Abstract
Although the outcome of respiratory infection alters with age, nutritional status, and immunologic competence, there is a growing body of evidence that we all develop a unique but subtle inflammatory profile. This uniqueness is determined by the sequence of infections or antigenic insults encountered that permanently mold our lungs through experience. This experience and learning process forms the basis of immunologic memory that is attributed to the acquired immune system. But what happens if the pathogen is not homologous to any preceding it? In the absence of cross-specific acquired immunity, one would expect a response similar to that of a subject who had never been infected with anything before. It is now clear that this is not the case. Prior inflammation in the respiratory tract alters immunity and pathology to subsequent infections even when they are antigenically distinct. Furthermore, the influence of the first infection is long lasting, not dependent on the presence of T and B cells, and effective against disparate pathogen combinations. We have used the term “innate imprinting” to explain this phenomenon, although innate education may be a closer description. This educational process, by sequential waves of infection, may be beneficial, as shown for successive viral infections, or significantly worse, as illustrated by the increased susceptibly to life-threatening bacterial pneumonia in patients infected with seasonal and pandemic influenza. We now examine what these long-term changes involve, the likely cell populations affected, and what this means to those studying inflammatory disorders in the lung.
Keywords: lung inflammation, heterologous immunity, respiratory tract, influenza, innate immunity
The lower respiratory tract is often referred to as part of the common mucosal immune system when in fact it is entirely different from other common members, especially the gut. The bronchioles and alveoli do not have a resident commensal microbial flora, and the lung is essentially sterile below the larynx. Sterility is maintained by, for example, an array of antimicrobial factors and mucus, which captures particulate matter that is then transported out of the respiratory tract by ciliated epithelium. This sterility is necessary for efficient gaseous exchange. Oxygen already has to traverse the epithelial and endothelial cell membranes and associated basement membranes before even reaching a red blood cell. Commensals attached to respiratory epithelium and immune cells to keep them in check would impede this process. In the gut, commensals are vital to compete with potentially more pathogenic organisms for attachment sites and nutrients; some even secrete their own antimicrobial substances. The lung, however, assumes that more inherent physical and chemical barriers will exclude pathogenic microorganisms. Unlike the gastrointestinal tract, the lower airways contain only sparse lymphoid tissue, which appears to limit inflammation rather than promote it. Takabayshi and colleagues (1) have recently reported that alveolar macrophage homeostasis is maintained by transforming growth factor (TGF)-β, which is tethered to epithelial cells by the integrin αvβ6. During successful infection of the lower respiratory tract, TGF-β is released and the alveolar macrophage is allowed to perform its antimicrobial and inflammatory roles. Homeostasis is restored when macrophage-released matrix metalloproteinases transform latent TGF-β into its active form. Dendritic cells (DCs), although abundant in the healthy respiratory tract juxtaposed with the basolateral surface of epithelial cells, are present in an immature form that is less capable of priming naive T cells. Microbial sampling, potentially via dendritic projections through the epithelial cells and into the airway lumen, can cause their activation, maturation, and migration, arming them to support potent T-cell responses (2). The mechanism that returns these cells to homeostasis is not known but may rely on the level of Toll-like receptor (TLR) signals and/or the influence of paired inhibitory receptors (3). Isolated B-cell follicles are also observed in the healthy lung, the so-called inducible bronchus associated lymphoid tissue (iBALT). As the name suggests, it is inducible and associated with the larger airways. Recent work suggests that this lymphoid structure also restrains its inflammatory potential. Respiratory influenza infection of wild-type mice leads to excessive inflammation that is deleterious to the host. If peripheral immunity is removed by deleting the gene for lymphotoxin, iBALT remains intact. Furthermore, infection of these mice with influenza results in a smaller immune response that clears the pathogen without bystander tissue damage (4). Less inflammation in this case is more; a concept that is supported by our data showing that a significant reduction (5–7), but not elimination (8), of T cells does not impede pathogen clearance or the deposition of sufficient immune memory to rapidly blunt subsequent encounters.
These observations suggest that the problem in the respiratory tract lies with primary, not secondary, immune responses. During a primary response, the frequency of antigen-specific cells is low and so pathogens are allowed to replicate unchecked for a significant period of time. This causes bystander tissue damage and the release of high levels of inflammatory cytokines and chemokines. The wave of cells recruited as a consequence, coupled with the high antigen load, causes occlusion of the airspaces and extensive bystander damage. During a secondary response, however, antibody and memory T cells are poised to reduce the antigen load. Reduced inflammatory signals and a smaller antigen load results in a more contained and short-lived immune response (see Figure 1). Collateral damage is significantly reduced. Immunologic memory, in the context of respiratory infections, is therefore beneficial. This acquisition of effectiveness with time is attributed to the adaptive immune response leading to immunologic memory. However, there is increasing evidence in the literature that this time-dependent maturation occurs even when infections are antigenically distinct.
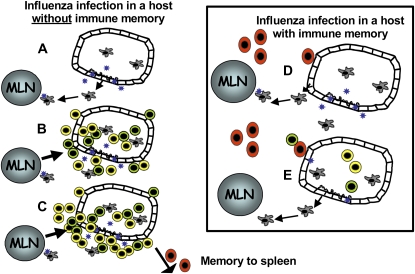
Figure 1.
The outcome of respiratory infection depends on whether immunologic memory exists. In a naive host, infection induces a highly inflammatory microenvironment, and antigen-presenting cells loaded with antigen track to the draining lymph node where they activate CD4+ and CD8+ T cells (A). Further inflammatory cytokines, produced due to unchecked viral replication, recruit a large inflammatory infiltrate (B) that eventually occludes the airways. Memory B and T cells migrate to the spleen (C). In hosts with cross-reactive T and B cells, however, antigen-specific cell migration occurs very quickly (D). Viral replication is rapidly controlled leading to less inflammation (E). MLN = mediastinal lymph node.
HETEROLOGOUS IMMUNITY AND THE IMPACT IN THE RESPIRATORY TRACT
In recent years, epidemiologic and animal model data have demonstrated that one respiratory infection alters subsequent immune responses to unrelated pathogens in the same site—an effect that is long lasting (9–11). Prior lung infection with influenza, for example, protects against respiratory syncytial virus (RSV)-induced immunopathology and lymphocytic choriomeningitis virus (LCMV)-infected mice exhibit heightened clearance of the unrelated Vaccinia virus (11–13). The outcome of infection history depends on the precise sequence of pathogens encountered. Influenza, for example, inhibits Vaccinia replication but enhances LCMV and murine cytomegalovirus (MCMV). The critical determinant appears to be the level of lymphocytic infiltrate during the second infection (13). Bacterial bacille Calmette-Guérin infection in the lung also leads to a better outcome for subsequent Cryptococcus neoformans infection, most likely because it skews the immune response from a nonprotective Th2 to a protective Th1 response (14). In most, but not all, cases, the improved outcome to the second infection is not explained by cross-reactivity in T- and B-cell antigens. Rather, we believe the alteration is in the lung environment itself or in innate immune cells.
