Abstract
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are important events in the natural history of this chronic lung disorder. These events can be caused by a large number of infectious and noninfectious agents and are associated with an increased local and systemic inflammatory response. Their frequency and severity have been linked to progressive deterioration in lung function and health status. Infectious pathogens ranging from viral to atypical and typical bacteria have been implicated in the majority of episodes. Most therapeutic regimens to date have emphasized broad, nonspecific approaches to bronchoconstriction and pulmonary inflammation. Increasingly, therapy that targets specific etiologic pathogens has been advocated. These include clinical and laboratory-based methods to identify bacterial infections. Further additional investigation has suggested specific pathogens within this broad class. As specific antiviral therapies become available, better diagnostic approaches to identify specific pathogens will be required. Furthermore, prophylactic therapy for at-risk individuals during high-risk times may become a standard therapeutic approach. As such, the future will likely include aggressive diagnostic algorithms based on the combination of clinical syndromes and rapid laboratory modalities to identify specific causative bacteria or viruses.
Keywords: chronic obstructive pulmonary disease, acute exacerbations, virus, bacteria, therapy
It has been estimated that approximately 6% of the United States adult population has chronic obstructive pulmonary disease (COPD) (1), whereas the prevalence of chronic bronchitis has been estimated at 3.2% (2). Acute exacerbations of disease (AE-COPDs) in these patients account for about 13 million office visits each year (3, 4). Although the topic remains controversial (5), a generally accepted definition of an AE-COPD is “a sustained worsening of the patient's condition, from the stable state and beyond normal day-to-day variations, that is acute in onset and necessitates a change in regular medication in a patient with underlying COPD” (6). Exclusion of alternate diagnoses, including congestive heart failure, pneumothorax, and pulmonary emboli, among others, has been considered important (3, 7, 8).
The frequency and severity of exacerbations are quite variable among patients with COPD (9). This variability, in part, reflects the nature of data collection (prospective vs. retrospective), disease severity, medications administered, vaccinations, and smoking status of the population studied (9). Reports that define AE-COPD by use of daily diary cards tend to identify more episodes per year (10, 11). Similarly, studies that include patients with more severely impaired pulmonary function find a greater number of yearly episodes (12).
Numerous groups have documented the negative effects of AE-COPDs, particularly on health-related quality of life (HRQL) (13, 14). Cross-sectional studies have reported reduced HRQL during an AE-COPD, whereas longitudinal studies have documented that HRQL improves from exacerbation to recovery (13). The greatest improvement in HRQL after a single episode occurs during the first 4 weeks, although HRQL continues to improve over 26 weeks; a recurrence of an AE-COPD results in markedly attenuated improvement (15). A 2-year prospective, longitudinal study of 336 patients with severe COPD confirmed that more frequent exacerbations had a deleterious effect on health status (16).
AE-COPD episodes result in measurable acute deteriorations on pulmonary function (10, 17, 18). Longitudinal effects of repeated AE-COPDs have also been reported. Among continuing smokers, lower respiratory tract infections (LRTIs) have been associated with additional decline in lung function, averaging an additional decline of 7 ml/year in FEV1 for every one LRTI per year (19). Two separate groups reported decreased lung function in patients with frequent exacerbations compared with those with infrequent exacerbations (20, 21). These data indicate that there are measurable negative short- and long-term impacts on pulmonary function in AE-COPDs.
AE-COPDs are also major source of health care expenditure (22); they were estimated to result in a total treatment cost of $1.2 billion in patients 65 years and older and $419 million in those younger than 65 years in the United States in 1995 (4). A prospective Spanish study of 2,414 patients with COPD identified after an acute exacerbation noted that 507 patients (21%) relapsed after therapy (23). Of these, 161 patients required emergency department treatment with 84 requiring hospitalization; these latter patients accounted for 58% of the total cost. Thus, AE-COPD episodes, particularly those that require hospitalization, result in major health care expenditures. Given the totality of these data, it has become increasingly evident that appropriate management of AE-COPDs is of paramount importance.
PATHOGENESIS
An augmented inflammatory response has been shown to be a central event in an AE-COPD (24). Multiple stimuli can acutely increase airway and parenchymal inflammation, leading to increased bronchial tone, bronchial wall edema, and mucous hypersecretion (25). The methodologic approaches to examining the inflammatory response have included sputum analyses, bronchoalveolar lavage, bronchial biopsy, and blood markers. The most robust evidence of an augmented inflammatory response comes from analysis of sputum. Neutrophilic and eosinophilic inflammation has been described. Similarly, a multitude of inflammatory mediators have been implicated. In an elegant prospective study, Fujimoto and colleagues followed 68 patients with emphysematous COPD for 2 to 3 years; 30 patients developed an acute exacerbation and expectorated sputum adequate for analysis (26). During an acute exacerbation total sputum cells, lymphocytes, neutrophils, eosinophils, IL-8, neutrophil elastase, eosinophilic cationic protein, and CCL5/RANTES increased compared with the stable state. This inflammatory response is associated with an increased oxidative stress (27, 28).
Bronchoscopic data are more limited, with some investigators confirming increased expression of CCL5/RANTES in both the surface epithelium and subepithelial mononuclear cells during an acute exacerbation of chronic bronchitis (29). Subsequent studies confirmed increased numbers of neutrophils in AE-COPDs, as well as increases in CXCL5 (ENA-87), CXCL8 (IL-8), and CXCR2 (28, 30). These data confirm an inflammatory process involving both neutrophils and eosinophils with an additional imbalance in oxidant status during exacerbations.
A systemic inflammatory response during an AE-COPD has been noted with increases in plasma fibrinogen, IL-6, C-reactive protein (CRP), and endothelin-1, all reported to increase during exacerbations (31–34). Interestingly, one group has confirmed that the degree of systemic inflammation, shown by rising serum IL-6 and CRP levels, correlated with sputum and nasal inflammation (35). In a separate report, only CRP seemed to exhibit reasonable diagnostic accuracy for an AE-COPD, particularly when combined with increased dyspnea, sputum volume, or purulence (34). These data support that the majority of exacerbations are associated with an intensified inflammatory response. The relationship of specific etiologies and the nature of the inflammatory response is a source of intense investigation.
ETIOLOGY
AE-COPDs appear to be triggered by a variety of infectious and noninfectious stimuli. Numerous environmental pollutants can provoke an inflammatory response in patients with COPD (36–38). Importantly, epidemiologic studies have confirmed increased respiratory symptoms and mortality during times of increased air pollution (39–41). Nevertheless, most AE-COPDs are believed to be related to infection, either viral or bacterial (25).
Viruses
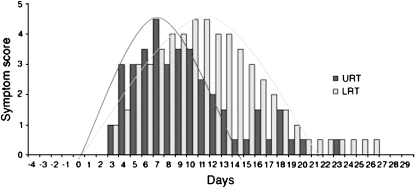
Clinical data confirming an important role for viral infection are quite robust (42, 43). Recent studies using advanced methodology have implicated numerous viral pathogens (44). Greenberg documented that 27% of exacerbations in 62 prospectively followed patients with COPD were related to a viral etiology (45); picornaviruses, parainfluenza virus, and coronaviruses accounted for the majority of infections in this study. Other investigators have suggested that up to 60% of exacerbations have been associated with viral infections, including picornaviruses, respiratory syncytial virus (RSV), influenza, and human metapneumoviruses (46–51). One prospective study of hospitalized patients with AE-COPDs found a viral pathogen in more than 48% of episodes (vs. 6.25% in the stable state), with a similar distribution of viral pathogens as previously noted (52). The most compelling data confirming a role for viruses in the genesis of an AE-COPD come from the experimental model of human rhinovirus (HRV) infection recently published by Mallia and colleagues (53). These investigators administered low doses of rhinovirus 16 (RV16) to four subjects with moderate COPD; all developed symptomatic colds with the lowest dose of virus challenge. Figure 1 illustrates that upper tract symptoms preceded lower tract symptoms by several days. In addition, there were reductions in peak flow and FEV1 in relation with lower tract symptoms. These data clearly confirm that rhinovirus can precipitate typical symptoms of an AE-COPD; the lag between upper and lower tract symptoms suggests an opportunity for intervention.
Figure 1.
Upper (URT) and lower respiratory tract (LRT) symptoms of patients with chronic obstructive pulmonary disease in vivo experimentally infected with rhinovirus. A 3- to 4-day gap between the peak of cold symptoms and the peak of lower respiratory symptoms was documented. Reproduced by permission from Reference 54.
Importantly, viral infections have been suggested to augment the inflammatory response in COPD (54). Rhinovirus infection of the bronchial epithelium induces expression of numerous proinflammatory genes (55–58). Induction of nuclear factor (NF)-κB and other transcription factors has been clearly demonstrated with several viruses including HRV, RSV, and influenza (59–64). Rhinovirus and RSV infection of bronchial epithelial cell lines results in the production of eotaxin, eotaxin-2, and RANTES (65, 66). Clinically, the role of RSV infection in COPD has become better defined, with several groups demonstrating that the inflammatory process is augmented in the presence of such infection (67, 68). One of these groups has suggested that persistence of infection may be particularly important in progression of the underlying obstructive process (67), although this remains controversial (69). It is evident that viral infection could account for the inflammatory response previously described as typical of an AE-COPD. In fact, a longitudinal study suggested that virus-associated exacerbations were associated with higher systemic inflammatory marker levels (46).
