Abstract
The incidence of finding evidence of both emphysema and pulmonary fibrosis in the same patient has received increased attention. Several investigators have found on biopsy the presence of emphysema of the upper zones and diffuse parenchymal disease with fibrosis of the lower zones of the lung, especially associated with current or previous heavy smokers. Believed previously to be two different disease mechanisms, there are now data to implicate some common pathways of cell and molecular activation leading to the different morphologic and physiologic outcomes. According to a current view, emphysema may originate from a protease/antiprotease imbalance, whereas a role for antiproteases has been proposed in the modulation of fibrosis. Overexpression of transforming growth factor β (TGF-β) in experimental rodent models leads to progressive pulmonary fibrosis, accompanied with marked up-regulation of protease inhibitors, such as tissue inhibitor of metalloproteinases (TIMP) and plasminogen activator inhibitor-1 (PAI-1) genes, along with excessive matrix accumulation. It may be that a “matrix degrading” pulmonary microenvironment, one in which metalloproteinase activities prevail, favors the development of emphysema, whereas a “matrix nondegrading” microenvironment, with enhanced presence of TIMPs, would lead to matrix accumulation and fibrosis. Surprisingly, although Smad3 null mice, deficient in TGF-β signal transmission, are resistant to bleomycin- and TGF-β–mediated fibrosis, they develop spontaneous age-related airspace enlargement, consistent with emphysema, with a lack of ability to repair tissue damage appropriately. A common element is tissue damage and repair, with TGF-β and the Smad signaling pathway playing prominent molecular roles. Both changes can be followed in experimental models with noninvasive imaging and physiologic measurements.
Keywords: chronic obstructive pulmonary disease, emphysema, fibrosis, Smad, transforming growth factor β
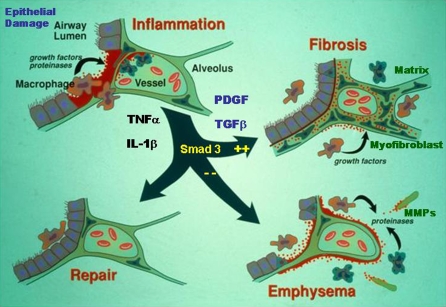
One of the important aspects of the host response to tissue injury is the immediate and crucial engagement of the inflammatory response as the host strives to limit the tissue damage and initiate tissue repair to return the organ to normal function. When one considers the lung and airway, it is damage to the epithelium that appears to initiate the process of inflammation and repair. As the epithelium undergoes damage, with ensuing necrosis and/or apoptosis, there are a number of factors that are released into the tissue and which initiate cell and molecular responses. Prominent factors include chemokines, such as interleukin 8 (IL-8) and monocyte chemotactic peptide 1 (MCP-1), and the highly inflammatory cytokines tumor necrosis factor α (TNF-α) and IL-1β. Although these are believed to be released mostly from activated macrophages, the parenchymal cells and the epithelium represent a very significant source of these factors, particularly in the lung. With the influx of granulocytes and monocytes, and the more recently described circulating fibrocyte and/or epithelial cell precursor cells (1–6), the inflammatory response provides further stimulation and subsequent release of repair or growth factors, such as vascular endothelial growth factor, keratinocyte growth factor, nerve growth factor, platelet-derived growth factor, and transforming growth factor β (TGF-β), all aimed at repairing the damage and restoring the epithelium and vascular and nerve connections, while sealing off the damaged areas from further insult. What usually ensues is the return of the lung to normal function; on some occasions, however, the repair function continues and leads to chronic scarring or fibrosis, whereas on other occasions, the repair function fails and damage to the alveolar walls continues with development of emphysema and chronic obstructive pulmonary disease (COPD; Figure 1). Although inflammation has been believed to be a central issue in both fibrosis and emphysema, the two disorders were believed to be fundamentally different in downstream pathogenesis—one a mechanism of enhanced matrix deposition, the other an imbalance of protease activity over inhibition. It is only recently that these two disorders have been recognized to exist in the same lung, in both human and animal models of disease, which raises the issue of overlapping mechanisms of progression or common pathways of activation.
Figure 1.
Initial epithelial injury is associated with inflammation and release of various cytokines and growth factors. The response to the balance of the released cytokines generates either a “matrix degrading” or “matrix nondegrading” microenvironment in which repair, emphysema, or fibrosis might occur. IL = interleukin; PDGF = platelet-derived growth factor; TNF = tumor necrosis factor. Adapted by permission from Bonner JC (59).