Evidence for long-term modification of the innate immune compartment is provided by studies where microbial products, such as CpG DNA or a modified bacterial labile toxin (LTK63), afford protection against an array of subsequent respiratory pathogens (15, 16). This phenomenon of “innate imprinting” or “innate education” can be defined as “the long-term modification of a microenvironment, which will consequently lead to a nonspecific, but more protective, immune phenotype to a subsequent pathogen.” LTK63 reduces inflammation and prevents immunopathology and illness associated with RSV and influenza infection (16). Furthermore, elimination of RSV is not compromised and clearance of influenza is actually improved. The protective effect conferred by LTK63 lasts up to 12 weeks after administration and is associated with the maturation of myeloid cells. The protective effect conferred by LTK63 does not depend on T and B cells, because innate education can be induced in mice lacking T and B cells (RAG knockout mice). This clearly defines a long-lasting modulation that has occurred at the level of the environment or innate immune compartment. Modulation of innate immunity in this manner and in a site as sensitive as the respiratory tract has clear therapeutic potential and may even be beneficial in patients with immune deficiency.
In some parts of the world, an altered outcome to respiratory infection by prior events may be due to a spill over of more chronic infections. Helminthic infections, for example, are highly prevalent in rural areas of Africa and generally induce excessive Th2 cytokine–dominated immunity that could impact on Th1-associated respiratory infections (17, 18). We have recently reported that a colonic, restricted Citrobacter rodentium infection modulates the usual Th2-driven pathology to lung C. neoformans infection by skewing the immune response toward a Th1 cytokine profile (19). Similarly, the induction of regulatory T cells to one infection may impact on others in a bystander fashion. Lung IL-10 production is enhanced after a secondary pneumococcal or meningococcal challenge in mice that have experienced an influenza infection, which in turn impairs the neutrophils' ability to clear the bacteria (20, 21). Interestingly, IL-10 appears late in the immune response to influenza and is sustained after clearance of the virus. Mycobacteria, malaria, and parasitic protozoa and helminths induce high levels of immuno suppressive cytokines (TGF-β and IL-10) that could reduce immunity to subsequent or concurrent infection in the respiratory tract. Vaccines proven to be efficient in high-income countries might perform less well in low-income countries (22) for the very same reasons.
Influences may also exist due to retention of acute pathogens, in whole or in part. This is mostly attributed to more chronic pathogens, such as Mycobacterium tuberculosis, but limited evidence also implicates acute viruses. M. tuberculosis, together with other bacteria capable of establishing latent infections, such as Chlamydia species, are reported to survive within lung DCs, impairing their antigen-presentation capability and subsequent capacity to stimulate T cells (23, 24). Conversely, in some instances, the low-level inflammation required to keep a latent infection in check can lead to increased inflammatory responses to a second insult, as demonstrated for adenovirus infection (25). Guinea pigs latently infected with adenovirus have greater inflammatory responses after exposure to cigarette smoke than do uninfected control animals (26). Similarly, in patients with chronic obstructive pulmonary disease, an association exists between increased inflammatory responses and the presence of adenoviral protein and DNA in the lungs (27). However, not all latent infections have a deleterious effect on the host. Persistence of MCMV or murine γ-herpesvirus 68 (γHV68) in DCs and macrophages induces resistance to infection with multiple unrelated bacterial pathogens. This protective effect is not restricted to the infected cells themselves but also uninfected macrophages via the sustained production of IFN-γ, which activates them (28).
Persistence of respiratory syncytial virus by identification of viral mRNA has been described in murine models (29), and live virus can live indefinitely within macrophages (30), but evidence in humans is lacking. Analysis of patients with chronic obstructive pulmonary disease implies an ongoing infection as the cause of exacerbation rather than influences of a persistent virus (31). Persistence of rhinovirus, however, has been demonstrated after asthma exacerbation (32) as have influenza antigens (33, 34), but not the viral genome (35).
The counterregulation between latent infections and the host immune response is complex, and to a large extent its effect on subsequent infections remains unknown. Considering that this interplay between the latent pathogen and the host persists in many cases for the lifetime of the infected individual, it presents yet another potential mechanism by which the innate immune response can be altered over time.
In all of these situations, we are not describing cross-reactive acquired immunity but nonspecific influences from either what has gone before or what is currently present. We are therefore all unique because prior local or distal antigenic experiences have shaped our responsiveness to future antigenic insults, all in the absence of cross-reactive acquired immunity.
INNATE IMMUNITY—CAN IT REMEMBER?
We attribute “memory” to the adaptive immune response, but research in invertebrates (36), in particular, implies that the innate compartment is also modified long term by prior events. Aging, and the associated exposure to more numerous infections over time, correlates with increased innate immune activation in fruit flies, mice, and humans (37–39). Furthermore, injection of the bacterial cell wall component LPS provides long-lasting antimicrobial resistance in mealworm beetles when subsequently challenged with a heterologous natural fungal pathogen (40). Similarly, mosquitoes previously infected with bacteria demonstrate a short-term reduction in infectivity of a particular malarial parasite strain, which is believed to involve the up-regulation of antimicrobial peptides by the first infection (41). Antimicrobial peptides (abundant in the lung) alter with age (39), with older Drosophila producing higher diptericin levels after live bacteria infection than young adult flies. In contrast, and somewhat surprisingly, the opposite is true after exposure to killed bacteria. Although effects that alter with age are attributed to senescence, another interpretation is that innate immunity is more tightly regulated as time progresses due to the experience of a greater number of infections. Thus, the older flies have a more experienced innate immune system, and therefore more focused production of diptericin, responding vigorously only in the presence of an infectious pathogen. Similar alterations in innate antibacterial peptides may also occur in humans.
The innate immune system in invertebrates therefore learns. This learning process, in the absence of adaptive immunity, also occurs with homologous or closely related pathogens. Copepods, which are miniature crustaceans, infected with a natural parasitic tapeworm are capable of specific memory responses and react more efficiently upon subsequent rechallenge with antigenically similar pathogens (42). Likewise, Streptococcus pneumoniae–infected Drosophila are protected indefinitely against an otherwise lethal second challenge with the same bacteria (43). The mechanisms underlying this educated innate immunity are currently unclear but are believed to require phagocytes and the Toll receptor pathway. If this can happen in invertebrates, then why not in vertebrates?
