Abstract
There are valid scientific and ethical considerations for using a control group in a clinical trial. Placebo-controlled trials are justifiable when they are supported by sound methodologic consideration and when their use does not expose research participants to excessive risk of harm. Consideration should be given to “best available therapy” control groups in the evaluation of a new therapy or intervention over an existing therapy. Investigators should keep in mind that one should not sacrifice the scientific merit of a trial to include a best-available-therapy control group as long as the placebo control group poses little harm to participants and, importantly, the trial offers potential benefit to the subject. The pros and cons of using placebo versus best-available-therapy control groups are discussed.
Keywords: control groups, placebo, trials
The investigator trying to decide the optimal study design to address a specific scientific hypothesis has to consider various options for control groups. There are valid scientific and ethical considerations for using a control group in a clinical trial. In deciding the optimal trial design, one may choose to have no control group, a placebo or sham control group, a usual-care group, an active treatment group using an approved therapy or intervention, or a control group that receives “best available therapy.” The selection of which control group to use is critical to the success of any trial investigating a new therapy or intervention.
RATIONALE FOR CONTROL GROUP
What is the rationale for the use of a control group in clinical research? The guidelines for biomedical research published by the Council for International Organizations of Medical Sciences have established that a clinical trial cannot be justified ethically unless is it capable of producing scientifically reliable results (1). Similarly, scientifically invalid research is unethical in that it exposes research subjects to risk without any possible benefit. Therefore, it is important to keep in mind that one must have a scientifically valid study in the selection of a control group. In addition, it is important to note that a randomized controlled trial is not a form of individualized medical therapy. It is a scientific tool for evaluating treatments in groups of research participants with the aim of improving the care of patients in the future (2).
There are numerous examples in the literature regarding studies that have demonstrated initial findings that were subsequently proven wrong because of a lack of a control group. An interesting example is a recent cardiology study that examined the effect of a cardiac pacemaker on the risk of vasovagal syncope (3). Previous small, unblinded, controlled trials of pacemaker therapy demonstrated a decrease in the incidence of syncope when compared with standard treatment. The results of these trials were interpreted as being favorable for the use of a pacemaker to reduce vasovagal syncope. However, in a follow-up study, which compared an active pacemaker with a pacemaker for sensing only (surgical sham control), active pacing did not reduce the risk of recurrent syncope in patients with vasovagal syncope. Therefore, the use of a control group in this instance was ethically justifiable. If the original study had used a sham pacemaker, it would have likely resulted in a different outcome and fewer patients would have been exposed to risk of complications from inserting a pacemaker.
RATIONALE FOR BEST AVAILABLE THERAPY AS A CONTROL GROUP
The World Medical Association (WMA) Declaration of Helsinki states specific concepts with regard to the use of a control group:
The benefits, risks, burdens, and effectiveness of the new methods should be tested against those of the best current prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of placebo or no treatment in studies where no proven prophylactic, or therapeutic methods exist. (Reference 4, paragraph 2a)
In a Note of Clarification on paragraph 29 added in 2002, the WMA stated that
… extreme care must be taken in making use of a placebo control trial and that, in general, this methodology should only be used in the absence of existing proven therapy. However, a placebo controlled trial may be ethically acceptable, even if proven therapy is available, under the following circumstances: (a) where for compelling and scientifically sound methodologic reasons its use is necessary to determine the efficacy or safety of a prophylactic, diagnostic, or therapeutic method; (b) where a prophylactic, diagnostic or therapeutic method is being investigated for a minor condition in the patients who receive placebo will not be subject to any additional risk of serious or irreversible harm.
This revised statement regarding the use of placebos still leaves certain concepts open to interpretation. For example, it is not clear when a therapy is considered “proven” because most clinical trials are highly selective and eligibility criteria exclude many patients with a given disorder. Also, there is ambiguity about what constitutes a “minor” condition, and the possibility of serious or irreversible harm may not always be easily appreciated.