COPD AND FIBROSIS CAN COEXIST
Pulmonary fibrosis, particularly idiopathic pulmonary fibrosis (IPF), also known as cryptogenic fibrosing alveolitis, is a chronic, progressive, fatal disorder of the lung associated with alveolitis and enhanced deposition of extracellular matrix, including collagen and fibronectin, within the parenchyma. There is damage to the alveolar epithelium and the presence of an altered fibroblast phenotype, the myofibroblast, seen as a parenchymal cell that expresses contractile elements, such as α-smooth muscle actin (7), and is typical of the process of chronic fibrogenesis (8–11). In IPF, myofibroblasts and fibroblasts are found in subepithelial aggregations, or fibroblastic foci, and these elements are believed to be both diagnostic of IPF and the sites of major matrix production and deposition. The presence of fibroblastic foci, usually associated with areas of alveolar epithelial damage, is seen as a critical element in prognosis of IPF and correlates with survival (12, 13). The sources of the fibroblast/myofibroblast in fibroblastic foci are believed to be local dividing and differentiating fibroblasts, circulating bone marrow mesenchymal cell precursors (1–3), or the more recently described epithelial–mesenchymal transition cells (14, 15). Whether the circulation pool of mesenchymal precursor cells is beneficial or detrimental in IPF is unknown.
The majority of patients with emphysema or IPF show morphologic features specific to their disease, with enlarged alveolar airspace in emphysema and deposition of matrix components in the parenchyma in IPF. However, a recent series of studies has called attention to the presence of both fibrosis and emphysema in lungs of some patients with a diagnosis of IPF, particularly in smokers with severe dyspnea on exertion. The fibrosis tends to be found in the lower zones of the lung, whereas the emphysematous changes appear to localize in the upper regions of the lung (16–19). To the extent that the presence of emphysema confounds pulmonary function indices, a composite physiologic index has been suggested as a way of compensating for the presence of COPD and IPF (17, 19). The presence of both processes in the same lung is not easily explained with current understanding of the pathobiology of these disorders (see below), and in IPF it is critical to differentiate traction bronchiectasis and honeycomb cysts from true emphysematous changes. However, although it makes sense that fibrotic lung tissue has high collagen content, it is also known that emphysematous lung tissue has an equally raised collagen expression, and gene expression studies show matrix-associated genes are up-regulated in both COPD and lung fibrosis (20–22). Moreover, although the existence of both processes in the same lung in adults may have eluded detection until recently, there is ample evidence in human neonates with bronchopulmonary dysplasia that adjacent tissues within the lung can show evidence of dense fibrogenesis and greatly enlarged alveolar spaces, similar to emphysema (23); and in animal models of fibrosis, emphysematous changes appear independent of traction bronchiectasis (24).
The presence of both emphysema and fibrosis is not restricted to humans, as seen in a recent study of TNF-α transgenic mice, bred to overexpress TNF-α from the surfactant C promoter, with pathologic changes consistent with fibrosis and emphysema (25). Our own and others' work using gene transfer of active TGF-β1 to the lung of neonatal rats also demonstrates the induction of a phenotype that is identical to human bronchopulmonary dysplasia, with fibrosis and emphysema being found in adjacent parts of the lung (26, 27). In addition, studies with DBA/2 mice and a smoking regime showed they developed evidence of both emphysema and fibrosis and that neutrophil elastase may be a “missing link” between these two disease processes (24). Similar to issues surrounding circulating mesenchymal progenitor cells in IPF, there are reports that endothelial progenitors may be decreased in patients with COPD, suggesting some common links with precursor cell participation in the two disorders (28). Taken together, these data suggest that smoking may also be a common element linking fibrosis with emphysema (29) and this raises issues regarding common mediators, signaling systems, and/or cellular elements in the pathogenesis of these disorders. We believe the data outlined below provide such a link through TGF-β and the Smad signaling pathway to explain aspects of microenvironments that may enhance chronic damage and/or overrepair being present in the same tissue.
TGF-β IN IPF
From early studies with immunohistochemistry and in situ hybridization on human IPF lung biopsies (7, 30, 31) and on experimental fibrosis in rats (32), it is clear that TGF-β plays an important, if not pivotal, role in IPF. Direct evidence for this role has been provided by transgenic studies, both with a controlled lung tissue–specific expression system in mice (33) and our own work with adenovirus vectors used to transfect epithelial cells in vivo in rats and express active TGF-β for a period of 7 to 10 d (34–36). It is clear from these data that the presence of active TGF-β at enhanced levels and for extended periods of time will induce a progressive fibrosis, with chronic matrix deposition, parenchymal tissue distortion with “honeycombing,” induction of “fibroblastic foci” within the lung, and compromise to lung function (34). Although there are a number of other interleukins, growth factors and chemokines found in IPF tissue, TGF-β appears the likely common element through which tissue damage results in tissue repair.