OTHER INNATE PARAMETERS THAT ARE (OR LIKELY TO BE) PERMANENTLY MODIFIED IN THE LUNG MICROENVIRONMENT
It is often assumed that homeostatic mechanisms restraining innate immunity are overcome during infection but that, upon elimination of the antigen, peace is fully restored. However, this may not make evolutionary sense. If the innate immune response to a particular pathogen almost killed the host on first encounter, why would it repeat the excessiveness on encounter with a homologous or closely related organism? In the context of the lung, we are proposing that subtle long-term changes occur in a multitude of different cell types, evidence for which we now discuss.
Altered Epithelial Cells
Infections, disease, or environmental stresses are all capable of inducing alterations in epithelial cells that may modify their response to further stimulation. Influenza infection, for example, enhances susceptibility to secondary bacterial infection by exposing cryptic bacterial adhesins or receptors on epithelial cells (44), and adenovirus enhances subsequent bacterial adherence (45). The longevity of these alterations is assumed to be transient but has never been investigated. Long-term changes in epithelial cell phenotype and homeostasis are observed in diseases such as asthma where structural changes increase epithelium fragility and shedding and goblet cell hyperplasia (46). However, although these are believed to be related to persistent uncontrolled inflammation, their reversibility in the absence of inflammation has not been investigated. There is certainly a decline in the capacity of the elderly to resist infectious disease, which may suggest defects in the barrier function of epithelium or in its ability to sense them.
Epithelial cells up-regulate TLRs during RSV and influenza infection, which may be mediated by the release of IFN by infected macrophages (47–49). Whether such up-regulation has an impact on secondary infection is unclear (50). Many of these receptors share signaling pathway components and so changes in the expression level or phosphorylation status of the molecules within these pathways may interfere with the recognition of subsequent pathogens. This may synergize and exacerbate the inflammatory responses, as recently shown during bacterial coinfection (51), or could lead to an attenuation of the signal. In support of the latter, we have evidence for a long-term modification in the responsiveness of lung epithelial cells to pathogen-associated molecular patterns (PAMPS) after clearance of an acute viral infection that results in poor recruitment of innate immune cells and subsequent clearance of respiratory bacteria (data not shown). This influence may occur during resolution of the first infection when epithelial integrity is regenerated by bone marrow progenitors or local stem cells in a process controlled by interaction with underlying sentinel mesenchymal cells, such as fibroblasts (52). These mesenchymal cells may be altered by the primary infection and maintain the “memory” of these inflammatory events to educate newly regenerated epithelium.
The transformation of airway epithelial cells into secretory epithelium containing mucus-secreting cells (mucous cell metaplasia) is a feature of many bronchial diseases in humans and is also observed in numerous rodent experimental systems after exposure to inhaled sulfur dioxide (53), cigarette smoke (54, 55), endotoxin (56–58), ozone (59), or neutrophil elastase (60–62). These alterations are frequently long-lasting and persist for weeks to months after exposure to the inhaled agent has ceased. Airway metaplasia can persist in ex-cigarette smokers 2 or more years after they have stopped smoking (63). Such long-term changes are likely to affect innate immune reactivity to subsequent infections. Further evidence that epithelial cells are modified for prolonged periods is provided by the development of resistance to peroxide exposure through long-term changes in epithelial cell biology and architecture, with changes in cytoskeletal structure and new molecular forms of p53 and heat shock proteins observed. There are also reports of long-term genome-wide reprogramming of gene expression in epithelial cells in vitro (64). Epithelial 293 cells exposed to cellular extracts from T cells take on T-cell properties through long-term transcriptional changes, which persist for approximately 80 population doublings in culture. If this occurs in the lung in vivo, then epithelial cell heterogeneity would be extensive and depend on the type of prior antigenic insult and precise composition of the cellular response.
Alteration of Endothelial Cells
Endothelial cells are known to display different characteristics depending on their organ of origin within an individual, and these characteristics are maintained in culture, suggesting that endothelium can be irreversibly altered and “remember” the conditions under which it differentiated even when those conditions change (65). Even within the same organ, endothelial cells show remarkable heterogeneity in size, shape, antigen responsiveness, and susceptibility to disease such as atherosclerosis (66). These differences are proposed to result from exposure to varying conditions during the life of an individual. There may be location-specific events—for example, the rate of flow of fluid surrounding cells, or differences in conditions relating to the health or behavior of the individual. For example, ethanol-fed rats display increased susceptibility to acute respiratory distress syndrome due to decreased endothelial antioxidant activity and increased endothelial sensitivity to tumor necrosis factor (TNF). Giant cell arteritis increases the expression of cellular adhesion molecules on endothelial cells, suggesting that this pathology can leave tissues more susceptible to inflammatory infiltrate (67), and a high pathogen burden correlates with increased susceptibility to atherosclerosis as a result of endothelial dysfunction (68). Finally, age-related deterioration of endothelial function (possibly by prior infection) is known to affect the nitric oxide signaling pathway, impairing vasodilation and vasoconstriction, which can be reduced by exercise (69).
Permanent Alterations in Lymphatics
Even lymphatics do not escape the learning process and impact on responsiveness to sequential waves of infectious disease. Inflammation during respiratory infection causes the release of fluid from blood vessels into the tissues (70), which the lymphatic drainage system needs to clear to prevent obstruction to oxygen transfer, breathlessness, and cough. Recently, evidence shows that pulmonary Mycoplasma pulmonis infection causes vascular endothelial growth factor (VEGF)-C–and VEGF-D (produced by infiltrating macrophages, neutrophils, and airway epithelial cells)–dependent genesis of new lymphatic vessels, allowing interstitial fluid to be cleared efficiently before edema can occur in the bronchioles (71). This in turn decreases the amount of inflammatory cytokines and leukocytes that may otherwise cause bystander damage to the lung. Because newly developed lymphatic vessels persist for prolonged periods of time, the precise infection history of the host may determine the efficiency of something as fundamental as fluid drainage.
In addition to the growth of new lymphatic vessels, new lymphoid structures develop in the lung after infection (4, 72). We alluded to iBALT earlier, which is sited at the peribronchial, perivascular, and interstitial areas of the lung. In the absence of peripheral lymphoid tissue, immune responses generated in iBALT efficiently clear an influenza infection, with delayed kinetics but lower bystander tissue damage (72). Thus, previous infections, which induce the development of new lymphoid aggregates, may alter the response to subsequent unrelated pathogens. The development of these ectopic lymphoid structures is not always beneficial. In autoimmune diseases, such as rheumatoid arthritis and Sjögren's syndrome, iBALT in the lungs of patients is linked to increased tissue damage due to enhanced collagen deposition and the development of fibrosis (73). A previous lung infection may therefore exacerbate pulmonary damage in patients with other chronic illnesses.