Bacteria
The role of bacterial infection in individual AE-COPD episodes has been a subject of much controversy (44). Recent comprehensive reviews of this topic suggest that much of this controversy may reflect evolving diagnostic methods (25, 70). These methods have included sputum culture, bronchoscopic sampling, molecular epidemiologic studies of bacterial pathogens, identification of an immune response, and recording a response to antimicrobial therapy. Sputum cultures have been the classic approach to identifying potentially pathogenic bacteria in AE-COPD; the organisms most frequently isolated are nontypeable Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae (44). However, the relationship between identification of these potentially pathogenic organisms and an etiologic diagnosis in AE-COPD has been questioned (71, 72). Unfortunately, sputum cultures have important limitations—for example, seriously underestimating colonization with nontypeable H. influenzae in comparison to polymerase chain reaction (PCR)–based detection (73).
Bronchoscopically collected samples have confirmed that potentially pathogenic microorganisms are identified in many patients with COPD at baseline and during AE-COPDs (74–78). A recent review pooled data from six published studies suggesting that there was a clear shift to organisms with a higher pathogenic potential (79). A bronchoscopic study has confirmed that patients with COPD colonized with potentially pathogenic bacteria exhibit increased neutrophil counts, IL-8, matrix metalloproteinase-9, and endotoxin (80).
Recent longitudinal cohort studies, using analyses of surface antigen diversity, have demonstrated that acquisition of a bacterial strain with which the patient had not been previously infected was associated with a greater than twofold increase in exacerbation risk (81, 82). Interestingly, new H. influenzae strains associated with symptomatic exacerbations resulted in increased neutrophil recruitment in a mouse model of airway bacterial infection as well as greater adherence to epithelial cells and induction of IL-8 release than strains not associated with such a clinical response (82). Similar data have been published regarding M. catarrhalis (83). Further support for the importance of bacterial infection in the etiology of AE-COPD comes from recent studies that confirmed a systemic immune response to homologous strains of H. influenzae and M. catarrhalis isolated simultaneously from sputum of patients during evaluation at time of stability and with symptomatic exacerbations (25, 84–86). Taken together, these data strongly support a pathogenic role for bacterial pathogens in many AE-COPD episodes (70).
Atypical infectious agents, including Chlamydia pneumoniae, Mycoplasma pneumoniae, and Legionella species, have also been reported to cause AE-COPD. Based on IgG and IgM antibody titers, C. pneumoniae has been reported as an etiologic factor in 4 to 34% of AE-COPDs (87–91). Disparities in reported rates likely relate to differences in serologic methods used to identify C. pneumoniae infection and in the prevalence of infection reported in patients with stable COPD disease (92). By contrast, M. pneumoniae has been identified in only a minority (<1–14%) of AE-COPDs (71, 89, 93, 94).
A biological interaction between respiratory viruses and bacterial pathogens has been well described. Infection with RSV, human parainfluenza virus, or influenza virus increases bacterial adhesion to epithelial cell lines (95). A similar process has been noted with rhinovirus infection (96). The clinical impact of such interaction has been prospectively examined in 39 patients with COPD during 56 exacerbations; those with both HRV and H. influenzae infection exhibited higher bacterial load and serum IL-6 (97). Bandi and colleagues extended these findings by identifying AE-COPDs caused by H. influenza and noting that an acute viral infection was seen in 45.7% of the events (98). The recent prospective study of Papi and colleagues provides important insight (52). Independent of bacterial versus viral infection, sputum neutrophils were increased in AE-COPD compared with baseline, whereas eosinophilic inflammation was seen in patients with viral or mixed viral/bacterial infection. It is evident that the etiology of AE-COPDs likely influences the type and magnitude of the inflammatory response that results.
THERAPEUTIC APPROACHES
General
Optimal therapy for an AE-COPD is multidisciplinary. Inhaled β-agonists and anticholinergic agents have been documented to decrease obstruction during an AE-COPD. Importantly, systematic reviews have suggested that both short-acting β-agonists and anticholinergic inhaled bronchodilators have comparable effects on spirometry and a greater effect than parenterally administered bronchodilators (99). Although the combination of an anticholinergic and a β-agonist has the potential for increased therapeutic benefit, studies combining agents from these classes have yielded varying results; on average, these results do not support the routine use of multiple agents for AE-COPDs (99).
The use of systemic corticosteroids for AE-COPD has been studied by numerous investigators (100). A systematic review suggested that systemic steroids result in physiologic improvement over the first 72 hours and reduced the odds of a treatment failure over the subsequent 30 days, although the risk of adverse drug reaction was increased (101). The largest study evaluated 271 patients from 25 Veterans Affairs medical centers (102). Patients were randomized to placebo or one of two steroid treatment arms (Solumedrol 125 mg/d for 3 d followed by either a 15-d or 8-wk taper). Both corticosteroid groups were associated with a faster improvement in FEV1, a lower number of treatment failures, and a shorter length of hospital stay. Patients in the corticosteroid groups were also more likely to experience complications of treatment; hyperglycemia was the most common.
Subsequently, a separate investigative group randomized patients hospitalized with an AE-COPD to methylprednisolone, 0.5 mg/kg every 6 hours for 3 days followed by either no further steroids or a taper completed on Day 10 (103). Patients treated with a longer course of corticosteroids experienced a greater improvement in FEV1. A separate study randomized 56 patients admitted with an AE-COPD to a smaller dose of prednisone (30 mg daily for 14 d) versus placebo (104). Patients treated with prednisone had a faster and greater improvement in FEV1. The median length of stay was also shorter in the steroid-treated group. In a study of 27 outpatients with an AE-COPD, Thompson and colleagues randomized patients to treatment with 9 days of prednisone (60 mg for 3 d, 40 mg for 3 d, and 20 mg for 3 d) or placebo (105). Patients treated with prednisone exhibited a faster and greater improvement in oxygenation and FEV1, while experiencing fewer treatment failures (0 vs. 57%, P = 0.002). An emergency room–based study randomized 147 of 202 eligible patients to prednisone (40 mg daily for 10 d) versus placebo; all patients received an oral antimicrobial agent and inhaled bronchodilators (106). Patients treated with prednisone experienced a reduced rate of relapse at 30 days, as well as a prolonged time to relapse, improved dyspnea, and improved pulmonary function. Recent comprehensive reviews of these data suggest that systemic steroids be used in all AE-COPD patients requiring hospitalization and in those outpatients with “appreciably worse” dyspnea (100, 107).
Pathogen-specific Therapy
Given the role of infectious pathogens in the genesis of many AE-COPD episodes, pathogen-specific therapy would be ideal. Novel approaches are under active investigation to optimize such a focused approach to therapy.
Antibacterial.
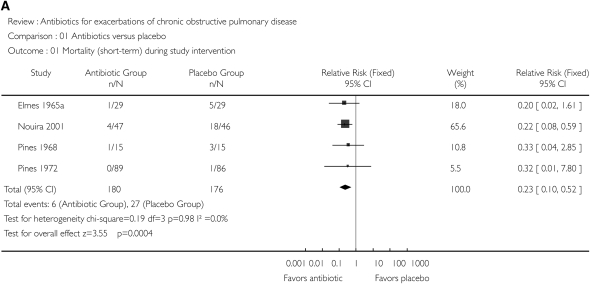
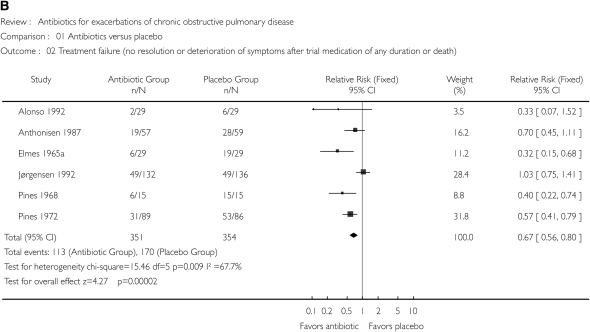
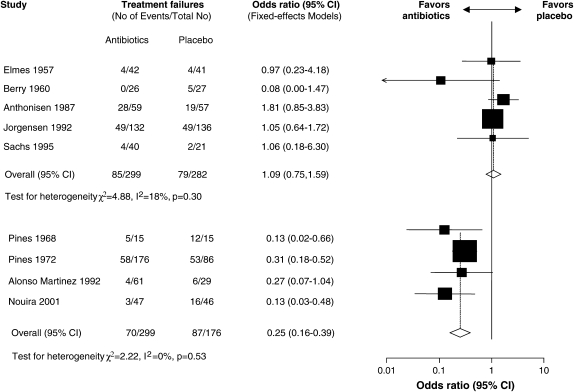
Because bacterial infection may be causative in approximately 50% of AE-COPDs, it is not surprising that antimicrobial therapy has been intensively studied in this disease. Numerous placebo-controlled trials of antibiotics in AE-COPDs have been published, with systematic reviews of these trials suggesting a beneficial treatment effect (108–110). Figure 2 illustrates the effect of antimicrobial therapy on short-term mortality (Figure 2A) and treatment failure (Figure 2B) (109). A significant benefit to antimicrobial therapy was suggested. The beneficial effect on reducing treatment failure appears to be better in studies including more severe AE-COPDs (Figure 3) (110). Unfortunately, there has been significant heterogeneity in the results of the individual studies, with none designed in an optimal fashion (44). Ongoing studies will provide additional data to refine recommendations for antimicrobial therapy, although most international guidelines have interpreted these data to suggest that antimicrobial agents provide additional benefit in selected patients (111).
Figure 2.