In this regard, it is important to note that TGF-β is synthesized as a large precursor molecule that is cleaved within the intracellular environment by enzymes such as furin to yield the active form and a latent associated peptide (LAP) (37). The cleaved products homodimerize and link covalently, but remain associated with each other in the extracellular space. Here the latent molecule associates further through a covalent linkage to a latent TGF-β binding protein (LTBP) and this large complex (TGF-β–LAP-LTBP) is incorporated into the extracellular matrix where it remains as a stored element (38–40). Activation of this complex to release the active form of TGF-β appears to be cell specific and may be accomplished in several ways. These include alteration by integrins, such as αvβ6; involvement of proteases, including plasmin, metalloproteinase 9, elastase, or cathepsins; and the binding of thrombospondin, all able to release active TGF-β from the matrix microenvironment around the cell (40–45). The fact that proteases such as elastase may be involved in release of TGF-β as well as directly damaging alveolar elastin elements should provide further stimulus to understand the differing outcome of involvement leading to fibrosis or emphysema (24).
MECHANISMS OF PROGRESSIVE FIBROSIS
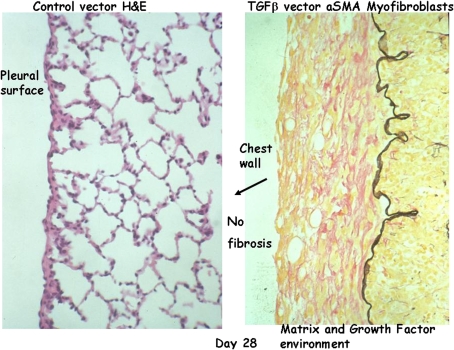
When active TGF-β is expressed from an adenovirus vector in the lungs of rats, we see the induction of altered alveolar and parenchymal structures, enhanced matrix deposition, development of “honeycomb” lung, and the presence of myofibroblast and fibroblast foci under the epithelium (34–36). The process begins within 1 or 2 d in the peribronchial tissues where the active TGF-β is being secreted into the extracellular environment. Over the period of a few days, the involvement gradually extends to the entire lung, eventually including the pleural surface of the lung, where extensive thickening begins to be seen around 10 to 15 d after transfection of the bronchial epithelium with the vector (Figure 2). Eventually, the entire lung is involved in this progressive fibrogenic response. Although this model demonstrates a chronic progressive element to the fibrosis and implicates TGF-β in the pathogenesis of IPF, one must recognize the highly elevated TGF-β presence for prolonged periods in the lung of experimental animals and thus the relationship of the model to human IPF remains limited.
Figure 2.
Lung histology sections of control vector (left panel) and AdTGF-β1 (right panel) rats 28 d after administration of the vector. Severe pleural fibrosis is observed at Day 28 (right panel) compared with control rats (left panel). However, no fibrosis can be observed on the chest wall, indicating that the fibrogenic signals do not transfer across a barrier to adjacent tissues or organs. H&E = hematoxylin and eosin; TGF = transforming growth factor.
However, one notes that there is both a temporal and spatial relationship in the development of this pleural thickening, which suggests mechanisms for progressive fibrosis. The episode (TGF-β release) that causes the response (pleural thickening) occurred some considerable time and distance (bronchial epithelium) away, and there is no evidence that tissues other than the lung are involved. Indeed, most, if not all, fibrotic disorders appear to be limited to one organ (e.g., pulmonary fibrosis does not induce liver fibrosis or renal fibrosis or vice versa), suggesting the mechanism of progression needs to account for this limited distribution. Moreover, when we examine the lung and adjacent tissues, we note that the chest wall abutting the thickened pleura is not itself thickened and thus the mechanism of progressive fibrosis needs to account for the inability to cross “boundaries,” and argues for a mechanism involving pathways other than soluble factor release and simple diffusion.