Can Natural Killer Cells Be Modified?
There is even evidence to suggest that natural killer (NK) cells (which clear pathogen-infected cells and tumor cells through direct cytotoxicity and secretion of cytokines) increase in the circulation (74) and display phenotypic alterations (75) with age. The decreased lytic activity of NK cells in the elderly (76) has been attributed to age, but may represent a refinement of reactivity due to past experiences. This is supported by a long-term clinical study of healthy elderly patients in Japan, which shows a positive correlation between decreased NK cell lytic activity and prior infections (77). Patients who had experienced an infectious episode in the 33 months before assessment had lower NK cell lytic activity than those free of infection. Again, these results suggest long-term changes to the innate immune response as a consequence of prior infectious events.
Modification of Antigen-presenting Cells
It is often assumed that antigen-presenting cells (APCs) return to their resting state once the pathogen is cleared. However, CD11c-expressing cells are maintained in the lung and display enhanced antigen presentation long after the resolution of an influenza or RSV primary infection (78, 79), which may influence subsequent immunity to unrelated pathogens. In the case of influenza infection, DCs that remain in the lung several weeks after infection have a more activated phenotype and enhanced ability to promote T-cell priming, a process that is dependent on IFN-γ (78) and controlled by cytotoxic γδ T cells recruited during the resolution phase of a bacterial infection (80). The activation of APCs is conditioned by the local environment in which they are primed, which may influence the development of Th1/Th2 responses to subsequent pathogens (81). Interestingly, purified microbial products, such as TLR ligands or toxins, induce a sustained activation of APCs in the lungs and provide generic protection to subsequent pathogenic insult due to ameliorated T-cell priming (15, 16, 82). DC migration can also be modulated by infection. For example, Legge and Braciale showed that DC migration to the draining lymph node is increased during the first 24 hours of infection but is then impaired for prolonged periods (83).
Alveolar macrophages express a particular profile of pattern recognition receptors, reside in the alveolar lumen (84), and play a dynamic role in initiating as well as controlling inflammation. They differentiate and functionally adapt depending on the environmental factors and stimuli present in the tissue, but in a steady state display a suppressive phenotype mediated by the secretion of IL-10, nitric oxide, or TGF-β (85, 86). Alveolar macrophages also produce a state of reversible T-cell inactivation that is mediated either by defective expression of costimulatory molecules (87) or an increased expression of CD80 (88), a receptor that preferentially binds to the negative T-cell receptor CTLA-4. In the introduction we alluded to the possibility that TGF-β plays a role in suppressing alveolar macrophage activity. It is likely that secretion of granulocyte macrophage–colony stimulating factor (GM-CSF), produced most probably by mesenchymal cells, does the opposite (89). The activity of alveolar macrophages will therefore dictate the immune responsiveness of the lung to future infection and will be influenced by prior inflammatory events.
During the course of infection, the population of alveolar macrophages in the airway consists not only of resident phagocytes but also of new monocyte-derived macrophages recruited into the airway. Monocyte chemoattractant protein (MCP)-1/chemokine (C-C) ligand 2 (CCL2) is essential for the recruitment of monocytes, which differentiate into phagocytes and clear residual apoptotic neutrophils present during the resolution of acute bacterial pneumonia (90). CD11b-high subpopulations of monocytes also populate the airway after pneumococcal challenge and are involved in the resolution of infection by clearing cell debris. These cells are relatively long-lasting and their expression of CD11b is dependent on the abundance of GM-CSF (91). Whether or not GM-CSF is involved in prolonging the presence of these cells in the airway is not known, but it is not unreasonable to suggest that prior infection may influence the number and activation of lung APCs and, in turn, affect how subsequent pathogens are handled.
IS LUNG REMODELING A FEATURE OF ACUTE INFECTION?
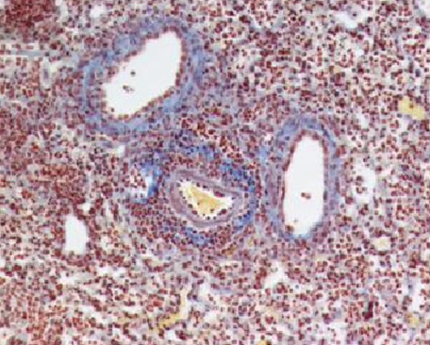
The tissue damage caused by some severe acute infections leads to a repair process that modifies the matrix composition of the lung (such as collagen and fibronectin deposition). To maintain barrier function, the epithelium is regenerated either from bone marrow progenitors or local stem cells in a controlled dynamic and bidirectional cross-talk with mesenchymal cells such as fibroblasts, which could also be potentially altered by a previous infection (92). Whether the regenerated epithelium responds differently in this context is unknown. Airway remodeling is very often restricted to chronic conditions such as asthma or fibrosis, and it is unclear whether some level of remodeling occurs upon tissue injury induced by an acute infection. We have observed excessive collagen deposition during respiratory C. neoformans infection (see Figure 2) and are currently assessing whether this is maintained after resolution of inflammation. Remodeling is classically controlled by TGF-β, a molecule also involved in immunosuppression. Whether local TGF-β controls the innate immune response to a secondary challenge has not been directly addressed.
Figure 2.
Collagen deposition during Cryptococcus neoformans infection. Mice were infected intranasally with C. neoformans and lung inflated and fixed in formalin 12 days later. Paraffin-embedded sections were stained with periodic acid Schiff, and collagen deposition (blue) and cellular infiltrate (brown) identified by light microscopy. Original magnification, × 200.
“INNATE ANTIBODY”
Innate “natural” antibodies are found in the circulation and at mucosal surfaces, including the lung. They are predominantly IgM, but IgA and IgG isotypes also exist (93, 94), all of which are principally produced by B-1 cells, but immature/transitional 1 B (93) and marginal zone B cells are also implicated. The principal advantage of innate antibody is immediate defense by binding pathogens (nonspecifically) for opsonization, neutralization, and epithelial transfer while more classical acquired T- and B-cell responses develop (reviewed in Reference 95). Cells producing innate antibodies are long-lived and self-replenishing, but do not expand after respiratory infections, although some dispute this. In the case of influenza infection, B-1 cells will produce IgM that is reactive to the virus; however, these cells do not undergo class switching or recombination; they therefore do not undergo affinity maturation or induce “memory” (96). However, in the case of S. pneumoniae infections, these cells undergo limited isotype switching, but no affinity maturation, and therefore would develop a type of “innate memory” (97). As this subset of cells undergoes selection, the innate antibody response to subsequent infections would be altered.