(A) Effect of antibiotics versus placebo on short-term mortality. (B) Effect of antibiotics versus placebo on treatment failure, defined as no resolution or deterioration of symptoms after trial medication of any duration or death. CI, confidence interval. Reprinted by permission from Reference 109.
Figure 3.
Forest plot showing nine studies grouped according to severity of exacerbation. The upper five studies included patients with mild to moderate exacerbations and the four studies below included patients with severe exacerbations. The x axis represents the odds ratio for treatment failure. Reprinted by permission from Reference 110.
The role of specific agents remains unclear, with limited data available to allow robust conclusions. Most recent antibiotic trials in AE-COPD have been comparative studies performed to demonstrate equivalence of newer antibiotics with established ones. These studies have generally been designed as noninferiority trials for registration purposes. As such, these studies provide limited information regarding clinical response for various antimicrobial classes. In addition, these studies have involved a wide variety of study designs with response assessed well after therapy was completed, which limits the ability to interpret differences in efficacy between antimicrobial classes. A recent systematic review of 19 of these trials suggests equivalent clinical outcomes in randomized studies comparing quinolones, macrolides, and amoxicillin–clavulonate tested against each other (112). Interestingly, in microbiologically evaluable patients, macrolides performed worse than quinolones (112).
Recently, studies have been designed that test novel endpoints. Some investigators have suggested that a more rapid resolution of symptoms may be seen with quinolones in contrast to comparators (113, 114). Antimicrobial therapy does not necessarily result in complete eradication of the pathogen, a process that has been associated with a persistent inflammatory response (115). As such, antimicrobials that decrease the bacterial load in the lower airways should result in greater symptomatic resolution and improve the time between exacerbations, the disease free interval (DFI) (116, 117). More recent AE-COPD clinical trials have assessed how different antibiotics affect the DFI (117). Although the data are somewhat conflicting, quinolones seem to be associated with a longer DFI (112, 117). Additional data are required to better establish this novel endpoint as a primary basis for future comparative studies.
Antiviral.
Given the important role of viruses, particularly rhinovirus and influenza, in AE-COPDs, it is important to review the potential specific antiviral therapies that are available. Recently, a large number of approaches have been taken, particularly to rhinovirus, which include targeting viral cell susceptibility, viral attachment and receptor blockade, viral uncoating, viral RNA replication, and viral protein synthesis (Table 1) (118, 119). Interferons have protean effects that are mediated through cell receptor signal transduction pathways. Intranasal interferon alpha 2b has been demonstrated to be effective in both experimental and natural colds when provided prophylactically; it has little effect on the development of infection or symptoms when administered after infection (119). In addition, the presence of numerous local side effects, including nasal mucosal bleeding, has limited its clinical utility. Because 90% of HRV serotypes use the intercellular ahesion molecule 1 (ICAM-1) receptor for attachment to cells, preventing binding to this receptor has been explored therapeutically (119). Tremacamra, a soluble ICAM-1, was studied in a series of double-blinded studies where the agent was administered as a nasal spray solution or inhaled powder (120). It reduced the severity of experimental colds when administered less than 12 hours after HRV challenge. Dosing six times daily and its poor tolerability limited clinical development.
TABLE 1.
THERAPEUTIC APPROACHES TO RHINOVIRUS INFECTION
| Targets | Compounds |
|---|---|
| Cell susceptibility | Interferons |
| Viral attachment/binding | Monoclonal antibodies to cellular ICAM-1 |
| Tremacamra (soluble ICAM-1) | |
| RNA inhibition/viral replication | Enviroxime |
| Viral protein synthesis inhibitors | 3C protease inhibitors |
| AG7088 | |
| Viral uncoating/capsid function | Capsid-function inhibitors (pleconaril, R61837, pirodavir, SCH48973, SDZ35-682, chalcone compounds) |
The most promising of the agents developed to date have targeted the HRV capsid. Numerous agents from diverse molecular classes exhibit antiviral activity by preventing viral attachment and/or uncoating. The agent that came furthest in clinical development was pleconaril. In two large phase II, placebo-controlled, natural cold trials, pleconaril was demonstrated to reduce the time to alleviation of illness in patients with an onset of a typical cold within 36 hours of respiratory symptoms during the months between July and December; this clinical response was noted in subjects who were HRV positive (121). Furthermore, subsequent analyses confirmed that clinical response to pleconaril was limited to those infected with pleconaril-sensitive HRV strains (122). Induction of reduced pleconaril susceptibility in HRV isolates was seen in greater than 10% of patients, although the clinical course of illness in these subjects was less protracted than in the overall group. A subsequent 6-week prophylaxis study documented that pleconaril induced CYP3A4, which led to intermenstrual bleeding and related menstrual abnormalities in women taking estrogen-based oral contraceptives (121, 123). In March 2002, a U.S. Food and Drug Administration Antiviral Drugs Advisory Committee voted to not recommend pleconaril approval based on these drug interactions and possible transmission of resistance isolates. Given the potential advantage of pleconaril treatment in patients with underlying lung disease, a nasal preparation is currently under development. The ability of this agent to decrease disease duration in conjunction with the data suggesting a delayed onset of lower respiratory tract symptoms following upper tract symptoms after experimental HRV infection suggests that pleconaril may be promising in patients with COPD as a prophylactic agent.
The HRV 3C protease is an enzyme responsible for post-translational cleavage of viral precursor polyproteins (124). Several different compounds have been developed targeting this enzyme. Ruprintivir is an agent that has been developed as a nasal spray. In experimentally induced HRV colds in healthy volunteers, it was able to moderate disease severity and reduce viral load (125). Unfortunately, a subsequent natural infection study was not able to reproduce these findings (126).
Influenza has major repercussions in patients with underlying lung disease (127). A number of agents with activity against this pathogen are licensed for use. Inhibitors of M2, a membrane protein in influenza A, are available for clinical use (amantidine and rimantidine). They reduce duration of fever and symptoms in patients by approximately 24 hours. Their high incidence of side effects in elderly patients and the rapid development of resistance during treatment have limited their use (127). Neuraminidase inhibitors, zanamivir and oseltamivir, are also available for clinical use. Clinical trials have confirmed that these agents reduce symptoms and speed disease resolution (128). Data in patients with chronic lung disease have confirmed these findings. Murphy and colleagues reported a randomized, placebo-controlled study of zanamivir in 525 patients 12 years or older with asthma or COPD (∼21–24% with asthma/COPD or COPD alone) (129). Zanamivir reduced the median time to alleviation of symptoms (5.5 vs. 7.0 d; difference, 1.5 d; 95% confidence interval, 0.5–3.25; P = 0.009), overall symptom scores, nights of sleep disturbance, and total incidence of complications. No adverse effect on pulmonary function was noted. A retrospective analysis of high-risk patients (COPD, asthma, cardiovascular disease, age ⩾ 65) in several zanamivir, placebo-controlled trials confirmed a 2.5-day reduction in median time to alleviation of clinical symptoms and a 3-day reduction in time to return to normal activities (130). A similar analysis in 10 placebo-controlled studies of oseltamivir has been reported (131). In at-risk patients (age ⩾ 65, COPD/asthma, or cardiovascular disease), oseltamivir reduced antibiotic use by 34% and hospitalizations by 50% in influenza-infected patients. These data confirm that neuraminidase inhibitors can be beneficial in patients with chronic respiratory disease who are infected with influenza and present within 36 hours of the onset of symptoms.
The therapy for RSV is largely supportive, although aerosolized ribavirin has been licensed for use in infants (69). This agent is a guanosine analog with broad antiviral properties (69). Experience with this agent in adults is limited, although it has been administered safely to elderly patients (132). Several other anti-RSV agents interfere with RNA, and small-molecule fusion inhibitors are under active development (69). As better therapeutic options become available, it will likely become feasible to give high-risk individuals prophylactic agents during at-risk times to prevent AE-COPDs.
Pathogen-directed approaches.
Given the various etiologies for AE-COPDs, clinical guidelines have struggled with the optimal approach to treating specific etiologies, whether bacterial or viral. The majority of recent international guidelines have provided recommendations on which patients with an AE-COPD are more likely to have bacterial infection and are, therefore, more likely to benefit from an antimicrobial agent. Most have heavily relied on the sentinel study of Anthonisen and colleagues (133). These investigators randomized 173 patients during 362 episodes of AE-COPDs to an antibiotic (trimethoprim–sulfamethoxazole, amoxicillin, or doxycycline), stratifying the results based on the number of symptoms at presentation (133). Patients with at least two cardinal symptoms (increase in dyspnea, increase in sputum production, and/or change in sputum color) experienced a benefit with antibiotic therapy. Importantly, recent data using bronchoscopic sampling in hospitalized patients stratified by the Anthonisen criteria confirmed a greater likelihood of bacterial infection in patients with multisymptom AE-COPDs (134). Thus, antibiotic therapy is more likely to be advantageous in patients with AE-COPDs suffering from multiple symptoms.