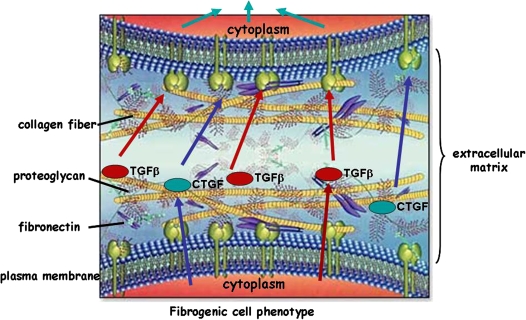
We propose an explanation as outlined in Figure 3. Here, a “fibrogenic” cell (one that has already been switched to a phenotype involved in fibrosis; i.e., enhanced matrix activation and expression of cytokine and growth factors) releases factors including TGF-β and connective tissue growth factor (CTGF), a downstream gene from TGF-β stimulation involved in matrix regulation. Parenchymal cells are never found in an isolated state; rather, they are tethered to the matrix. TGF-β and CTGF are known to have strong affinity for matrix molecules and are normally found tightly bound to them such that the enhanced content of growth factors adjacent to the surface of the “fibrogenic” cell would allow the signal for growth and matrix induction to be passed to a contiguous cell, thereby inducing the second cell to become “fibrogenic.” Growth factors are known to be more efficiently presented to receptors in the context of matrix proteins, and the altered matrix deposition could result in greater concentration of growth factors in one area versus another (profibrotic microenvironment) through these binding mechanisms. In this manner, the process of progressive fibrosis can account for both temporal and spatial restrictions and also explain the inability of the pathogenic process to cross a physical or spatial barrier, thus restricting the progression to a single organ. Moreover, it would be possible for matrix alterations to create local profibrotic microenvironments in some lung tissue while tissue destruction is ongoing in other parts, resulting in simultaneous presence of alveolar damage and emphysema-like lesions. Obviously, the coexistence of two such different microenvironments would not be a common occurrence but could explain the presence of fibrosis and emphysema in those limited instances seen in humans.
Figure 3.
Fibrogenic parenchymal cells, tethered to extracellular matrix, release and activate cytokines and growth factors that can be efficiently presented in the context of matrix by contiguous cells, inducing a fibrogenic phenotype to progress from one cell to the next. CTGF = connective tis-sue growth factor. Modified by permission from Biomedica Laboratories.
INFLAMMATION AND PROGRESSION IN FIBROSIS
Historically and realistically, inflammatory responses are believed to drive the repair response and contribute in an ongoing manner to the chronic phase of disease as depicted in Figure 1 (6, 10, 46). However, although there is considerable evidence linking inflammation to initiation of fibrosis, there is debate as to the role played by these mechanisms in chronic progression as seen in IPF (11, 35, 36, 47). We have addressed this directly by administration of an adenovirus vector expressing IL-1β to the lungs of rats (48), bypassing the systems needed to initiate tissue injury and exposing the lung to an inflammatory and profibrotic cytokine. In these studies, we saw that IL-1β overexpression caused extensive tissue damage and inflammation. This cytokine, alone among many we have studied, caused very extensive tissue disruption and led to ongoing progressive fibrosis of the lung, with similar features of honeycombing and fibroblastic foci, as seen with TGF-β expression, out to 60 d (48). The progression extended well beyond the time when IL-1β was expressed (the vector system leading to only a transient—up to 10 d—expression of transgene). Examination of many factors showed only TGF-β in the lung was extendedly up-regulated and suggests that IL-1β can induce inflammation and progressive fibrosis, but is likely mediated by TGF-β (36).
TGF-β AND Smad SIGNALING IN FIBROSIS
With the emphasis on TGF-β as the important “coalescent” mediator in the progression to fibrosis, it is important to examine downstream events that may clarify how extracellular events lead to fibroblast phenotype alteration and matrix gene regulation. Binding of the TGF-β active form to the type II TGF-β receptor (TGF-βRII) leads to a dimer of TGF-βRII assembling with a dimer of TGF-βRI, both of which are transmembrane serine/threonine kinase receptors. TGF-βRI is also known as activin-like kinase 5 (ALK5), and within the heterotetrameric complex, the kinase domain of ALK5 is phosphorylated by TGF-βRII. Subsequently, ALK5 phosphorylates and activates the intracellular receptor-activated (R) Smads (Smad2 and Smad3). These then interact with the comediator, Smad4, to enter the nucleus and provide activation for a series of genes involved in matrix expression and cell differentiation and proliferation (49–51). It is important to note that TGF-β signals exclusively through ALK5 in fibroblasts, although ALK1 in endothelial cells and ALK2 in epithelial cells have been shown to also engage TGF-β (52). We have used two approaches to show the critical involvement of the Smad pathway in fibrosis. First, we have had access to a small-molecular-weight, orally acting inhibitor of the TGF-βRI (ALK5) kinase. This drug is a potent inhibitor of the kinase and blocks TGF-β–mediated fibrosis in the lung when administered to rats undergoing either bleomycin- or gene vector–initiated fibrosis (53). Second, we have used the Smad3 null mouse to demonstrate that, in the absence of Smad3, the ALK5 receptor cannot transmit message through to the nucleus and thus cannot up-regulate matrix gene expression in either bleomycin or TGF-β gene–mediated fibrotic stimulation (54, 55). Taken together, these data indicate that only when there is an intact TGF-βRI signaling mechanism and/or intact Smad3 signaling can the progressive nature of fibrosis proceed.