B-1 cells are selected during the neonatal period to have low reactivity to self-antigens, a process that maintains self-replenishment. Such cells are more prevalent in the aging host as they are selected for linearly throughout life (98). This evolution pushes a more selective innate response and will certainly be affected by the precise sequence of prior infections. Each person may therefore end up with their own unique repertoire of innate antibodies.
CROSS-REACTIVE T-CELL REPERTOIRES AND CELLULAR ATTRITION—SOMETHING HAS TO GIVE
In the preceding sections, we have attempted to highlight how individual innate or resident cell populations adapt due to prior infectious events in the lung and present evidence to suggest that this may impact on subsequent unrelated organisms. There is another phenomenon that is worth considering in this respect (albeit briefly in this article): the influence of cross-reactive T cells and immune senescence.
T cells recognize only a few contact points within a major histocompatability complex (MHC) bound epitope. This, together with conformational changes that occur when the TCR binds to MHC (99), means that an individual T-cell receptor may recognize up to 106 different peptides (100). During infection, this leads to cross-reactivity (101), which may be advantageous (i.e., producing a diverse immune repertoire) or detrimental and lead to pathologies such as autoimmunity (102, 103). Evidence in murine models suggests we cannot house all of the altered or expanded cell repertoires after resolution of infection due to space constraints. Some populations therefore have to be sacrificed during each successive inflammatory event. This is clearly evident within the T-cell compartment but may also be true between cellular subsets. This may be especially important when different subsets use similar growth factors (e.g., IL-2 for NK and T cells) or are dependent on the same chemokines for migration into inflamed sites. Should the growth factor be limiting and one populations arrive in the lung before the other, and in greater numbers, then it is more likely to use the growth factor to its advantage at the expense of the later population of cells.
Making way for new memory T-cell populations has been elegantly demonstrated by Selin and colleagues (9) in murine models, although evidence in humans is still limited. The early attrition of memory T cells observed in the periphery during numerous viral infections, including respiratory, is mediated by type 1 IFN (104). This attrition is believed to reduce potential cross-reactive memory cells that could otherwise skew the T-cell repertoire and prevent the efficient clearance of the new pathogen (104). Longer term erosion of preexisting T-cell memory by waves of infection is apparent in multiple infectious models (105) and driven by homeostatic forces driving deletion to accommodate others (106), an effect believed to be most significantly mediated by the early attrition phenomenon mentioned above. The precise pools of memory cells affected may depend on whether cross-reactive epitopes exist between the current and previous pathogen (107) (see Figure 3). At present, however, attrition does not seem to be affected by antigen specificity.
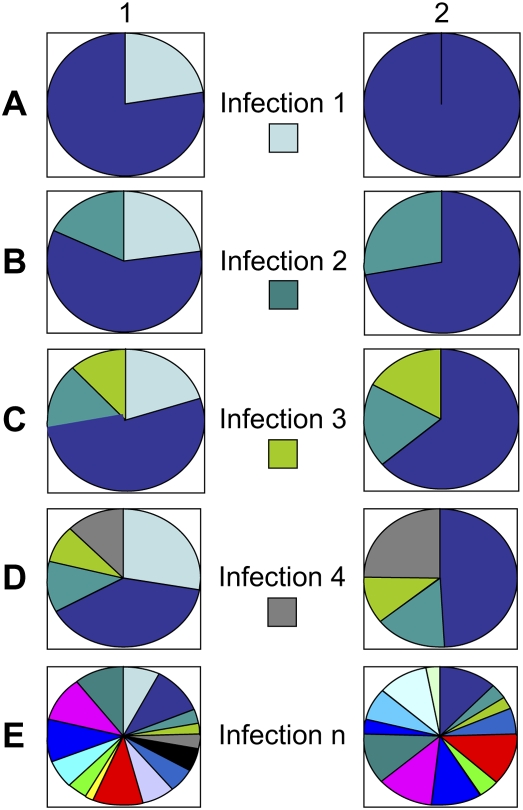
Figure 3.
The influence of infection on memory T-cell repertoires in monozygotic twins. Despite the genetic similarities, established memory T-cell pools will vary depending on the infections encountered, the degree of attrition that may be affected by the severity of infection, and private T-cell specificities. In this rather simplified schematic in (A), twin 1 becomes infected with virus 1, whereas twin 2 does not. Twin 1 will therefore have resident immune memory to virus 1. In (B), both twins experience infection 2, but the response to this in twin 1 is less because of innate adapted influences from the first infection. Furthermore, in twin 2, homeostatic suppression of T-cell expansion may be less. In (C), both twins experience infection 3, which reduces the memory T-cell pool to infection 1 (in twin 1) and infection 2 (in both twins) due to attrition (see text). In (D), infection 4 gives rise to immunologic memory in both twins but also an expansion of memory cells to infection, one due to cross-reactive epitopes between viruses 1 and 4. (E) The heterogeneity in the memory T-cell pools after multiple infections in identical twins is shown. This heterogeneity and influence of previous infections could also easily extend to innate immunity.
In summary, as soon as the first antigen enters the lung, the environment, cellular composition, and ability to react to subsequent challenges are permanently modified. This modification is not necessarily fixed but will adapt to each new situation, although the lung will never recover its original form. This adaptation may be beneficial in some cases and prevent bystander tissue damage (due to excessive inflammation) or, in other cases, detrimental, allowing, for example, bacterial colonization in the wake of an influenza infection. We are therefore all unique, due to not only our genetic makeup and private T- and B-cell specificities but also the precise sequence and severity of infections and other diseases encountered throughout life. Alteration of immune reactivity with time is often attributed to simple aging, but this may also be influenced by the accumulation of antigenic experience with time. This heterogeneity, developed through experience, probably represents an evolutionary strategy ensuring survival of at least some of the population. It also explains the diversity of immune pathologies experienced during a single infection, the reason why monozygotic twins do not succumb to the same diseases, and the variability in response to vaccination.
Supported by the Medical Research Council, the Biotechnology and Biological Sciences Research Council, the National Institutes of Health, and the European Union.
