Abstract
Contribution of transplanted bone marrow has, in many models, led to the appearance of marrow-derived epithelial cells in a variety of organs, including the lung. Following the initial descriptions of these cells, many questions remain about the mechanisms by which bone marrow adopts an epithelial phenotype in the murine lung. Data from other epithelial lineages, such as those of the kidney and colon, suggest that one mechanism is fusion of transplanted marrow with host pneumocytes. This process appears to require severe damage and may not be the only mechanism by which mature lung epithelia can derive from marrow. This article discusses the processes leading to the appearance of marrow-derived pneumocytes and highlights the therapeutic potential of bone marrow to fuse with or differentiate into epithelial cells of the lung.
Keywords: Bone marrow, stem cell plasticity
We and others have demonstrated that bone marrow–derived cells (BMDCs) can develop into epithelial cells in the lung, liver, gastrointestinal (GI) tract, and skin. These findings, initially reported in laboratory animals, have also been reported in humans. It is not yet clear which subpopulation(s) of the bone marrow (BM) has the ability to become epithelial cells. Another unresolved question regarding this BM to epithelial transition is the mechanism by which this occurs. Data suggest that cell–cell fusion can occur but is not required. We hypothesize that both the degree of epithelial engraftment and the mechanism by which this occurs in the lung are dependent on the degree and type of damage induced. Here, we review the data regarding the mechanisms underlying the BM to epithelial transition with an emphasis on the role of cell fusion. In addition, we present data regarding the feasibility of using BM cells as a vehicle to deliver gene therapy to the lung.
BM CELLS CAN DEVELOP INTO EPITHELIAL CELLS IN MICE
In the earliest studies demonstrating marrow-derived epithelial cells, Petersen and colleagues (1) and Theise and coworkers (2) showed that female rats and mice that had been lethally irradiated and transplanted with male BM developed Y chromosome–positive hepatocytes. These data were obtained by other laboratories as well, and the reported level of engraftment varied widely, from 0.01 to 2%. This is likely due to multiple variables, including the degree and type of injury induced in the liver, the cell population transplanted, and the method of detection used. BMDCs have also been reported to take on the gene expression pattern of hepatocytes in vitro when cocultured with hepatocytes (3) or with hepatocyte growth factor (4).
Methods of detection of marrow-derived epithelial cells have improved since the earliest reports. In our initial studies, we reported that more than 1% of hepatocytes were marrow derived after lethal irradiation using Y chromosome fluorescence in situ hybridization (Y-FISH) and immunofluorescence for cytokeratin as our detection methods (2). However, this may have been an overestimation due to overlay of blood cells in the thin sections analyzed. When we added anti-CD45 to the staining protocol together with anticytokeratin and Y-FISH, the incidence of hepatocytes that were marrow derived was approximately 1 in 1,000. In a mouse model of fatal hereditary tyrosinemia type I, in which the gene for fumarylacetoacetate hydrolase (FAH) has been knocked out, transplanted wild-type bone marrow cells could comprise up to 30% of hepatocytes and save the mice from liver failure as discussed in more detail below (5).
Data for engraftment of marrow-derived cells as lung epithelial cells have been reported by our group as well as others (6–14), and some forms of lung injury promote mobilization of hematopoietic stem and progenitor cells from the BM into the circulation (14, 15). There exists some controversy in this area, because a number of investigators using alternate approaches have found no evidence of BM contribution to epithelial repair in the murine lung (16–18). All of these studies used a green fluorescent protein (GFP) reporter gene to track marrow-derived cells, and none of them used chromosomal analysis to identify marrow-derived lung epithelium. Furthermore, in one of the models used, the baseline reporter gene expression was extremely low (16), and in another, no lung injury was induced during the preconditioning phase (18). Because transgene expression has been shown to be relatively insensitive for the identification of marrow-derived epithelial cells, and injury is known to be a critical event for the appearance of these cells, the significance of these negative data is unknown. However, they do illustrate the need for rigorous and consistent study design. Current standards in this field demand either confocal or single-cell analysis of marrow-derived epithelial cells to rule out the possibility of overlay. The addition of CD45 and/or other hematopoietic antigens to staining protocols is appropriate in many cases. Last, where technically feasible, phenotypic analysis with cell-specific markers is indicated to ensure that the appearance of epithelial cells derived from marrow is not the result of microscopy artifact.