An exacerbation associated with sputum purulence has been suggested to be more likely to benefit from antibiotic treatment. Stockley and colleagues noted that 32 of 34 patients with an exacerbation and mucoid sputum, as defined by a semiquantitative scoring system, resolved their exacerbation without antibiotic therapy; patients with purulent sputum were more likely to have polymorphonuclear cells and organisms in sputum, with 77 of 87 patients resolving their AE-COPDs with antibiotic therapy (135). A multicenter Italian study expanded these findings by noting that increasing sputum purulence, as defined by a semiquantitative colorimetric scale, was associated with bacterial growth (136). In addition, deepening sputum color was associated with increased yield of gram-negative bacteria, including P. aeruginosa/Enterobacteriaceae. Importantly, the definition of color by subjects and investigators was concordant in only 68% of cases without the aid of an objective color stick. These data suggest that new purulent sputum may identify a patient more likely to benefit from antibiotic therapy, although additional data defining how this concept can be incorporated in daily decision making are required. Despite these uncertainties, numerous major multispecialty societies have incorporated these concepts into the identification of patients who should be treated with antimicrobial therapy (Table 2).
TABLE 2.
RECOMMENDATIONS REGARDING TIMING OF ANTIMICROBIAL THERAPY IN RECENT SPECIALTY-SOCIETY RECOMMENDATIONS
| Guideline Source, Year (Reference) | Recommendation* |
|---|---|
| Canadian Thoracic Society, 2003 (143) | “The Panel proposes that antibiotics should only be considered for use in patients with purulent exacerbations.” |
| American Thoracic Society/European Respiratory Society, 2004 (169) | “[Antibiotics] may be initiated in patients with altered sputum characteristics.” |
| National Institute for Clinical Excellence, 2004 (107) | “Antibiotics should be used to treat exacerbations of COPD associated with a history of more purulent sputum.” |
| European Respiratory Society, 2005 (170) | “[Hospitalized patients with COPD exacerbations should receive antibiotics if] |
| I. Patients with all three of the following symptoms: increased dyspnea, sputum volume and sputum purulence (a type I Anthonisen exacerbation). | |
| II. Patients with only two of the above three symptoms (a type II Anthonisen exacerbation) when increased purulence of sputum is one of the two cardinal symptoms. | |
| III. Patients with a severe exacerbation that requires invasive or non-invasive mechanical ventilation. | |
| IV. Antibiotics are generally not recommended in Anthonisen type II without purulence and type III patients (one or less of the above symptoms).” | |
| GOLD, 2006 (144) | “Antibiotics are only effective when patients with worsening dyspnea and cough also have increased sputum volume and purulence.” |
Definition of abbreviation: GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Italics added for emphasis.
Biomarkers have been highly sought after to complement or replace symptom-based methods to identify patients in whom bacteria are pathogenic during an AE-COPD. As such, an evolving role for procalcitonin level to define AE-COPD patients with a higher likelihood of bacterial infection has been suggested (137). This small protein (116 amino acid, 13 kD) is normally undetectable in plasma (138), but increases in bacterial infections. Data from single-center, cluster-randomized, single-blinded studies suggest that procalcitonin-guided therapy can be used safely to reduce antibiotic use in patients with LRTI at low likelihood of bacterial infection (137, 139). Stolz and coworkers randomized 208 consecutive patients admitted to the hospital with an AE-COPD to usual care (management based on standard criteria without access to procalcitonin levels) or to a procalcitonin-guided group where antibiotic use was based on procalcitonin level at time of admission (140). In the procalcitonin-guided therapy group, a level of less than 0.1 μg/L was considered to be nonbacterial, and antimicrobial use was discouraged. In those with a procalcitonin level greater than 0.25 μg/L, bacterial infection was believed to be present and antimicrobial therapy was encouraged. For patients with levels between 0.1 and 0.25 μg/L, the use of antimicrobial agents was based on the clinical situation at admission and during early follow-up. Total antimicrobial use was decreased during the hospitalization (72% in usual-care group compared with 40% in procalcitonin-guided therapy), at short-term follow-up and through 6 months. No difference was noted in clinical success rate during the index hospitalization, antimicrobial use during the subsequent 6 months, or in time to the next exacerbation. Additional investigation is required to assess if patients with low procalcitonin levels require antimicrobial therapy or if similar results can be achieved in a properly designed multicenter trial (141).
The choice of antimicrobial agent remains nebulous. Increasingly, guidelines have suggested stratifying patients according to the risk of treatment failure (142–144). These stratification schemes have generally suggested features for a high likelihood of infection with organisms that are not covered with standard antibiotic regimens (e.g., P. aeruginosa, drug-resistant bacteria) or host factors that predict treatment failure. The latter include worse lung function, increased frequency of exacerbation/office visits, ischemic heart disease, and other comorbid conditions (145, 146). Prospective data supporting such an approach are quite limited, however. A recent study prospectively confirmed that patients with a complicated AE-COPD experienced inferior clinical response rate compared with those with an uncomplicated AE-COPD (114). A tailored approach for the initial antimicrobial regimen selection in individuals at increased risk for treatment failure requires additional prospective validation.
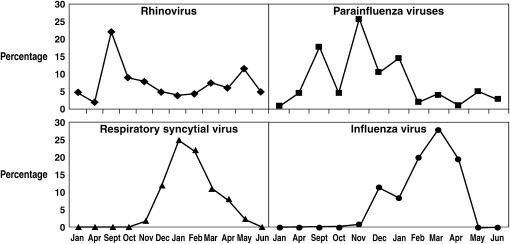
As a clearer picture develops of which patients are infected with viral pathogens, effective antiviral agents can be considered. The benefit of such a targeted approach will depend on the ability to rapidly identify the causative viral pathogen. The science behind this endeavor has been evolving rapidly, with approaches to diagnosis that range from an appreciation of seasonal distribution of specific viridae, clinical syndromes, and rapid diagnostic laboratory testing. It is well known that many common respiratory viruses associated with respiratory illnesses are most evident during specific times of the year (Figure 4) (147, 148). The combination of these seasonal patterns with clinical syndromes has been used in clinical trials and advocated for clinical use (148). Influenza serves as a prototypical agent for this approach. A retrospective, pooled analysis of phase II and III clinical trials that enrolled mainly adults (mean age, 35 yr) with an “influenza-like illness” (defined as fever or feverishness and at least 2 of the following symptoms: headache, myalgia, cough, or sore throat) has been published (149). Of the 3,744 subjects enrolled, 2,470 (66%) were confirmed to have influenza infection. Infected subjects were more likely to have fever, cough, and nasal congestion but less likely to have sore throat. In multivariate modeling, the best positive predictive value (PPV) was seen with the combination of fever and cough: the higher the temperature, the higher the PPV. A smaller prospective study yielded similar results (150). Unfortunately, the PPV of cough and fever seems to be lower in elderly hospitalized patients (151), a problem that could be particularly problematic in patients with COPD.
Figure 4.
Proportion for each of four viral pathogens isolated during each calendar month over a 6-month period of study in Tecumseh, Michigan. Reprinted by permission from Reference 147.
HRV infection also presents with a well-described symptom complex. Unfortunately, significant overlap with other infectious agents is present. Two large studies recruited adults (age > 18 yr) with self-diagnosed colds including moderate–severe rhinorrhea and more than one other respiratory symptom (nasal congestion, cough, sore throat) that was moderate or greater in severity (121). Importantly, subjects were excluded if they had a fever (oral temperature > 37.8°C). Of the 2,096 enrolled subjects, 1,363 (65%) were reverse transcriptase–PCR (RT-PCR) positive for a picornavirus infection. Clearly, a clinical definition is not foolproof in diagnosing rhinovirus infection. In a well-defined cohort of patients with COPD followed prospectively, 10 of 43 exacerbations were associated with rhinovirus isolation in either induced sputum or nasopharyngeal cultures (152). Increased nasal discharge/nasal congestion (“colds”) was present in 26 (61%), sore throat in 13 (30%), and increased sputum volume in 21 (49%) of all exacerbations. The simultaneous presence of cold and increased sputum volume was the symptom combination most strongly associated with the isolation of rhinovirus (odds ratio, 6.15; P = 0.036); importantly, however, two of the rhinovirus exacerbations (20%) were not associated with colds.
Clinical features of RSV infection overlap significantly with those of other winter respiratory viruses (69). One group contrasted clinical syndromes for elderly or otherwise high-risk adults admitted to hospital with respiratory illness during four consecutive winters (153). A total of 118 RSV and 133 influenza-infected patients were available; they were generally elderly (mean age, 76), female (∼60%), with 49 to 54% having underlying heart disease and 55 to 58% chronic lung disease. Subtle differences were noted in clinical characteristics (Table 3). Unfortunately, even the combination of these features did not markedly improve operating characteristics. Additional diagnostic modalities, as described below, will be required to improve RSV detection.
TABLE 3.
SENSITIVITY AND SPECIFICITY OF INDIVIDUAL AND COMBINED CLINICAL CHARACTERISTICS BETWEEN RESPIRATORY SYNCYTIAL VIRUS– AND INFLUENZA-INFECTED ELDERLY OR HIGH-RISK PATIENTS ADMITTED WITH RESPIRATORY ILLNESS DURING FOUR CONSECUTIVE WINTERS
| Clinical Characteristic | Sensitivity % | Specificity % |
|---|---|---|
| Nasal congestion | 63 | 55 |
| Sputum production | 79 | 32 |
| Wheezing by history | 68 | 43 |
| Feverishness | 46 | 56 |
| Rhinorrhea on examination | 11 | 93 |
| Wheezing on examination | 75 | 38 |
| Temperature > 37.9° C | 60 | 30 |
| Combined nasal congestion, wheezing on examination and temperature > 37.9° C | 13 | 91 |
Adapted from Reference 153.