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Takabayshi K, Corr M, Hayashi T, Redecke V, Beck L, Guiney D, Sheppard D, Raz E. Induction of a homeostatic circuit in lung tissue by microbial compounds. Immunity 2006;24:475–487. [DOI] [PubMed] [Google Scholar]
- 2.Hammad H, Lambrecht BN. Lung dendritic cell migration. Adv Immunol 2007;93:265–278. [DOI] [PubMed] [Google Scholar]
- 3.Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol 2002;23:285–290. [DOI] [PubMed] [Google Scholar]
- 4.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 2004;10:927–934. [DOI] [PubMed] [Google Scholar]
- 5.Humphreys IR, Walzl G, Edwards L, Rae A, Hill S, Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med 2003;198:1237–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussell T, Snelgrove R, Humphreys IR, Williams AE. Co-stimulation: novel methods for preventing viral-induced lung inflammation. Trends Mol Med 2004;10:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussell T, Pennycook A, Openshaw PJM. Inhibition of tumour necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol 2001;31:2566–2573. [DOI] [PubMed] [Google Scholar]
- 8.Humphreys IR, Edwards L, Snelgrove RJ, Rae AJ, Coyle AJ, Hussell T. A critical role for ICOS co-stimulation in immune containment of pulmonary influenza virus infection. Eur J Immunol 2006;36:2928–2938. [DOI] [PubMed] [Google Scholar]
- 9.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev 2006;211:164–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamradt T, Goggel R, Erb KJ. Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trends Immunol 2005;26:260–267. [DOI] [PubMed] [Google Scholar]
- 11.Walzl G, Tafuro S, Moss P, Openshaw PJ, Hussell T. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J Exp Med 2000;192:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol 2001;2:1067–1076. [DOI] [PubMed] [Google Scholar]
- 13.Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol 2003;163:1341–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walzl G, Humphreys IR, Marshall BG, Edwards L, Openshaw PJ, Shaw RJ, Hussell T. Prior exposure to live Mycobacterium bovis BCG decreases Cryptococcus neoformans-induced lung eosinophilia in a gamma interferon-dependent manner. Infect Immun 2003;71:3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards L, Williams AE, Krieg AM, Rae AJ, Snelgrove RJ, Hussell T. Stimulation via Toll-like receptor 9 reduces Cryptococcus neoformans-induced pulmonary inflammation in an IL-12-dependent manner. Eur J Immunol 2005;35:273–281. [DOI] [PubMed] [Google Scholar]
- 16.Williams AE, Edwards L, Humphreys IR, Snelgrove R, Rae A, Rappuoli R, Hussell T. Innate imprinting by the modified heat-labile toxin of Escherichia coli (LTK63) provides generic protection against lung infectious disease. J Immunol 2004;173:7435–7443. [DOI] [PubMed] [Google Scholar]
- 17.Maizels RM. Infections and allergy: helminths, hygiene and host immune regulation. Curr Opin Immunol 2005;17:656–661. [DOI] [PubMed] [Google Scholar]
- 18.Furze RC, Hussell T, Selkirk ME. Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infect Immun 2006;74:1924–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams AE, Edwards L, Hussell T. Colonic bacterial infection abrogates eosinophilic pulmonary disease. J Infect Dis 2006;193:223–230. [DOI] [PubMed] [Google Scholar]
- 20.Alonso JM, Guiyoule A, Zarantonelli ML, Ramisse F, Pires R, Antignac A, Deghmane AE, Huerre M. van der WS, Taha MK. A model of meningococcal bacteremia after respiratory superinfection in influenza A virus-infected mice. FEMS Microbiol Lett 2003;222:99–106. [DOI] [PubMed] [Google Scholar]
- 21.van der Sluijs KF, Nijhuis M, Levels JH, Florquin S, Mellor AL, Jansen HM. van Der PT, Lutter R. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J Infect Dis 2006;193:214–222. [DOI] [PubMed] [Google Scholar]
- 22.Rook GA, Dheda K, Zumla A. Immune systems in developed and developing countries: implications for the design of vaccines that will work where BCG does not. Tuberculosis (Edinb) 2006;86:152–162. [DOI] [PubMed] [Google Scholar]
- 23.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol 2007;179:2509–2519. [DOI] [PubMed] [Google Scholar]
- 24.Rey-Ladino J, Jiang X, Gabel BR, Shen C, Brunham RC. Survival of Chlamydia muridarum within dendritic cells. Infect Immun 2007;75:3707–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogg JC. Role of latent viral infections in chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med 2001;164:S71–S75. [DOI] [PubMed] [Google Scholar]
- 26.Vitalis TZ, Kern I, Croome A, Behzad H, Hayashi S, Hogg JC. The effect of latent adenovirus 5 infection on cigarette smoke-induced lung inflammation. Eur Respir J 1998;11:664–669. [PubMed] [Google Scholar]
- 27.Higashimoto Y, Yamagata Y, Itoh H. Complex effect of adenovirus early region proteins on innate immune system. Inflamm Allergy Drug Targets 2006;5:229–237. [DOI] [PubMed] [Google Scholar]
- 28.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007;447:326–329. [DOI] [PubMed] [Google Scholar]
- 29.Schwarze J, O'Donnell DR, Rohwedder A, Openshaw PJ. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med 2004;169:801–805. [DOI] [PubMed] [Google Scholar]
- 30.Guerrero-Plata A, Ortega E, Ortiz-Navarrete V, Gomez B. Antigen presentation by a macrophage-like cell line persistently infected with respiratory syncytial virus. Virus Res 2004;99:95–100. [DOI] [PubMed] [Google Scholar]
- 31.Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kling S, Donninger H, Williams Z, Vermeulen J, Weinberg E, Latiff K, Ghildyal R, Bardin P. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin Exp Allergy 2005;35:672–678. [DOI] [PubMed] [Google Scholar]
- 33.Dubrovina TY, Ivanova IA, Shidlovskaya NK, Polyak RY. Significance of long lasting persistence of influenza virus antigens at the portal of infection and in the spleen of mice. Acta Virol 1992;36:450–458. [PubMed] [Google Scholar]
- 34.Bramley AM, Vitalis TZ, Wiggs BR, Hegele RG. Effects of respiratory syncytial virus persistence on airway responsiveness and inflammation in guinea pigs. Eur Respir J 1999;14:1061–1067. [DOI] [PubMed] [Google Scholar]
