes at higher temperatures (up to 31 degrees C) than did group C antibodies (up to 14 degrees C). In addition, S. pneumoniae type XIV antibodies did not discriminate between I- or i-bearing human erythrocytes to a significant extent. The results obtained provide substantial evidence that autoreactive cold agglutinin antibodies produced by immunization with these vaccines represent subpopulations of bacterial carbohydrate-specific antibodies that cross-react with mammalian carbohydrate structures.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. J., Kasper D. L. Microcapsule of type III strains of group B Streptococcus: production and morphology. Infect Immun. 1976 Jan;13(1):189–194. doi: 10.1128/iai.13.1.189-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Colling R. G. Properties of cold agglutinin and group carbohydrate-specific antibodies isolated from group C streptococcal antisera. Mol Immunol. 1982 Mar;19(3):457–465. doi: 10.1016/0161-5890(82)90212-7. [DOI] [PubMed] [Google Scholar]
- Carey R. B., Eisenstein T. K., Shockman G. D., Greber T. F., Swenson R. M. Soluble group- and type-specific antigens from type III group B Streptococcus. Infect Immun. 1980 Apr;28(1):195–203. doi: 10.1128/iai.28.1.195-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Kapadia A., Feizi T. Expression of blood group I and i active carbohydrate sequences on cultured human and animal cell lines assessed by radioimmunoassays with monoclonal cold agglutinins. Eur J Immunol. 1980 May;10(5):379–384. doi: 10.1002/eji.1830100512. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Fraser B. A., Kindt T. J. A disaccharide hapten from streptococcal group C carbohydrate that cross-reacts with the Forssman glycolipid. J Immunol. 1977 Jan;118(1):6–11. [PubMed] [Google Scholar]
- Colling R. G., Brown J. C. The appearance of IgM and IgG cold agglutinins in rabbits hyperimmunized with group C streptococcal vaccine. J Immunol. 1979 Jan;122(1):202–208. [PubMed] [Google Scholar]
- Costea N., Yakulis V. J., Heller P. The mechanism of indction of cold agglutinins by mycoplasma pneumoniae. J Immunol. 1971 Mar;106(3):598–604. [PubMed] [Google Scholar]
- Fisher J. N., Shahshahani M. N., Kitabchi A. E. Diabetic ketoacidosis: low-dose insulin therapy by various routes. N Engl J Med. 1977 Aug 4;297(5):238–241. doi: 10.1056/NEJM197708042970502. [DOI] [PubMed] [Google Scholar]
- Herd Z. L., Spragg J. The occurrence of homogeneous antibodies and antigenic selection of kappa light chains to streptococcal carbohydrates in Monash rabbits. Aust J Exp Biol Med Sci. 1972 Apr;50(2):225–243. doi: 10.1038/icb.1972.19. [DOI] [PubMed] [Google Scholar]
- Honda M., Sakane T., Steinberg A. D., Kotani H., Tsunematsu T., Moriyama K., Fukase M. Studies of immune functions of patients with systemic lupus erythematosus: antibodies to desialized, rather than intact, T cells preferentially bind to and eliminate suppressor effector T cells. J Clin Invest. 1982 Apr;69(4):940–949. doi: 10.1172/JCI110533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Rosell K. G., Kasper D. L. Structural determination and serology of the native polysaccharide antigen of type-III group B Streptococcus. Can J Biochem. 1980 Feb;58(2):112–120. doi: 10.1139/o80-016. [DOI] [PubMed] [Google Scholar]
- Lau F. O., Rosse W. F. The reactivity of red blood cell membrane glycophorin with "cold-reacting" antibodies. Clin Immunol Immunopathol. 1975 May;4(1):1–8. doi: 10.1016/0090-1229(75)90032-x. [DOI] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Wood E., Lecomte J., Childs R. A., Feizi T. A radioimmunoassay for the measurement of blood group Ii activities: its application to glycoconjugates, oligosaccharides and intact cells. Mol Immunol. 1979 Oct;16(10):813–819. doi: 10.1016/0161-5890(79)90160-3. [DOI] [PubMed] [Google Scholar]