Although not strictly a pathogen-directed therapeutic approach, vaccination has assumed an important role in the patient with COPD. The most promising data to date are those surrounding influenza vaccination. Large population-based studies have suggested that influenza vaccination improves COPD mortality (154). Six placebo-controlled trials in patients with COPD have been reviewed; a reduction in the total number of exacerbations was described, particularly in AE-COPDs occurring after 3 or 4 weeks (155). Although data are limited, the clinical effect does not appear to vary by spirometric disease severity (156), nor does the immune response vary by the concomitant use of corticosteroids (157). As such, influenza vaccination has been strongly recommended in the latest international guideline (144). Data regarding the effect of pneumococcal vaccination are more limited. Retrospective studies have suggested decreased risk of pneumonia hospitalization and death in elderly patients with chronic lung disease, an effect that was additive to that of influenza vaccination (158) or in veterans with asthma or COPD (159). Very little information is available from randomized, controlled trials. A recent Cochrane review identified a paucity of such studies, with no convincing evidence of clinical benefit (160). The largest available study suggested that benefit may be most likely in those younger than 65 years or with in those with more severe spirometric disease (FEV1 < 40% predicted) (161), a recommendation that has been incorporated with more modest enthusiasm in the latest international guideline (144). Oral bacterial vaccines for the prevention of AE-COPDs are under intensive study (162).
Novel Approaches
Given the limitations of seasonal variation and syndrome definitions, specific diagnostic strategies continue to evolve, including the use of inflammatory markers, viral cultures, antigen identification, and molecular assays (including RT-PCR) (69, 118). The recent identification of differing inflammatory patterns in the sputum samples of hospitalized patients with an exacerbation suggests a possible additional approach to identify viral versus bacterial etiology; sputum eosinophil values greater than 1.68 × 106/g exhibited a sensitivity of 0.82 and specificity of 0.77 for a viral etiology (52). An extension of this approach has been proposed by the incorporation of sputum inflammatory markers (tumor necrosis factor-α and IL-8) in segregating exacerbations caused by “common bacteria” compared with Pseudomonas or nonbacterial etiologies (163).
For the identification of specific viral pathogens, viral cultures remain the “gold standard” but exhibit limited sensitivity and take days to return, limiting the practical value in direct clinical care. Improved diagnostic approaches have been best developed for influenza infection. Numerous laboratory approaches have been advocated with rapid antigen diagnostic studies available on the market (164); unfortunately, their sensitivity varies. Despite this limitation, one group has suggested that rapid antigen test results can aid in decision making in hospitalized adults (165). One group has compared multiple diagnostic modalities in 1,033 patients enrolled in clinical trials of zanamivir (166); in 692 patients, the results of viral culture, hemagglutinin inhibition serology, or RT-PCR agreed. In 791 (77%) of the patients, a positive result for one of the three tests was identified. It was evident that diagnostic accuracy of the tests varied, although RT-PCR exhibited 92% sensitivity and 84% specificity compared with the other two diagnostic modalities. The value of RT-PCR in the diagnosis of picornavirus (167) and RSV (69) infection has increasingly been recognized. In fact, advanced, comprehensive microarray systems have been developed that can detect the majority of respiratory viruses in clinical samples (168). It is likely that straightforward, relatively inexpensive, and accurate diagnostic modalities for specific viral pathogens will be developed in the near future.
CONCLUSIONS
Acute exacerbations of COPD are important events in the natural history of this disease. These events can be caused by a large number of infectious and noninfectious agents. Infectious pathogens range from viral to atypical to typical bacterial pathogens. Increasingly, approaches targeting pathogen-specific etiologies have been developed. These include clinical and laboratory-based methods to identify bacterial versus viral infections. Further additional investigation has suggested specific pathogens within these broad classes. The goal should be to better target specific antibacterial or antiviral therapies to individual episodes.
Supported, in part, by National Institutes of Health NHLBI grant 5 P50 HL56402, NIH/NHLBI NO1-HR-46162, NHLBI U10 HL080371, NIH/NHLBI R01 HL073728, NHLBI 2K24HL04212, and 1 K23 HL68713.
Conflict of Interest Statement: F.J.M. is a consultant for Altana Pharma and has received compensation greater than $10K. He has been a member of several advisory boards, CME committees, and the speaker's bureau for Boehringer Ingelheim, Pfizer, and GlaxoSmithKline. His total compensation per company is greater than $10K. In addition, he is on an advisory board for Novartis and speaker's bureau for Sepracor and AstraZeneca, receiving less than $10K per company. He has been an investigator for industry-sponsored studies for GlaxoSmithKline, Boehringer Ingelheim, and Actelion.
References
- 1.Mannino D. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care 2003;48:1185–1191. [PubMed] [Google Scholar]
- 2.Soriano J, Davis K, Coleman B, Visick G, Mannino D, Pride N. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the UK. Chest 2003;124:474–481. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S. Etiology and management of infection in chronic obstructive pulmonary disease. Clin Pulm Med 1999;6:327–332. [Google Scholar]
- 4.Niederman M, McCombs J, Unger A, Kumar A, Popovian R. Treatment cost of acute exacerbations of chronic bronchitis. Clin Ther 1999;21:576–591. [DOI] [PubMed] [Google Scholar]
- 5.Pauwels R, Calverley P, Buist A, Rennard S, Fukuchi Y, Stahl E, Lofdahl C. COPD exacerbations: the importance of a standard definition. Respir Med 2004;98:99–107. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000;117:398S–401S. [DOI] [PubMed] [Google Scholar]
- 7.Tillie-Leblond I, Marquette C, Perez T, Scherpereel A, Zanetti C, Tonnel A, Remy-Jardin M. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Ann Intern Med 2006;144:390–396. [DOI] [PubMed] [Google Scholar]
- 8.Abroug F, Ouanes-Besbes L, Nciri N, Sellami N, Addad F, Hamda K, Amor A, Najjar M, Knani J. Left heart dysfunction and severe exacerbation of COPD: diagnostic performance of cardiac biomarkers. Am J Respir Crit Care Med 2006;174:990–996. [DOI] [PubMed] [Google Scholar]
- 9.Burge S, Wedzicha J. COPD exacerbations: definitions and classifications. Eur Respir J 2003;21:46s–53s. [DOI] [PubMed] [Google Scholar]
- 10.Seemungal T, Donaldson G, Bhowmik A, Jeffries D, Wedzicha J. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:1608–1613. [DOI] [PubMed] [Google Scholar]
- 11.Seemungal T, Donaldson G, Paul E, Bestall J, Jeffries D, Wedzicha J. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–1422. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson G, Seemungal T, Patel I, Lloyd-Owen S, Wilkinson T, Wedzicha J. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J 2003;22:931–936. [DOI] [PubMed] [Google Scholar]
- 13.Schmier J, Halpern M, Higashi M, Bakst A. The quality of life impact of acute exacerbations of chronic bronchitis (AECB): A literature review. Qual Life Res 2005;14:329–347. [DOI] [PubMed] [Google Scholar]
- 14.Doll H, Miravitlles M. Health-related QOL in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literature. Pharmacoeconomics 2005;23:345–363. [DOI] [PubMed] [Google Scholar]
- 15.Spencer S, Jones P; GLOBE Study Group. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax 2003;58:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala J, Masa F, Verea H, Murio C, Ros F, Vidal R; IMPAC Study Group. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004;59:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker C, Voduc N, Aaron S, Webb K, O'Donnell D. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J 2005;26:420–428. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson N, Walker P, Costello R, Calverley P. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:1510–1516. [DOI] [PubMed] [Google Scholar]
- 19.Kanner R, Anthonisen N, Connett J. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:358–364. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson G, Seemungal T, Bhowmik A, Wedzicha J. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makris D, Moschandereas J, Damianaki A, Ntaoukakis E, Siafakas N, Emili J, Tzanakis N. Exacerbations and lung function decline in COPD: new insights in current and ex-smokers. Respir Med 2006;101:1305–1312. [DOI] [PubMed] [Google Scholar]
- 22.Halpern M, Higashi M, Bakst A, Schmier J. The economic impact of acute exacerbations of chronic bronchitis in the United States and Canada: a literature review. J Manag Care Pharm 2003;9:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miravitlles M, Murio C, Guerrero T, Gisbert R, for the DAFNE Study Group. Costs of chronic bronchitis and COPD: a 1-year follow-up study. Chest 2003;123:784–791. [DOI] [PubMed] [Google Scholar]