More in-depth analyses of the kinetics and degree of engraftment of marrow-derived epithelial cells were then performed (19). We showed that BM engraftment as type II pneumocytes occurred within 1 to 2 wk after intravenous infusion, suggesting that these cells may have become part of the lung architecture during recovery of the lung from the radiation-induced pneumonitis. A greater understanding of how the lung repairs itself after irradiation, including the clearance of apoptotic cells and their replacement with newly formed epithelial cells, will allow us to better understand how marrow-derived cells become epithelial cells in the lung.
BMDCs CAN DEVELOP INTO EPITHELIAL CELLS IN HUMANS
We also examined whether BMDCs could differentiate into hepatocytes in humans (20). We obtained archived liver tissue samples from two women who had undergone BM transplantation with marrow from a male donor, and from four men who had undergone liver transplantation with livers from female donors. In all six samples, Y chromosome–positive hepatocytes were identified, with the highest numbers (12% in the periportal regions) identified in the transplanted female-derived liver of a male recipient who had been transplanted for hepatitis C and who had developed recurrent hepatitis C in the transplanted female liver. These findings not only confirmed that marrow-derived cells could become hepatocytes in humans but also that severe injury may increase the degree of engraftment of marrow-derived hepatocytes.
Marrow-derived epithelial cells have also been reported in human lungs (21–23). Consistent with the potential contribution of circulating BMDCs to lung injury repair, several studies have shown that different forms of lung injury lead to high levels of circulating hematopoietic and endothelial stem and progenitor cells from the BM (24).
THE DEGREE OF LUNG EPITHELIAL CELL ENGRAFTMENT FROM BMDCs IS DEPENDENT ON THE DEGREE AND TYPE OF LUNG DAMAGE INDUCED
The level of engraftment of marrow-derived epithelial cells has been assessed in response to different forms of lung damage. As discussed above, lethal doses of irradiation (> 1,000 cGy) result in engraftment of BM-derived epithelial cells. However, until recently, no study had directly addressed the relationship between lung tissue injury and the degree of epithelial cell engraftment from BMDCs. We performed such a study (25) based on the following facts: (1) lung injury in response to irradiation is dose dependent, with a threshold of approximately 750 cGy necessary for lung damage, and (2) BM engraftment can be attained using sublethal doses of irradiation. In this study, female mice received male BM intravenously after being exposed to 0, 300, 600, or 1,000 cGy irradiation. As had been reported previously with high-dose irradiation, maximal lung damage was apparent 3 to 4 d after 1,000 cGy irradiation, with resolution by Day 7. We quantitated the lung damage by assessing the percentage of cytokeratin-positive lung cells that were apoptotic by TdT-mediated dUTP-biotin nick end labeling analysis. In response to 1,000 cGy, approximately 30% of epithelial cells were apoptotic on Day 3. In contrast, there was no increase in the percentage of apoptotic epithelial cells above background (0 cGy) in mice that received 300 or 600 cGy. Next, we assessed the BM engraftment in mice that received different doses of irradiation. All of the mice that received 300, 600, or 1,000 cGy had significant (> 80%) BM engraftment by 1 mo post-transplant, consistent with previous reports. To determine the percentage of marrow-derived lung epithelial cells, we analyzed lung tissue sections and separate lung cells that had been digested using collagenase/dispase, and then cytospun directly onto microscope slides to analyze discrete individual cells without the risk of overlay artifact that could occur in tissue sections. In all cases, we detected no marrow-derived epithelial cells in the lungs of mice that had received 0, 300, or 600 cGy irradiation. In contrast, approximately 0.2% of type II epithelial cells were marrow derived in the mice that had received 1,000 cGy. These data are important for several reasons. First, they show that a threshold level of lung damage is necessary for engraftment of lung epithelial cells from BMDCs, and second, they show that overlay with marrow-derived hematopoietic cells, which were present at equally high levels in mice that received 300, 600 or 1,000 cGy, was not causing a false-positive interpretation of marrow-derived epithelial cells.