RATIONALE FOR PLACEBO AS A CONTROL GROUP
Despite the recommendations by the World Medical Association, there is substantial support for the use of a placebo control group in clinical trials. There are several methodologic reasons to include a placebo-controlled group as opposed to an active control group. First, the use of a placebo group in a double-blind, randomized, controlled trial is the most rigorous test of treatment efficacy for evaluating a medical therapy. Second, placebo-controlled trials can be conducted with fewer patients than active control trials. This is because trials with a placebo group offer the opportunity to compare outcomes under conditions in which there is maximal “treatment separation” (group exposed to an investigational treatment vs. group not exposed to the same investigational treatment), increasing the likelihood of detecting beneficial and/or harmful treatment-related effects (i.e., increased statistical power). This has ethical implications because fewer subjects are potentially exposed to toxic or ineffective treatments. Third, a placebo group can be used as an “add on” to standard of care in comparison to an investigational treatment added to standard of care. Therefore, the true added benefit (or risk) of the new therapy could be evaluated. Fourth, when one compares a new treatment to a standard-of-care therapy, if there is a high rate of placebo response or if the standard treatments are only partially effective a placebo-controlled trial is justified. Last, placebo-controlled trials are essential in the selection of trial endpoints when subjective measures (vs. objective measures) are used because there are often immense variations in the way persons perceive and report patient-reported outcomes. This is especially true for studies involving pain relief, depression, and asthma.
There may also be indirect benefits from trials in general that are particularly applicable to placebo groups. For example, participants often benefit in the placebo control group from the attention that they receive from the study investigators and staff as well as from the ancillary treatments and diagnostics they receive as part of a study trial. In addition, medications are provided as part of the study to which the patients would not otherwise have access.
RATIONALE FOR PLACEBO AS A CONTROL GROUP IN ASTHMA TRIALS
Let us further evaluate the use of the placebo-controlled trials in the evaluation of new therapies for the treatment of asthma. It was once believed that airway bronchoconstriction was a physiologic response that was an objective measure and unlikely to be induced by suggestion. However, studies have now shown that saline inhalation (placebo used in most inhalation studies) can cause bronchoconstriction when the subjects were told that the saline therapy was a bronchoconstrictor (5, 6). Interestingly, anticholinergic agents blocked this placebo-induced bronchoconstriction. Furthermore, placebo bronchoconstriction can be reversed by inhaling saline if it is portrayed as a bronchodilator (7). The magnitude of response to active bronchoconstrictors or bronchodilators is also influenced by the information that is given to the subjects about what they should expect. Furthermore, in numerous trials, subjects with asthma who have been assigned to placebo have demonstrated improvement in symptoms, quality of life, and even in lung function, such as FEV1. In general, the placebo effect in asthma can be as great as 30 to 50% depending on which endpoint is chosen.
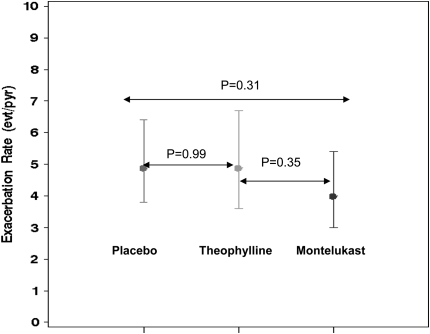
With this large effect from placebo, the use of a “best available therapy” as a control group may be flawed. If there is a large effect from the investigational treatment and from the best available therapy, one may incorrectly conclude that both therapies are effective. However, the same magnitude of effect could have been seen with a placebo group as well. For example, a recent study by the American Lung Association Asthma Clinical Research Centers compared “add on” low-dose theophylline, montelukast, and placebo; all three arms were added to standard-of-care therapy (8). Using the primary endpoint of “episodes of poor asthma control,” the rates (event/person/year) were 4.9 (95% confidence interval [CI], 3.6–6.7) with low-dose theophylline, 4.0 (95% CI, 3.0–5.4) with montelukast, and 4.9 (95% CI, 3.8–6.4) with placebo (Figure 1). If the only comparison arm had been montelukast, one could have incorrectly concluded that the low-dose theophylline was as good as the montelukast. Instead, the correct conclusion was that neither low-dose theophylline nor montelukast was different than placebo.
Figure 1.
Comparing best available therapy with placebo-controlled group. Annualized rates of exacerbations by treatment assignment in the Low-Dose theophylline Or montelukast (LODO) trial. Reprinted by permission from Reference 8.