- 24.Wedzicha J. Exacerbations: etiology and pathophysiologic mechanisms. Chest 2002;121:136S–141S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sethi S. Bacteria in exacerbations of chronic obstructive pulmonary disease: phenomenon or epiphenomenon? Proc Am Thorac Soc 2004;1:109–114. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto K, Yasuo M, Urushibata K, Hanaoka M, Koizumi T, Kubo K. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J 2005;25:640–646. [DOI] [PubMed] [Google Scholar]
- 27.Tsoumakidou M, Tzanakis N, Chrysofakis G, Siafakas N. Nitrosative stress, hemo oxygenase-1 expression and airway inflammation during severe exacerbations of COPD. Chest 2005;127:1911–1918. [DOI] [PubMed] [Google Scholar]
- 28.Drost E, Skwarski K, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005;60:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, Qiu Y, Majumdar S, Gamble E, Matin D, Turato G, Fabbri L, Barnes N, Saetta M, Jeffery P. Exacerbations of bronchitis: bronchial eosinophilia and gene expression for interleukin-4, interleukin-5, and eosinophil chemoattractants. Am J Respir Crit Care Med 2001;164:109–116. [DOI] [PubMed] [Google Scholar]
- 30.Qiu Y, Zhu J, Bandi V, Atmar R, Hattotuwa K, Guntupalli K, Jeffery P. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;168:968–975. [DOI] [PubMed] [Google Scholar]
- 31.Dev D, Wallace E, Sankaran R, Cunniffe J, Govan JRW, Wathen CG, Emmanuel FXS. Value of C-reactive protein measurements in exacerbations of chronic obstructive pulmonary disease. Respir Med 1998;92:664–667. [DOI] [PubMed] [Google Scholar]
- 32.Wedzicha J. The heterogeneity of chronic obstructive pulmonary disease. Thorax 2000;5:631–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roland M, Bhowmik A, Sapsford R, Seemungal T, Jeffries D, Warner T, Wedzicha J. Sputum and plasma endothelin-a levels in exacerbations of chronic obstructive pulmonary disease. Thorax 2001;56:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurst J, Donaldson G, Perera W, Wilkinson T, Bilello J, Hagan G, Vessey R, Wedzicha J. Utility of plasma biomarkers at exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:867–874. [DOI] [PubMed] [Google Scholar]
- 35.Hurst J, Perera W, Wilkinson T, Donaldson G, Wedzicha J. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;173:71–78. [DOI] [PubMed] [Google Scholar]
- 36.Devalia J, Rusznak C, Herdman M, Trigg C, Tarraf H, Davies R. Effect of nitrogen dioxide and sulphur dioxide on airway response of mild asthmatic patients to allergen inhalation. Lancet 1994;344:1668–1671. [DOI] [PubMed] [Google Scholar]
- 37.Ohtoshi T, Takizawa H, Okazaki H, Kawasaki S, Takeuchi N, Ohta K, Ito K. Diesel exhaust particulates stimulate human airway epithelial cells to produce cytokines relevant to airway inflammation in vitro. J Allergy Clin Immunol 1998;101:778–785. [DOI] [PubMed] [Google Scholar]
- 38.Rudell B, Blomberg A, Helleday R, Ledin M, Lundback B, Stjernberg N, Horstedt P, Sandstrom T. Bronchoalveolar inflammation after exposure to diesel exhaust: comparison between unfiltered and particulate trap filtered exhaust. Occup Environ Med 1999;56:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunyer J, Saez M, Murillo C, Castellsague J, Martinez F, Anto J. Air pollution and emergency room admissions for chronic obstructive pulmonary disease: a 5-year study. Am J Epidemiol 1993;137:701–705. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Aymerich J, Tobias A, Anto J, Sunyer J. Air pollution and mortality in a cohort of patients with chronic obstructive pulmonary disease. J Epidemiol Community Health 2000;54:73–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunyer J, Schwartz J, Tobias A, Macfarlane D, Garcia J, Anto J. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. Am J Epidemiol 2000;151:50–56. [DOI] [PubMed] [Google Scholar]
- 42.Glezen W, Greenberg S, Atmar R, Piedra P, Couch R. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 2000;283:499–505. [DOI] [PubMed] [Google Scholar]
- 43.El-Sahly H, Atmar R, Glezen W, Greenberg S. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis 2000;31:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez F, Han M, Flaherty K, Curtis J. Role of infection and antimicrobial therapy in acute exacerbations of chronic obstructive pulmonary disease. Expert Rev Anti Infect Ther 2006;4:101–124. [DOI] [PubMed] [Google Scholar]
- 45.Greenberg S. Viral respiratory infections in elderly patients and patients with chronic obstructive pulmonary disease. Am J Med 2002;112:28S–32S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seemungal T, Harper-Owen R, Bhowmic A, Moric I, Sanderson G, Message S, MacCallum P, Meade T, Jeffries D, Johnston S, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1618–1623. [DOI] [PubMed] [Google Scholar]
- 47.Rhode G, Wiethege A, Borg I, Kauth M, Bauer T, Gillissen A, Bufe A, Schultze-Werninghaus G. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax 2003;58:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan W, Xiang X, Qiu D, Ng T, Lam S, Hegele R. Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am J Med 2003;115:272–277. [DOI] [PubMed] [Google Scholar]
- 49.Pletz MW, Ioanas M, de Roux A, Burkhardt O, Lode H. Reduced spontaneous apoptosis in peripheral blood neutrophils during exacerbation of COPD. Eur Respir J 2004;23:532–537. [DOI] [PubMed] [Google Scholar]
- 50.Cameron R, de Wit D, Welsh T, Ferguson J, Grissell T, Rye P. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intens Care Med 2006;32:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beckham J, Cadena A, Lin J, Piedra P, Glezen W, Greenberg S, Atmar R. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect 2005;50:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papi A, Bellettato C, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri L, Johnston S. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006;173:1114–1121. [DOI] [PubMed] [Google Scholar]
- 53.Mallia P, Message S, Kebadze T, Parker H, Kon O, Johnston S. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res 2006;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papi A, Contoli M, Gaetano C, Mallia P, Johnston S. Models of infection and exacerbations in COPD. Curr Opin Pharmacol 2007;7:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnston S, Papi A, Bates P, Mastronarde J, Monick M, Hunnighake G. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol 1998;160:6172–6181. [PubMed] [Google Scholar]
- 56.Biagioli M, Kaul P, Singh I, Turner R. The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Radic Biol Med 1999;26:454–462. [DOI] [PubMed] [Google Scholar]
- 57.Griego S, Weston C, Adams J, Tal-Singer R, Dillon S. Role of p38 mitogen-activated protein kinase in rhinovirus-induced cytokine production by bronchial epithelial cells. J Immunol 2000;165:5211–5220. [DOI] [PubMed] [Google Scholar]
- 58.Donninger H, Glashoff R, Haitchi H, Syce J, Ghildyal R, van Rensburg E, Bardin P. Rhinovirus induction of the CXC chemokine epithelial-neutrophil activating peptide-78 in bronchial epithelium. J Infect Dis 2003;187:1809–1817. [DOI] [PubMed] [Google Scholar]
- 59.Zhu J, Tang W, Gwaltney J Jr, Wu Y, Elias J. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol 1997;273:L814–L824. [DOI] [PubMed] [Google Scholar]
- 60.Papi A, Johnston S. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem 1999;274:9707–9720. [DOI] [PubMed] [Google Scholar]
- 61.Papi A, Johnston S. Respiratory epithelial cell expression of vascular cell adhesion molecule-1 and its up-regulation by rhinovirus infection via NF-kappaB and GATA transcription factors. J Biol Chem 1999;274:30041–30051. [DOI] [PubMed] [Google Scholar]
- 62.Mastronarde J, He B, Monick M, Mukaida N, Matsushima K, Hunnighake G. Induction of interleukin (IL)-8 gene expression by respiratory syncytial virus involves activation of nuclear factor (NF)-kappa B and NF-IL-6. J Infect Dis 1996;174:262–267. [DOI] [PubMed] [Google Scholar]
- 63.Mastronarde J, Monick M, Mukaida N, Matsushima K, Hunnighake G. Activator protein-1 is the preferred transcription factor for cooperative interaction with nuclear factor-kappaB in respiratory syncytial virus-induced interleukin-8 gene expression in airway epithelium. J Infect Dis 1998;177:1275–1281. [DOI] [PubMed] [Google Scholar]
- 64.Ludwig S, Ehrhardt C, Neumeier E, Kracht M, Rapp U, Pleschka S. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J Biol Chem 2001;276:10990–10998. [PubMed] [Google Scholar]
- 65.Papadopoulos N, Papi A, Meyer J, Stanciu L, Salvi S, Holgate S, Johnston S. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin Exp Allergy 2001;31:1060–1066. [DOI] [PubMed] [Google Scholar]
- 66.Noah T, Wortman I, Becker S. The effect of fluticasone propionate on respiratory syncytial virus-induced chemokines release by a human bronchial epithelial cell line. Immunopharmacology 1998;39:193–199. [DOI] [PubMed] [Google Scholar]
- 67.Wilkinson T, Donaldson G, Johnston S, Openshaw P, Wedzicha J. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:871–876. [DOI] [PubMed] [Google Scholar]
- 68.Falsey A, Formica M, Hennessey P, Criddle M, Sullender W, Walsh E. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Falsey A. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med 2007;28:171–181. [DOI] [PubMed] [Google Scholar]
- 70.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev 2001;14:336–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gump D, Phillips C, Forsyth B. Role of infections in chronic bronchitis. Am Rev Respir Dis 1976;113:465–473. [DOI] [PubMed] [Google Scholar]
- 72.McHardy V, Inglis J, Calder M, Crofton J, Gregg I, Ryland D, Taylor P, Chadwick M, Coombs D, Riddell R. A study of infective and other factors in exacerbations of chronic bronchitis. Br J Dis Chest 1980;74:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murphy T, Brauer A, Schiffmacher A, Sethi S. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:266–272. [DOI] [PubMed] [Google Scholar]