A SINGLE BMDC CAN DIFFERENTIATE INTO BLOOD AND EPITHELIAL CELLS IN VIVO
In an effort to determine which BMDC subpopulation is capable of becoming epithelial cells, our laboratory collaborated with that of Saul Sharkis at Johns Hopkins (7). Sharkis had already published data showing that hematopoietic stem cells could be fractionated using elutriation, and that hematopoietic stem cells home to the BM of lethally irradiated recipients within 48 h of intravenous infusion. In our collaborative work, he showed that, if BM cells are elutriated (E), then depleted of lineage committed blood cells (L), and then transplanted into a lethally irradiated recipient and recovered after homing (H) to the BM, one can obtain a population of cells that is highly enriched for hematopoietic stem cells. A single one of these ELH cells can long-term reconstitute the hematopoietic system when transplanted into a lethally irradiated recipient (together with a population of short-term hematopoietic reconstituting cells). In our studies, we used female recipient mice that had undergone BM transplantation with a single male-derived ELH cell together with 20,000 female-derived short-term reconstituting cells, and achieved long-term (11 mo) hematopoietic reconstitution with Y chromosome–positive cells. If we could detect Y chromosome–positive epithelial cells in these mice, then it would mean that a single marrow-derived cell could engraft not only hematopoietic cells but also as nonhematopoietic cells. Analysis of tissue using triple staining for cytokeratin, CD11b, and the Y chromosome revealed marrow-derived epithelial cells in the lung (including rare bronchiolar epithelial cells), liver, GI tract, and skin of these mice. In these mice, marrow-derived type II pneumocytes were identified by morphology, cytokeratin expression, and the detection of both transcription centers for surfactant protein-B (SP-B) as well as the Y chromosome. The potential mechanisms underlying this engraftment are discussed below.
MARROW-DERIVED EPITHELIAL CELLS ARE FUNCTIONAL
To determine whether marrow-derived epithelial cells can function normally, we showed that marrow-derived epithelial cells in the murine GI tract express the cystic fibrosis transmembrane regulator (Cftr) mRNA and protein, and can restore chloride transport through this channel (26). In collaboration with Dr. Marie Egan from the Department of Pediatrics at Yale School of Medicine, Dr. Emanuela Bruscia transplanted BM from GFP+ mice that had the wildtype Cftr gene into Cftr-null mice. Cftr, as its name implies, is a membrane channel that is not functional in patients with cystic fibrosis (CF). CF is a genetic disease in which the loss of CFTR-dependent Cl− transport from the apical membrane of epithelial cells in the airway and GI tract (as well as other organs) leads to hyperviscous secretions that cause pathology in the respiratory and GI tracts.
It is important to note that, in Cftr-null (CFTRtm1Unc) mice, the lungs are relatively normal, presumably due to the expression of a redundant chloride channel in mice, that does not compensate for CFTR loss in humans. However, Cftr-null mice do have severe GI tract pathology that closely resembles the GI epithelium dysfunction in patients with CF. Therefore, we focused our studies on the ability of marrow-derived GI epithelial cells to express functional Cftr. Our results have been very promising. We have shown that Cftr-null mice (n = 13) that receive BM from donors that are wild type at the Cftr locus have detectable levels of Cftr mRNA throughout their GI tracts, including the small and large intestines. We also occasionally observed Cftr mRNA in the lungs of recipient mice. To assess for Cftr protein expression in the transplanted Cftr-null mice, we analyzed tissue from the GI tract by co-immunofluorescence for GFP and Cftr. Although these cells were rare, they were found in most of the Cftr-null recipients. In contrast, none of the untransplanted Cftr-null mice had any Cftr mRNA or protein detected. Similarly, control Cftr-null mice that underwent BM transplantation with BM from Cftr-null donor mice also had no Cftr mRNA or protein.
With these exciting data on Cftr mRNA and protein expression, the next step was to assess for Cftr activity. Because Cftr+ epithelial cells line the GI tract, it is possible to assess for Cftr activity in vivo in mice by using a probe to measure the cyclic AMP–induced chloride channel activity in the rectum and distal colon. This allowed us to measure the Cftr activity in individual mice over time. We also performed in vitro electrophysiology assays (Ussing chamber analyses) on the mucosal surfaces of multiple organs harvested at the time of death. Some of the Cftr-null mice had a partial recovery of functional Cftr channel activity in the distal colon by 5 wk post–BM transplantation, which was maintained for up to 5 mo. Ussing chamber analysis confirmed that there was Cftr-specific channel activity in the large intestine of these transplanted Cftr-null mice, as well as in the small intestine, including the duodenum, jejunum, and ileum. Specificity of the channel activity was demonstrated not only by its characteristic response to agents that elevate intracellular cAMP levels but also by the loss of activity when a specific blocker of Cftr activity was administered. As for the expression data presented above, control mice, including Cftr-null mice that received Cftr-null BM (n = 7), showed no Cftr activity in vivo or in vitro. It is not clear why we were able to detect Cftr channel activity with the very low levels of engraftment of Cftr+ cells. Possibilities include that we are assessing the electrophysiologic function of a very large number of cells, that there is cell–cell communication that enhances channel activity along the epithelium, or that the levels of engraftment are underestimated by the detection approaches that we have used (e.g., transgene expression, Y-FISH, and immunofluorescence).