CLINICAL EQUIPOISE IN PLACEBO-CONTROLLED TRIALS
One argument proposed by Freedman (9) and Michels and Rothman (10) against placebo-controlled trials is that they potentially violate the concept of clinical equipoise when proven effective therapy is available. Clinical equipoise refers to the state where clinicians are unsure whether the new treatment or intervention is as good as the standard treatment. Those who reject the use of placebo-controlled trials argue that they violate the therapeutic obligation of physicians to offer optimal medical care. In other words, they compromise the patient's right to receive the best care possible and violate the ethical principle of therapeutic beneficence. Furthermore, these clinicians have argued that, when proven therapy exists, the use of a placebo-controlled trial lacks both scientific and clinical merit.
Miller and Brody have countered these arguments by stating that this rejection of placebo-controlled trials represents erroneous ethical guidance because it ignores two valid concepts (11). First, there is a distinction between clinical trials and treatment in clinical medicine. In other words, investigators are not offering personalized medical care to the participants in a clinical trial. Randomized, controlled clinical trials are very different from the clinical care that a patient receives in the patient–provider relationship. Second, there are methodologic limitations of active control trials that violate the condition of clinical equipoise. Placebo-controlled trials are ethically justified when they are supported by sound methodologic considerations and when their use does not expose research participants to excessive risk of harm. However, physician investigators have the obligation not to exploit participants for the sake of scientific investigation.
PRO: BEST-AVAILABLE-THERAPY CONTROL GROUP
There are several reasons to support the use of a best-available-therapy control group. Best-available-therapy control groups work well in late phase II and III trials where the goal is to test a new therapy in the planned manner of use in the general population (12). The use of best-available-therapy control groups also provides pivotal evidence of efficacy and provides further information with regard to potential side effects from that medication. This allows one to weigh the balance between efficacy versus side effects between two different therapies. Furthermore, the use of a best-available-therapy control group allows one to avoid the ethical concerns of not providing treatment, as in a placebo-controlled trial.
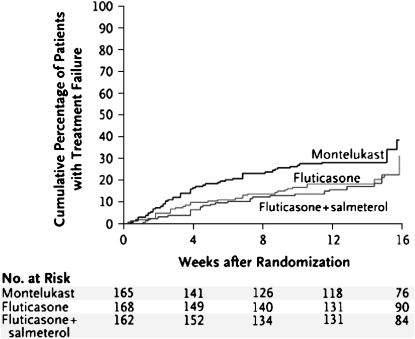
The use of best available therapy is appropriate when a standard of therapy already exists for a condition. In the Leukotriene Modifier or Corticosteroid or Corticosteroid–Salmeterol (LOCCS) trial, the researchers posed the following question: Can patients with mild asthma that is well controlled with a low-dose inhaled corticosteroid (fluticasone proprionate, 100 μg twice daily) be switched to an oral therapy (montelukast, 5 mg or 10 mg once daily) or combination therapy (fluticasone/salmeterol, 100/50 μg/d) without undergoing worsening asthma control or treatment failure (13)? In the LOCCS trial, 500 participants were randomized in a three-arm study to continuation of the active standard of care (inhaled fluticasone twice daily) or were randomized to “step down” therapy with an oral agent (monteleukast) once daily or a combination therapy (fluticasone/salmeterol) once daily (Figure 2). A best-available-therapy group used as the control group in the LOCCS trial (inhaled fluticasone twice per day) was compared with two alternatives for stepping down therapy (montelukast or inhaled combination fluticasone/salmeterol once per day). The investigators believed that the use of a placebo control group was not ethically justifiable given that inhaled corticosteroids (a long-term controller) is the standard treatment of mild asthma (14). Therefore, the withdrawal of inhaled corticosteroids after the run-in phase of this trial for those randomized to placebo was not believed to be ethically justifiable. The LOCCS trial demonstrated that montelukast in step-down therapy for mild asthma was inferior to fluticasone/salmeterol once daily or to continuation of inhaled fluticasone twice daily (best available therapy). Therefore, the withdrawal of one or more components of therapy before randomization is an important consideration in the selection of an active comparator versus the use of a placebo control.
Figure 2.
Comparing best available therapy without placebo control. LOCCS (Leukotriene Modifier or Corticosteroid or Corticosteroid–Salmeterol) treatment failures. Reprinted by permission from Reference 13.