- 35.Eichelberger MC, Wang ML, Allan W, Webster RG, Doherty PC. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4 depleted mice. J Gen Virol 1991;72:1695–1698. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol 2002;3:121–126. [DOI] [PubMed] [Google Scholar]
- 37.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol 2004;39:687–699. [DOI] [PubMed] [Google Scholar]
- 38.Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev 2005;126:365–379. [DOI] [PubMed] [Google Scholar]
- 39.Zerofsky M, Harel E, Silverman N, Tatar M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 2005;4:103–108. [DOI] [PubMed] [Google Scholar]
- 40.Moret Y, Siva-Jothy MT. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc Biol Sci 2003;270:2475–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowenberger CA, Kamal S, Chiles J, Paskewitz S, Bulet P, Hoffmann JA, Christensen BM. Mosquito-Plasmodium interactions in response to immune activation of the vector. Exp Parasitol 1999;91:59–69. [DOI] [PubMed] [Google Scholar]
- 42.Kurtz J, Franz K. Innate defence: evidence for memory in invertebrate immunity. Nature 2003;425:37–38. [DOI] [PubMed] [Google Scholar]
- 43.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog 2007;3:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis 2003;187:1000–1009. [DOI] [PubMed] [Google Scholar]
- 45.Hakansson A, Kidd A, Wadell G, Sabharwal H, Svanborg C. Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect Immun 1994;62:2707–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai TR, Knight DA. Structural changes in the airways in asthma: observations and consequences. Clin Sci (Lond) 2005;108:463–477. [DOI] [PubMed] [Google Scholar]
- 47.Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol 2006;176:1733–1740. [DOI] [PubMed] [Google Scholar]
- 48.Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem 2005;280:5571–5580. [DOI] [PubMed] [Google Scholar]
- 49.Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate Toll-like receptor gene expression in viral infections. Genes Immun 2001;2:349–355. [DOI] [PubMed] [Google Scholar]
- 50.Dessing MC, Knapp S, Florquin S, de Vos AF. van Der PT. CD14 facilitates invasive respiratory tract infection by Streptococcus pneumoniae. Am J Respir Crit Care Med 2007;175:604–611. [DOI] [PubMed] [Google Scholar]
- 51.Ratner AJ, Lysenko ES, Paul MN, Weiser JN. Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proc Natl Acad Sci USA 2005;102:3429–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torday JS, Rehan VK. The evolutionary continuum from lung development to homeostasis and repair. Am J Physiol Lung Cell Mol Physiol 2007;292:L608–L611. [DOI] [PubMed] [Google Scholar]
- 53.Lamb D, Reid L. Mitotic rates, goblet cell increase and histochemical changes in mucus in rat bronchial epithelium during exposure to sulphur dioxide. J Pathol Bacteriol 1968;96:97–111. [DOI] [PubMed] [Google Scholar]
- 54.Lamb D, Reid L. Goblet cell increase in rat bronchial epithelium after exposure to cigarette and cigar tobacco smoke. BMJ 1969;1:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hotchkiss JA, Evans WA, Chen BT, Finch GL, Harkema JR. Regional differences in the effects of mainstream cigarette smoke on stored mucosubstances and DNA synthesis in F344 rat nasal respiratory epithelium. Toxicol Appl Pharmacol 1995;131:316–324. [DOI] [PubMed] [Google Scholar]
- 56.Harkema JR, Hotchkiss JA. In vivo effects of endotoxin on intraepithelial mucosubstances in rat pulmonary airways: quantitative histochemistry. Am J Pathol 1992;141:307–317. [PMC free article] [PubMed] [Google Scholar]
- 57.Steiger D, Hotchkiss J, Bajaj L, Harkema J, Basbaum C. Concurrent increases in the storage and release of mucin-like molecules by rat airway epithelial cells in response to bacterial endotoxin. Am J Respir Cell Mol Biol 1995;12:307–314. [DOI] [PubMed] [Google Scholar]
- 58.Tesfaigzi Y, Harris JF, Hotchkiss JA, Harkema JR. DNA synthesis and Bcl-2 expression during development of mucous cell metaplasia in airway epithelium of rats exposed to LPS. Am J Physiol Lung Cell Mol Physiol 2004;286:L268–L274. [DOI] [PubMed] [Google Scholar]
- 59.Harkema JR, Hotchkiss JA, Barr EB, Bennett CB, Gallup M, Lee JK, Basbaum C. Long-lasting effects of chronic ozone exposure on rat nasal epithelium. Am J Respir Cell Mol Biol 1999;20:517–529. [DOI] [PubMed] [Google Scholar]
- 60.Jamil S, Breuer R, Christensen TG. Abnormal mucous cell phenotype induced by neutrophil elastase in hamster bronchi. Exp Lung Res 1997;23:285–295. [DOI] [PubMed] [Google Scholar]
- 61.Nields HM, Snider GL, Breuer R, Christensen T. Reversible pancreatic elastase-induced bronchial secretory cell metaplasia in the rat. Exp Pathol 1991;41:185–193. [DOI] [PubMed] [Google Scholar]
- 62.Lucey EC, Stone PJ, Christensen TG, Breuer R, Snider GL. An 18-month study of the effects on hamster lungs of intratracheally administered human neutrophil elastase. Exp Lung Res 1988;14:671–686. [DOI] [PubMed] [Google Scholar]
- 63.Wright JL, Lawson LM, Pare PD, Wiggs BJ, Kennedy S, Hogg JC. Morphology of peripheral airways in current smokers and ex-smokers. Am Rev Respir Dis 1983;127:474–477. [DOI] [PubMed] [Google Scholar]
- 64.Andley UP, Spector A. Peroxide resistance in human and mouse lens epithelial cell lines is related to long-term changes in cell biology and architecture. Free Radic Biol Med 2005;39:797–810. [DOI] [PubMed] [Google Scholar]
- 65.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res 2004;68:1–12. [DOI] [PubMed] [Google Scholar]
- 66.Thorin E, Shreeve SM. Heterogeneity of vascular endothelial cells in normal and disease states. Pharmacol Ther 1998;78:155–166. [DOI] [PubMed] [Google Scholar]
- 67.Cid MC. Endothelial cell biology, perivascular inflammation, and vasculitis. Cleve Clin J Med 2002;69:SII45–SII49. [DOI] [PubMed] [Google Scholar]
- 68.Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections: role of endothelial dysfunction. Circulation 2002;106:184–190. [DOI] [PubMed] [Google Scholar]
- 69.Spier SA, Delp MD, Stallone JN, Dominguez JM, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and NO. Am J Physiol Heart Circ Physiol 2007;292:H3119–H3127. [DOI] [PubMed] [Google Scholar]
- 70.Xu T, Qiao J, Zhao L, Wang G, He G, Li K, Tian Y, Gao M, Wang J, Wang H, et al. Acute respiratory distress syndrome induced by avian influenza A (H5N1) virus in mice. Am J Respir Crit Care Med 2006;174:1011–1017. [DOI] [PubMed] [Google Scholar]