POTENTIAL MECHANISMS UNDERLYING THE BM TO EPITHELIAL CELL TRANSITION
Several mechanisms may be responsible for the appearance of epithelial cells derived from BM. These are all applicable to the lung and are not mutually exclusive. In one scenario, BM with the ability to directly differentiate into nonhematopoietic cell types contains an unidentified population of pluripotent cells not yet committed to becoming blood. Alternative mechanisms feature “committed” cells that differentiate from one cell type to that of a completely different cell type either directly or with an intervening de-differentiated state. To date, none of these processes has been definitively shown. The only proven mechanism for the generation of epithelial cells from BM is the fusion of a BMDC with a tissue cell.
Cells arising from membrane fusion have at least three fates. Those that retain both nuclei are termed “heterokaryons” if the two initial cells were not identical. Other fused cells can develop a single fused nucleus and are termed “synkaryons.” A third possibility is that the cell could lose one nucleus or a fraction of its chromosomal material through reductive division. Only recently have cells from the BM been shown to fuse with nonhematopoietic cells, a process that leads to changes in gene expression. In the absence of therapeutic amplification strategies, the initial frequency of fused cells in any organ is very low.
FUSION UNDERLIES THE CURATIVE EFFECT OF BM TRANSPLANTATION IN A MOUSE MODEL OF TYROSINEMIA
The first in vivo evidence of fusion was presented in an investigation of marrow-derived hepatocytes in FAH-null mice (27, 28). In these studies, a complicated system of sex-mismatched hematopoietic stem cell (HSC) transplantation showed that the vast majority of marrow-derived hepatocytes contained chromosomal material from both the host and the donor. This was shown either through gene expression (27) or abnormal karyotypes (26). A small fraction of these hepatocytes did not appear to have arisen through fusion, though it was not determined whether reductive divisions had given rise to 2N hepatocytes in this model. Later work found that the marrow-derived cells with this ability are likely macrophages (29). The high number of fused cells detected in the FAH model is likely due to clonal proliferation of marrow-derived hepatocytes carrying a wild-type FAH gene. We have independently confirmed these data in our laboratory (Figure 1C).
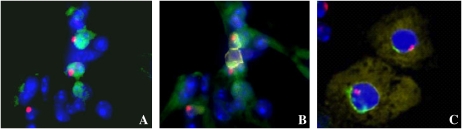
Figure 1.
(A) Alveolar tissue from a female recipient of male marrow stained for thyroid transcription factor 1 (TTF-1) (green) and Y chromosome (red), and counterstained with 4′,6-Diamidino-2-phenylindole (DAPI) (blue). The apparent colocalization of TTF-1 and Y gives the appearance of two marrow-derived type II pneumocytes. However, as B demonstrates, addition of CD45 staining shows that these cells are covered by two blood cells and cannot be considered to be donor-derived epithelial cells. This artifact is termed “overlay.” (C) Two hepatocytes isolated from the livers of female FAH-null recipients of wild-type male marrow that have undergone fluorescence in situ hybridization (FISH) for both the X and Y chromosomes. In this image, the characteristic autofluorescence of the liver cell is shown in yellow, the Y chromosome in red, the X chromosome in green, and the nucleus is counterstained with DAPI (blue). The cell on the left contains three X chromosomes and one Y chromosome, indicating that it arose through a fusion event. The cell on the right contains one X and one Y, indicating either differentiation or fusion followed by reductive division and loss of one set of sex chromosomes.
Data from the kidney (30) and the GI tract (31) as well as our own unpublished data show that fusion of marrow with mature epithelial cells can be followed by reductive divisions. The intracellular events governing this process as well as its stability and safety require further investigation but it appears that fusion is an effective means of gene transfer and cytotherapy.
FUSION IS NOT REQUIRED FOR BM TO EPITHELIAL TRANSITION
Although fusion is clearly sufficient for the appearance of BM-derived hepatocytes, it is not clear whether it is required. We sought to address this question using a relatively simple approach. By performing BM transplantation of Cre reporter strain marrow into mice that ubiquitously express the Cre recombinase, we could track the appearance of fused cells by reporter gene expression. Our approach was similar to that of Alvarez-Dolado and colleagues (32), but we took the extra step of looking for false-negative results based on transgene silencing.