- 74.Monso E, Ruiz J, Rosell A, Manterola J, Fiz J, Morera J, Ausina V. Bacterial infection in chronic obstructive pulmonary disease: a study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med 1995;152:1316–1320. [DOI] [PubMed] [Google Scholar]
- 75.Fagon J, Chastre J, Trouillet J, Domart Y, Dombret M, Bornet M, Gibert C. Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis: use of the protected specimen brush technique in 54 mechanically ventilated patients. Am Rev Respir Dis 1990;142:1004–1008. [DOI] [PubMed] [Google Scholar]
- 76.Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, Hernandez C, Rodriguez-Roisin R. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998;157:1498–1505. [DOI] [PubMed] [Google Scholar]
- 77.Pela R, Marchesani F, Agostinelli C, Staccioli D, Cecarini L, Basotti C, Sanguinetti C. Airways microbial flora in COPD patients in stable clinical conditions and during exacerbations: a bronchoscopic investigation. Monaldi Arch Chest Dis 1998;53:262–267. [PubMed] [Google Scholar]
- 78.Bandi V, Apicella M, Mason E, Murphy T, Siddiqi A, Atmar R, Greenberg S. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Respir Crit Care Med 2001;164:2114–2119. [DOI] [PubMed] [Google Scholar]
- 79.Rosell A, Monso E, Soler N, Torres F, Angrill J, Riise G, Zalacain R, Morera J, Torres A. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med 2005;165:891–897. [DOI] [PubMed] [Google Scholar]
- 80.Sethi S, Maloney J, Grove L, Wrona C, Berenson C. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sethi S, Evans N, Grant B, Murphy T. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;347:465–471. [DOI] [PubMed] [Google Scholar]
- 82.Chin C, Manzel L, Lehman E, Humlicek A, Shi L, Starner T, Denning G, Murphy T, Sethi S, Look D. Haemophilus influenzae from COPD patients with exacerbation induce more inflammation than colonizers. Am J Respir Crit Care Med 2005;172:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy T, Brauer A, Grant B, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med 2005;172:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Musher D, Kubitschek K, Crennan J, Baughn R. Pneumonia and acute febrile tracheobronchitis due to Haemophilus influenzae. Ann Intern Med 1983;99:444–450. [DOI] [PubMed] [Google Scholar]
- 85.Yi K, Sethi S, Murphy T. Human immune response to non-typable Haemophilus influenzae in chronic bronchitis. J Infect Dis 1997;176:1247–1252. [DOI] [PubMed] [Google Scholar]
- 86.Bakri F, Brauer A, Sethi S, Murphy T. Systemic and mucosal antibody response to Moraxella catarrhalis after exacerbations of chronic obstructive pulmonary disease. J Infect Dis 2002;185:632–640. [DOI] [PubMed] [Google Scholar]
- 87.Beaty C, Grayston J, Wang S, Kuo C, Reto C, Martin T. Chlamydia pneumoniae, strain Twar, infection in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1991;144:1408–1410. [DOI] [PubMed] [Google Scholar]
- 88.Blasi F, Legnani D, Lombardo V, Negretto G, Magliano E, Pozzoli R, Chiodo F, Fasoli A, Allegra L. Chlamydia pneumoniae infection in acute exacerbations of COPD. Eur Respir J 1993;6:19–22. [PubMed] [Google Scholar]
- 89.Mogulkoc N, Karakurt S, Isalska B, Bayindir U, Celikel T, Korten V, Colpan N. Acute purulent exacerbation of chronic obstructive pulmonary disease and Chlamydia pneumoniae infection. Am J Respir Crit Care Med 1999;160:349–353. [DOI] [PubMed] [Google Scholar]
- 90.Karnak D, Beng-sun S, Beder S, Kayacan O. Chlamydia pneumoniae infection and acute exacerbation of chronic obstructive pulmonary disease (COPD). Respir Med 2001;95:811–816. [DOI] [PubMed] [Google Scholar]
- 91.Seemungal T, Wedzicha J, MacCallum P, Johnston S, Lambert P. Chlamydia pneumoniae and COPD exacerbation. Thorax 2002;57:1087–1088. [Author reply, 1088–1089.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu L, Skinner S, Lambie N, Vuletic J, Blasi F, Black P. Immunohistochemical staining for Chlamydia pneumoniae is increased in lung tissue from subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:1148–1151. [DOI] [PubMed] [Google Scholar]
- 93.Smith C, Golden C, Kanner R, Renzetti A Jr. Association of viral and Mycoplasma pneumoniae infections with acute respiratory illness in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1980;121:225–232. [DOI] [PubMed] [Google Scholar]
- 94.Lieberman D, Lieberman D, Ben-Yaakov M, Lazarovich Z, Hoffman S, Ohana B, Friedman M, Dvoskin B, Leinonen M, Boldur I. Infectious etiologies in acute exacerbation of COPD. Diagn Microbiol Infect Dis 2001;40:95–102. [DOI] [PubMed] [Google Scholar]
- 95.Avadhanula V, Rodriguez C, DeVincenzo J, Wang Y, Webby R, Ulett G, Adderson E. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol 2006;80:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ishizuka S, Yamaya M, Suzuki T, Takahashi H, Ida S, Sasaki T, Inoue D, Sekizawa K, Nishimura H, Sasaki H. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis 2003;188:1928–1939. [DOI] [PubMed] [Google Scholar]
- 97.Wilkinson T, Hurst J, Perera W, Wilks M, Donaldson G, Wedzicha J. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 2006;129:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bandi V, Jakubowycz M, Kinyon C, Mason E, Atmar R, Greenberg S, Murphy T. Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzae. FEMS Immunol Med Microbiol 2003;10:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCrory D, Brown C. Anticholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;1. [DOI] [PMC free article] [PubMed]
- 100.Niewoehner D. The role of systemic corticosteroids in acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Med 2002;1:243–248. [DOI] [PubMed] [Google Scholar]
- 101.Wood-Baker R, Gibson P, Hannay M, Walters E, Walters J. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;1. [DOI] [PubMed]
- 102.Niewoehner D, Erbland M, Deupree R, Collins D, Gross N, Light R, Anderson P, Morgan N; Department of Veterans Affairs Cooperative Study Group. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1999;340:1941–1947. [DOI] [PubMed] [Google Scholar]
- 103.Saymer A, Aytemujr Z, Cirit M, Unsal I. Systemic glucocorticoids in severe exacerbations of COPD. Chest 2001;119:726–730. [DOI] [PubMed] [Google Scholar]
- 104.Davies L, Angus R, Calverley P. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456–460. [DOI] [PubMed] [Google Scholar]
- 105.Thompson W, Nielson C, Carvalho P, Charan N, Crowley J. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996;154:407–412. [DOI] [PubMed] [Google Scholar]
- 106.Aaron S, Vandemheen K, Hebert P, Dales R, Stiell I, Ahuja J, Dickinson G, Brinson R, Rowe B, Dreyer J, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med 2003;348:2618–2625. [DOI] [PubMed] [Google Scholar]
- 107.National Collaborating Center for Chronic Conditions. Chronic obstructive pulmonary disease: national clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59:141–143. [PMC free article] [PubMed] [Google Scholar]
- 108.Saint S, Bent S, Vittinghoff E, Grady D. Antibiotics in chronic obstructive pulmonary disease exacerbations: a meta-analysis. JAMA 1995;273:957–960. [PubMed] [Google Scholar]
- 109.Ram F, Rodriguez-Roisin R, Granados-Navarrete A, Garcia-Aymerich J, Barnes N. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;3. [DOI] [PubMed]
- 110.Puhan M, Vollenweider D, Latshang T, Steurer J, Steurer-Stev C. Exacerbations of chronic obstructive pulmonary disease: when are antibiotics indicated? A systematic review. Respir Res 2007;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blasi F, Ewig S, Torres A, Huchon G. A review of guidelines for antibacterial use in acute exacerbations of chronic bronchitis. Pulm Pharmacol Ther 2006;19:361–369. [DOI] [PubMed] [Google Scholar]
- 112.Siempos I, Dimopoulos G, Korbila I, Manta K, Falagas M. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Respir J 2007;29:1127–1137. [DOI] [PubMed] [Google Scholar]
- 113.Miravitlles M, Llor C, Naberan K, Cots J, Molina J. Effect of various antimicrobial regimens on the clinical course of exacerbations of chronic bronchitis and chronic obstructive pulmonary disease in primary care. Clin Drug Investig 2004;24:63–72. [Google Scholar]
- 114.Martinez F, Grossman R, Zadeikis N, Fisher A, Walker K, Ambruzs M, Tennenberg A. Patient stratification in the management of acute bacterial exacerbation of chronic bronchitis: the role of levofloxacin 750 mg. Eur Respir J 2005;25:1001–1010. [DOI] [PubMed] [Google Scholar]
- 115.White AJ, Gompertz S, Bayley DL, Hill SL, O'Brien C, Unsal I, Stockley RA. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax 2003;58:680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Anzueto A, Rizzo J, Grossman R. The infection-free interval: its use in evaluating antimicrobial treatment of acute exacerbations of chronic bronchitis. Clin Infect Dis 1999;28:1344–1345. [DOI] [PubMed] [Google Scholar]
- 117.Chodosh S. Clinical significance of the infection-free interval in the management of acute bacterial exacerbations of chronic bronchitis. Chest 2005;127:2231–2236. [DOI] [PubMed] [Google Scholar]
- 118.Anzueto A, Niederman M. Diagnosis and treatment of rhinovirus respiratory infections. Chest 2003;123:1664–1672. [DOI] [PubMed] [Google Scholar]
- 119.Patick A. Rhinovirus chemotherapy. Antiviral Res 2006;71:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Turner R, Wecker M, Pohl G, Witek T, McNally E, St. George R, Winther B, Hayden F. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA 1999;281:1797–1804. [DOI] [PubMed] [Google Scholar]