- 71.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest 2005;115:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suda T, Chida K, Hayakawa H, Imokawa S, Iwata M, Nakamura H, Sato A. Development of bronchus-associated lymphoid tissue in chronic hypersensitivity pneumonitis. Chest 1999;115:357–363. [DOI] [PubMed] [Google Scholar]
- 73.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest 2006;116:3183–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pawelec G, Solana R, Remarque E, Mariani E. Impact of aging on innate immunity. J Leukoc Biol 1998;64:703–712. [DOI] [PubMed] [Google Scholar]
- 75.Krishnaraj R, Svanborg A. Preferential accumulation of mature NK cells during human immunosenescence. J Cell Biochem 1992;50:386–391. [DOI] [PubMed] [Google Scholar]
- 76.Mariani E, Roda P, Mariani AR, Vitale M, Degrassi A, Papa S, Facchini A. Age-associated changes in CD8+ and CD16+ cell reactivity: clonal analysis. Clin Exp Immunol 1990;81:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ogata K, Yokose N, Tamura H, An E, Nakamura K, Dan K, Nomura T. Natural killer cells in the late decades of human life. Clin Immunol Immunopathol 1997;84:269–275. [DOI] [PubMed] [Google Scholar]
- 78.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol 2004;5:337–343. [DOI] [PubMed] [Google Scholar]
- 79.Beyer M, Bartz H, Horner K, Doths S, Koerner-Rettberg C, Schwarze J. Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J Allergy Clin Immunol 2004;113:127–133. [DOI] [PubMed] [Google Scholar]
- 80.Kirby A, Newton D, Carding S, Kaye P. Pulmonary dendritic cells and alveolar macrophages are regulated by gammadelta T cells during the resolution of S. pneumoniae-induced inflammation. J Pathol 2007;212:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 2003;3:984–993. [DOI] [PubMed] [Google Scholar]
- 82.Juffermans NP, Leemans JC, Florquin S, Verbon A, Kolk AH, Speelman P, van Deventer SJ. van Der PT. CpG oligodeoxynucleotides enhance host defense during murine tuberculosis. Infect Immun 2002;70:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 2003;18:265–277. [DOI] [PubMed] [Google Scholar]
- 84.Myrvik QN, Leake ES, Fariss B. Lysozyme content of alveolar and peritoneal macrophages from the rabbit. J Immunol 1961;86:133–136. [PubMed] [Google Scholar]
- 85.Thepen T, Kraal G, Holt PG. The role of alveolar macrophages in regulation of lung inflammation. Ann N Y Acad Sci 1994;725:200–206. [DOI] [PubMed] [Google Scholar]
- 86.Lipscomb MF, Pollard AM, Yates JL. A role for TGF-beta in the suppression by murine bronchoalveolar cells of lung dendritic cell initiated immune responses. Reg Immunol 1993;5:151–157. [PubMed] [Google Scholar]
- 87.Chelen CJ, Fang Y, Freeman GJ, Secrist H, Marshall JD, Hwang PT, Frankel LR, DeKruyff RH, Umetsu DT. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J Clin Invest 1995;95:1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balbo P, Silvestri M, Rossi GA, Crimi E, Burastero SE. Differential role of CD80 and CD86 on alveolar macrophages in the presentation of allergen to T lymphocytes in asthma. Clin Exp Allergy 2001;31:625–636. [DOI] [PubMed] [Google Scholar]
- 89.Bilyk N, Holt PG. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med 1993;177:1773–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol 2004;172:398–409. [DOI] [PubMed] [Google Scholar]
- 91.Kirby AC, Raynes JG, Kaye PM. CD11b regulates recruitment of alveolar macrophages but not pulmonary dendritic cells after pneumococcal challenge. J Infect Dis 2006;193:205–213. [DOI] [PubMed] [Google Scholar]
- 92.Torday JS, Rehan VK. The evolutionary continuum from lung development to homeostasis and repair. Am J Physiol Lung Cell Mol Physiol 2007;292:L608–L611. [DOI] [PubMed] [Google Scholar]
- 93.Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol 2007;178:3593–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 2000;288:2222–2226. [DOI] [PubMed] [Google Scholar]
- 95.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol 2005;26:347–362. [DOI] [PubMed] [Google Scholar]
- 96.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci USA 1999;96:2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Claflin JL, Berry J. Genetics of the phosphocholine-specific antibody response to Streptococcus pneumonia: germ-line but not mutated T15 antibodies are dominantly selected. J Immunol 1988;141:4012–4019. [PubMed] [Google Scholar]
- 98.Herzenberg LA, Baumgarth N, Wilshire JA. B-1 cell origins and VH repertoire determination. Curr Top Microbiol Immunol 2000;252:3–13. [DOI] [PubMed] [Google Scholar]
- 99.Wu LC, Tuot DS, Lyons DS, Garcia KC, Davis MM. Two-step binding mechanism for T-cell receptor recognition of peptide MHC. Nature 2002;418:552–556. [DOI] [PubMed] [Google Scholar]
- 100.Mason D. A very high level of crossreactivity is an essential feature of the T cell receptor. Immunol Today 1998;19:395–404. [DOI] [PubMed] [Google Scholar]
- 101.Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu Rev Immunol 2004;22:711–743. [DOI] [PubMed] [Google Scholar]
- 102.Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science 1998;279:1344–1347. [DOI] [PubMed] [Google Scholar]
- 103.Barnett LA, Fujinami RS. Molecular mimicry: a mechanism for autoimmune injury. FASEB J 1992;6:840–844. [DOI] [PubMed] [Google Scholar]
- 104.Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J Immunol 2006;176:4284–4295. [DOI] [PubMed] [Google Scholar]
- 105.Chapdelaine Y, Smith DK, Pedras-Vasconcelos JA, Krishnan L, Sad S. Increased CD8+ T cell memory to concurrent infection at the expense of increased erosion of pre-existing memory: the paradoxical role of IL-15. J Immunol 2003;171:5454–5460. [DOI] [PubMed] [Google Scholar]
- 106.Sad S, Krishnan L. Maintenance and attrition of T-cell memory. Crit Rev Immunol 2003;23:129–147. [DOI] [PubMed] [Google Scholar]
- 107.Smith DK, Dudani R, Pedras-Vasconcelos JA, Chapdelaine Y, van Faassen H, Sad S. Cross-reactive antigen is required to prevent erosion of established T cell memory and tumor immunity: a heterologous bacterial model of attrition. J Immunol 2002;169:1197–1206. [DOI] [PubMed] [Google Scholar]