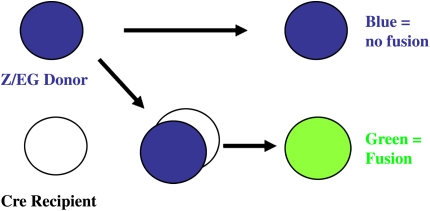
In our study (33), the Cre reporter strain was Z/EG. In the Z/EG mouse, the lac Z gene is driven by the β-actin promoter and followed by three SV40 poly-A tracts and in-frame stop codons. This construct is flanked by two lox P sites, and the GFP gene is located just 3′ of this loxP flanked cassette. When intact (i.e., in the absence of Cre recombinase), the lacZ gene is expressed constitutively, and the GFP is off since it is downstream of the stop codons. However, in the presence of the Cre recombinase, the loxP flanked DNA is excised such that the eGFP gene is under control of the ubiquitous β-actin promoter. Because, in this approach, the only way that GFP can be expressed is on the introduction of the host Cre recombinase into the nucleus of the donor Z/EG construct, detection of this reporter gene would imply fusion. Marrow-derived cells that formed by differentiation without cell fusion would contain unrecombined DNA and thus express lacZ. This strategy is illustrated in Figure 2.
Figure 2.
Simplified schematic of fusion events leading to GFP expression. When Z/EG marrow is transplanted into an ubiquitously expressing Cre mouse, differentiation and fusion can be determined based on reporter gene expression. In the case of a differentiation event, the Z/EG construct remains intact and lacZ is expressed (top right, blue). In the case of a fusion event, the Cre recombinase is introduced into a Z/EG-containing cell and loxP sites are excised, leading to activation of the GFP reporter transgene (bottom right, green).
With this rationale, we performed sex-mismatched BM transplantation of Z/EG marrow into Cre mice. At the time of death, organs from the recipient mice were analyzed for GFP on the mRNA and protein levels. Reverse transcriptase–polymerase chain reaction on the GI, kidney, liver, lung, diaphragm, skeletal muscle, and heart failed to detect GFP expression with a sensitivity of 1/106 cells. No GFP protein was detected by immunohistochemistry on liver, lung, and skin. Nor was GFP detected by fluorescence-activated cell sorter analysis on isolated lung epithelia. Marrow-derived epithelia were detected by combining immunohistochemistry for epithelial markers with lacZ or Y-FISH. Using this approach, low frequencies of marrow-derived epithelia were detected in the liver, lung, and GI tract. In addition, the donor-derived lung cells were found to contain only one copy of each sex chromosome. Taken together, these data demonstrate that fusion is not required for the appearance of marrow-derived epithelia.
Because this experimental system relied so heavily on transgene expression, we performed a number of systematic controls to rule out transgene failure as a cause of false-negative results. This included adenoviral assays for the presence of functional Cre recombinase, the ability of the Z/EG construct to undergo postembryonic recombination on exposure to Cre, and the ability of this system to detect in vivo fusion events following secondary injury after BM transplantation. These controls attest to the specificity in the use of this approach to detect fusion and showed that secondary injury after transplantation is sufficient to induce fusion events between marrow-derived cells and epithelial cells.
CAN BM-DERIVED CELLS BE USED TO DELIVER GENE THERAPY TO THE LUNG?
One potential clinical application of these findings is that, theoretically, marrow-derived cells could be used as vehicles for targeted transgene expression in epithelial cells of the lung. Such an approach could be used clinically in diseases caused by single-gene mutations, such as surfactant deficiencies, CF, or α1-antitrypsin deficiency. Dr. Joanna Grove performed experiments to directly address the question of whether autologous cells could be modified to deliver a gene to lung epithelial cells (34). For these studies, which were performed in collaboration with Drs. Don Kohn and Carolyn Lutzko from the Children's Hospital of Los Angeles, wild-type BM cells from male mice were infected with a retrovirus encoding the GFP transgene expressed on the constitutively active retroviral long terminal repeat promoter. The cells were transplanted into lethally irradiated female recipients, and GFP+ marrow-derived epithelial cells were detected using immunohistochemistry for GFP and cytokeratin, as well as Y-FISH and SP-B–FISH. Between 1 and 7% of cytokeratin-positive cells with the appearance of type II cells expressed GFP, indicating that the retroviral transgene was still expressed after the conversion of BMDCs to type II epithelial cells in the lung.
FUTURE STUDIES
Current and future work in our laboratories is focused on defining the functional phenotype of the marrow-derived epithelial cells, the mechanisms that underlie the BM to epithelial transition, and the development of feasible molecular approaches to amplify engraftment to levels that would be therapeutic for use in respiratory disorders.