CON: BEST-AVAILABLE-THERAPY CONTROL GROUP
There are several potential reasons for rejecting the use of the best available therapy as a control group in the design of a clinical trial despite the recommendations by the World Medical Association (4). First, a new therapy or intervention may not be superior to a best available therapy but offers advantages in terms of safety, tolerability, or convenience (compliance) (12). Second, there may be methodologic limitations in using a best-available-therapy control group. For example, there may be difficulty in demonstrating similarity to an existing therapy, as in a noninferiority trial, due to sample size issues or reduced efficacy of the therapy. Or, there may be difficulty in standardizing the treatment for all subjects in the trial. Third, without having a concurrent placebo arm to compare with, one has to rely on indirect evidence that the trial actually showed clinically important efficacy or difference in therapy. It could be that an “equivalent” outcome in a trial occurred because the best available therapy and the investigational treatments were not efficacious. Last, the quality of the trial may be impaired by the use of the best-available-therapy group in terms of demonstrating a consistent effect when using an active control group (12). For example, the active comparator may not have the same effect in the patient population that it had in previous trials (e.g., β-blockers in the treatment of hypertension) because the characteristics of the study population may have changed.
CONCLUSIONS
Placebo-controlled trials are justifiable when they are supported by sound methodologic consideration and when their use does not expose research participants to excessive risk of harm. However, investigators should always consider best-available-therapy control groups in the evaluation of a new therapy or intervention. This is especially true when one wants to provide evidence of improved efficacy of a new therapy over an existing therapy. Furthermore, one should consider the use of best-available-therapy control groups when the use of placebo control group would not be ethically justifiable, as in a situation in which patients would not receive the standard of therapy. Last, investigators should keep in mind that one should not sacrifice the scientific merit of a trial to include a best-available-therapy control group if the placebo control poses little harm to participants and, importantly, the trial offers potential benefit to the subject.
Supported by National Institutes of Health/NHLBI grant U10 HL074208.
Conflict of Interest Statement: M.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Council for International Organizations of Medical Sciences. International ethical guidelines for biomedical research involving human subjects. Geneva, Switzerland: CIOMS; 2002. [PubMed]
- 2.Horng S, Miller F. Is placebo surgery unethical? N Engl J Med 2002;347:137–139. [DOI] [PubMed] [Google Scholar]
- 3.Conolly S, Sheldon R, Thorpe K, Roberts R, Ellenbogen K, Wilkoff B, Morillo C, Gent M; VPS II Investigators. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope: Second Vasovagal Pacemaker Study (VPS II). JAMA 2003;289:2224–2229. [DOI] [PubMed] [Google Scholar]
- 4.World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. [internet]. Available at: www.wma.net/e/ethicsunit/helsinki.htm. [DOI] [PubMed]
- 5.Neild J, Cameron I. Bronchoconstriction in response to suggestion: its prevention by an inhaled anticholinergic agent. BMJ 1985;290:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spector S, Luparello T, Kopetzky M, Souhrada J, Kinsman R. Response of asthmatics to methacholine and suggestion. Am Rev Respir Dis 1976;113:43–50. [DOI] [PubMed] [Google Scholar]
- 7.McFadden EJ, Luparello T, Lyons H, Bleecker E. The mechanism of action of suggestion in the induction of acute asthma attacks. Psychosom Med 1969;31:134–143. [DOI] [PubMed] [Google Scholar]
- 8.American Lung Association Asthma Clinical Research Centers. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med 2007;175:235–242. [DOI] [PubMed] [Google Scholar]
- 9.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med 1987;317:141–145. [DOI] [PubMed] [Google Scholar]
- 10.Michels K, Rothman K. Update on unethical use of placebos in randomised trials. Bioethics 2003;17:925–926. [DOI] [PubMed] [Google Scholar]
- 11.Miller F, Brody H. What makes placebo-controlled trials unethical? Am J Bioeth 2002;2:3–9. [DOI] [PubMed] [Google Scholar]
- 12.Lewis J, Jonsson E, Kreutz G, Sampaio C, van Zwieten-Boot B. Placebo-controlled trials and the Declaration of Helsinki. Lancet 2002;359:1337–1340. [DOI] [PubMed] [Google Scholar]
- 13.American Lung Association Asthma Clinical Research Centers. Randomized comparison of strategies for reducing treatment in mild persistent asthma. N Engl J Med 2007;356:2027–2039. [DOI] [PubMed] [Google Scholar]
- 14.National Asthma Education and Prevention Program. Full report of the expert panel: guidelines for the diagnosis and management of asthma. Expert Panel report 2. Bethesda, MD: National Institutes of Health; 1997. (NIH publ. no. 97-4051)