- 121.Hayden F, Herrington D, Coats T, Kim K, Cooper E, Villano S, Liu S, Hudson S, Pevear D, Collett M, McKinlay M; Pleconaril Respiratory Infection Study Group. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis 2003;36:1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pevear D, Hayden F, Demenczuk T, Barone L, McKinlay M, Collett M. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob Agents Chemother 2005;49:4492–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fleischer R, Laessig K. Safety and efficacy evaluation of pleconaril for treatment of the common cold. Clin Infect Dis 2003;37:1722. [DOI] [PubMed] [Google Scholar]
- 124.Patick A, Potts K. Protease inhibitors as antiviral agents. Clin Microbiol Rev 1998;11:614–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hayden F, Turner R, Gwaltney J, Chi-Burris K, Gersten M, Hsyu P, Patick A, Smith G III, Zalman L. Phase II, randomized, double-blind, placebo-controlled studies of rupintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob Agents Chemother 2003;47:2907–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patick A, Brothers M, Maldonado F, Binford S, Maldonado O, Fuhrman S, Peterson A, Smith G III, Zalman L, Burns-Naas L, et al. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother 2005;49:2267–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mallia P, Contoli M, Caramori G, Pandit A, Johnston S, Papi A. Exacerbations of asthma and chronic obstructive pulmonary disease (COPD): focus on virus induced exacerbations. Curr Pharm Des 2007;13:73–97. [DOI] [PubMed] [Google Scholar]
- 128.Cooper N, Sutton A, Abrams K, Wailoo A, Turner D, Nicholson K. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analysis of randomised controlled trials. BMJ 2003;326:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murphy K, Eivindson A, Pauksens K, Stein W, Tellier G, Watts R, Leophonte P, Sharp S. Efficacy and safety of inhaled zanamivir for the treatment of influenza in patients with asthma or chronic obstructive pulmonary disease: a double-blind, randomised, placebo-controlled, multicenter study. Clin Drug Investig 2000;20:337–349. [Google Scholar]
- 130.Lalezari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch Intern Med 2001;161:212–217. [DOI] [PubMed] [Google Scholar]
- 131.Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med 2003;163:1667–1672. [DOI] [PubMed] [Google Scholar]
- 132.Liss H, Bernstein J. Ribavirin aerosol in the elderly. Chest 1988;93:1239–1241. [DOI] [PubMed] [Google Scholar]
- 133.Anthonisen N, Manfreda J, Warren C, Hersfield E, Harding G, Nelson N. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196–204. [DOI] [PubMed] [Google Scholar]
- 134.Soler N, Agusti A, Angrill J, Puig de la Bellacasa J, Torres A. Bronchoscopic validation of the significance of sputum purulence in severe exacerbations of chronic obstructive pulmonary disease (COPD). Thorax 2007;62:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stockley RA, O'Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest 2000;117:1638–1645. [DOI] [PubMed] [Google Scholar]
- 136.Allegra L, Blasi F, Diano P, Cosentini R, Tarsia P, Confalonieri M, Dimakou K, Valenti V. Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary disease. Respir Med 2005;99:742–747. [DOI] [PubMed] [Google Scholar]
- 137.Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay M, Huber P, Tamm M, Muller B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded interventional trial. Lancet 2004;363:600–607. [DOI] [PubMed] [Google Scholar]
- 138.Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Rev 2000;49:S57–S61. [PubMed] [Google Scholar]
- 139.Christ-Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber P, Zimmerli W, Harbath S, Tamm M, Muller B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med 2006;174:84–93. [DOI] [PubMed] [Google Scholar]
- 140.Stolz D, Christ-Crain M, Leuppi J, Miedinger D, Muller C, Huber P, Muller B, Tamm M. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007;131:9–19. [DOI] [PubMed] [Google Scholar]
- 141.Martinez F, Curtis J. Procalcitonin-guided antibiotic therapy in COPD exacerbations: closer but not quite there. Chest 2007;131:1–2. [DOI] [PubMed] [Google Scholar]
- 142.Martinez F. Acute bronchitis: state of the art diagnosis and therapy. Compr Ther 2004;30:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Balter M, La Forge J, Low D, Mandell L, Grossman R. Canadian guidelines for the management of acute exacerbations of acute exacerbations of chronic bronchitis. Can Respir J 2003;10:3B–32B. [DOI] [PubMed] [Google Scholar]
- 144.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global Strategy for the Diagnosis of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary. Am J Respir Crit Care Med 2007;176:532–555. [DOI] [PubMed] [Google Scholar]
- 145.Ball P. Epidemiology and treatment of chronic bronchitis and its exacerbations. Chest 1995;108:43S–52S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilson R, Kubin R, Ballin I, Deppermann K, Bassaris H, Leophonte P, Schreurs A, Torres A, Sommerauer B. Five day moxifloxacin therapy compared with 7 day clarithromycin therapy for the treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother 1999;44:501–513. [DOI] [PubMed] [Google Scholar]
- 147.Monto A, Ross H. Acute respiratory illness in the community: effect of family composition, smoking, and chronic symptoms. Br J Prev Soc Med 1977;31:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Monto A. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin Ther 2002;24:1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Monto A, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000;160:3243–3247. [DOI] [PubMed] [Google Scholar]
- 150.Boivin G, Hardy I, Tellier G, Maziade J. Predicting influenza infections during epidemics with use of a clinical case definition. Clin Infect Dis 2000;31:1166–1169. [DOI] [PubMed] [Google Scholar]
- 151.Walsh E, Cox C, Falsey A. Clinical features of influenza A virus infection in older hospitalized persons. J Am Geriatr Soc 2002;50:1498–1503. [DOI] [PubMed] [Google Scholar]
- 152.Seemungal T, Harper-Owen R, Bhowmik A, Jeffries D, Wedzicha J. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J 2000;16:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Walsh E, Peterson D, Falsey A. Is clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis 2007;195:1046–1051. [DOI] [PubMed] [Google Scholar]
- 154.Wang C, Wang S, Lai C, Lin L, Chou P. Impact of influenza vaccination on major cause-specific mortality. Vaccine 2007;25:1196–1203. [DOI] [PubMed] [Google Scholar]
- 155.Poole P, Chacko E, Wood-Baker R, Cates C. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;1:CD002733. [DOI] [PubMed] [Google Scholar]
- 156.Wongsurakiat P, Maranetra K, Wasi C, Kositanont U, Defsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest 2004;125:2011–2020. [DOI] [PubMed] [Google Scholar]
- 157.de Roux A, Marx A, Burkhardt O, Schwiger B, Borkowski A, Banzhoff A, Pletz M, Lode H. Impact of corticosteroids on the immune response to a MF59-adjuvanted influenza vaccine in elderly COPD-patients. Vaccine 2006;24:1537–1542. [DOI] [PubMed] [Google Scholar]
- 158.Nichol K, Baken L, Wourenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med 1999;159:2437–2442. [DOI] [PubMed] [Google Scholar]
- 159.Lee T, Weaver F, Weiss K. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. J Gen Intern Med 2007;22:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Granger R, Walters J, Poole P, Lasserson T, Mangtani P, Cates C, Wood-Baker R. Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev Issue 2006;4:CD001390. [DOI] [PubMed] [Google Scholar]
- 161.Alfageme I, Vazquez R, Reyes N, Munos J, Fernandez A, Hernandez M, Merino M, Perez J, Lima J. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 2006;61:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Arandjus C, Black P, Poole P, Wood Baker R, Steurer-Stey C. Oral bacterial vaccines for the prevention of acute exacerbations in chronic obstructive pulmonary disease and chronic bronchitis. Respir Med 2006;100:1671–1681. [DOI] [PubMed] [Google Scholar]
- 163.Dal Negro R, Micheletto C, Tognella S, Visconti M, Guerriero M, Sandri M. A two-stage logistic model based on the measurement of pro-inflammatory cytokines in bronchial secretions for assessing bacterial, viral, and non-infectious origin of COPD exacerbations. COPD 2005;2:7–16. [DOI] [PubMed] [Google Scholar]
- 164.Lynch J III, Walsh E. Influenza: evolving strategies in treatment and prevention. Semin Respir Crit Care Med 2007;28:144–158. [DOI] [PubMed] [Google Scholar]
- 165.Falsey A, Murata Y, Walsh E. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med 2007;167:354–360. [DOI] [PubMed] [Google Scholar]
- 166.Zambon M, Hays J, Webster A, Newman R, Keene O. Diagnosis of influenza in the community. Relationship of clinical diagnosis to confirmed virological, serologic, or molecular detection of influenza. Arch Intern Med 2001;161:2116–2122. [DOI] [PubMed] [Google Scholar]
- 167.Greenberg S. Rhinovirus and coronavirus infections. Semin Respir Crit Care Med 2007;28:182–192. [DOI] [PubMed] [Google Scholar]
- 168.Quann P-L, Palacios G, Jabado OJ, Conlan S, Hirschberg DL, Pozo F, Jack PJM, Cisterna D, Renwick N, Hui J, et al. Detection of respiratory viruses and subtype identification of influenza A viruses by GreeneChipResp oligonucleotide microarray. J Clin Microbiol 2007;45:2359–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Celli BR, MacNee W, Agusti A, Anzueto A, Berg AS, Buist AS, Calverley PMA, Chavannes N, Dillard T, Fahy B, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. [DOI] [PubMed] [Google Scholar]
- 170.Woodhead M, Blasi F, Ewig S, Huchon G, Leven M, Ortqvist A, Schaberg T, Torris A, van der Heijden G, Verheij TJM. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2005;26:1138–1180. [DOI] [PubMed] [Google Scholar]