Supported by NIH DK061846 and NIH HL073742 (D.S.K.).
Conflict of Interest Statement: E.L.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.S.K. was reimbursed by AstraZeneca for attending and speaking at a conference focused on chronic obstructive pulmonary disease, which was held in Lund, Sweden, in May 2006. She also received an honorarium of $2,500 for this speaking engagement.
References
- 1.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science 1999;284:1168–1170. [DOI] [PubMed] [Google Scholar]
- 2.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 2000;31:235–240. [DOI] [PubMed] [Google Scholar]
- 3.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol 2004;6:532–539. [DOI] [PubMed] [Google Scholar]
- 4.Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun 2000;279:500–504. [DOI] [PubMed] [Google Scholar]
- 5.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000;6:1229–1234. [DOI] [PubMed] [Google Scholar]
- 6.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 7.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 8.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 2004;556:249–252. [DOI] [PubMed] [Google Scholar]
- 9.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult marrow–derived cells. Am J Respir Crit Care Med 2005;173:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macpherson H, Keir P, Webb S, Samuel K, Boyle S, Bickmore W, Forrester L, Dorin J. Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo. J Cell Sci 2005;118:2441–2450. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr, Spees JL, Bertucci D, Peister A, Weiss DJ, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA 2005;102:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, Wang X, Hashimoto M, Sharp JG, Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med 2004;170:1158–1163. [DOI] [PubMed] [Google Scholar]
- 14.Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol 2004;172:1266–1272. [DOI] [PubMed] [Google Scholar]
- 15.Murakami S, Nagaya N, Itoh T, Iwase T, Fujisato T, Nishioka K, Hamada K, Kangawa K, Kimura H. Adrenomedullin regenerates alveoli and vasculature in elastase-induced pulmonary emphysema in mice. Am J Respir Crit Care Med 2005;172:581–589. [DOI] [PubMed] [Google Scholar]
- 16.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005;33:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 2002;297:2256–2259. [DOI] [PubMed] [Google Scholar]
- 18.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol 2005;33:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theise N, Henegariu O, Grove J, Jagirdar J, Kao P, Crawford J, Badve S, Saxena R, Krause D. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol 2002;30:1333–1338. [DOI] [PubMed] [Google Scholar]
- 20.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology 2000;32:11–16. [DOI] [PubMed] [Google Scholar]
- 21.Kleeberger W, Versmold A, Rothamel T, Glockner S, Bredt M, Haverich A, Lehmann U, Kreipe H. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol 2003;162:1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer H, Rampling D, Aurora P, Bonnet D, Hart SL, Jaffe A. Transbronchial biopsies provide longitudinal evidence for epithelial chimerism in children following sex mismatched lung transplantation. Thorax 2005;60:60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168: 318–322. [DOI] [PubMed] [Google Scholar]
- 24.Yamada M, Kubo H, Ishizawa K, Kobayashi S, Shinkawa M, Sasaki H. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax 2005;60:410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzog E, Van Arnam J, Hu B, Krause D. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells 2006;24:1986–1992. [DOI] [PubMed] [Google Scholar]
- 26.Bruscia E, Price J, Cheng E, Weiner S, Caputo C, Ferreira E, Egan ME, Krause DS. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice following bone marrow transplantation. PNAS 2006;103:2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003;422:897–901. [DOI] [PubMed] [Google Scholar]
- 28.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003;422:901–904. [DOI] [PubMed] [Google Scholar]
- 29.Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, Finegold M, Fleming WH, Grompe M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med 2004;10:744–748. [DOI] [PubMed] [Google Scholar]
- 30.Held PK, Al-Dhalimy M, Willenbring H, Akkari Y, Jiang S, Torimaru Y, Olson S, Fleming WH, Finegold M, Grompe M. In vivo genetic selection of renal proximal tubules. Mol Ther 2006;13:49–58. [DOI] [PubMed] [Google Scholar]
- 31.Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, Grompe M, Fleming WH, Wong MH. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci USA 2006;103:6321–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 2003;425:968–973. [DOI] [PubMed] [Google Scholar]
- 33.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 2004;305:90–93. [DOI] [PubMed] [Google Scholar]
- 34.Grove JE, Lutzko C, Priller J, Henegariu O, Theise ND, Kohn DB, Krause DS. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol 2002;27:645–651. [DOI] [PubMed] [Google Scholar]