INFLAMMATION AND THE Smad3 PATHWAY
The data above confirmed the central role of TGF-β and the Smad pathway for proceeding to progressive fibrosis; however, we sought to clarify whether inflammation could bypass this restriction and lead to fibrosis through alternate pathways of activation and/or signaling. This was done by administration of the adenovirus vector expressing IL-1β to the lung of Smad3 null mice. In these animals, the administration of IL-1β caused extensive inflammation and tissue damage in both the Smad3 null as well as wild-type mice. If anything, the Smad3 null mouse showed an even greater degree of inflammation. However, after 20 d, only the wild-type animal had progressed to fibrosis, whereas the Smad3 null mouse had no indication of fibrogenesis and was not markedly different from animals receiving only control vector (56). In the wild-type animals, there was extensive evidence of Smad2/3 phosphorylation, as seen by immunohistochemistry on lung tissue, as well as indication of the presence of TGF-β in the lung during progressive fibrosis. No such changes were seen in the Smad3 null mouse. Thus, despite an equivalent, or even enhanced, ability to generate inflammation, the progression from inflammation to fibrosis depends on the presence of an intact Smad signaling pathway and confirms that inflammation is linked to fibrosis through participation of TGF-β in the parenchymal tissue response.
EMPHYSEMA AND Smad3 SIGNALING
To this point, we have shown that TGF-β and the Smad signaling pathway are critical elements of progression from inflammation to chronic fibrosis. Lack of Smad signaling capacity is protective from fibrosis. However, despite the Smad3 null mutation being viable (the animals suffer from altered bone metabolism and some immune dysfunction), a Smad signaling pathway appears to be critical for protection from chronic tissue damage. The Smad3 null mouse develops spontaneous and likely environmentally induced emphysema over 4 to 6 mo, with markedly increased mean linear intercept measurements of alveolar spaces developing over time (55). Examination of lung tissue at 4 mo showed a markedly up-regulated expression and enhanced activity of matrix metalloproteinase 9 (MMP-9) and MMP-12, each of which can cause damage to alveolar structures. MMP-12 is mainly expressed in alveolar macrophage in the lung and TGF-β is known to suppress expression of this inhibitor in macrophages under normal circumstances. During normal development as the mice age in conventional animal quarters, the lung is likely exposed to levels of dust and particulate (e.g., bedding) that can activate tissue damage mechanisms, and without the proper signaling systems, these microtraumas may not be repaired properly, resulting in age-related progressive airspace enlargement. Notably, in these animals, the changes occur throughout the lung and studies with a separate Smad3 null mutation implied some element of developmental impairment in alveolar structure, but these studies also showed a progressive emphysema-like change to the lung over time (57). That TGF-β and the Smad pathway are involved in response to damage and repair is also seen in the αvβ6 integrin null mouse (a matrix interactive integrin), which is compromised in the ability to activate latent TGF-β and also develops an emphysema-like response, albeit at a slower rate than the Smad3 null mouse (58). This highlights the role that matrix (probably altered) plays in activation of growth factors and also provides a direct signaling system to regulate parenchymal cell differentiation.
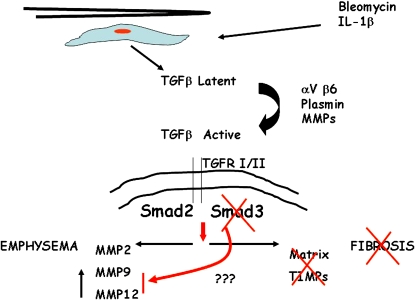
Figures 3 and 4 show a summary of the interactions between inflammation, TGF-β activation, and Smad3 signaling and the disorders of fibrosis and emphysema. However the injury or insult is initiated, mesenchymal and epithelial cells appear to respond and express enhanced levels of latent TGF-β. Activation of this mediator leads to binding with TGF-βRI/II and kinase activity with phosphorylation of Smad2/3. In the absence of Smad3 or blockade of TGF-βRII kinase activity, there is no progression to fibrosis and there are no enhanced levels of either matrix deposition or antiproteases, such as tissue inhibitors of metalloproteinases (TIMPs). This lack of inhibitory microenvironment means that, even if matrix was deposited, it would be digested readily and thus not remain as scar tissue. Smad3 signaling is crucial for progression to fibrosis. On the other hand, absence of proper Smad3 signaling results in an ineffective repair response to damage in the lung, reduction of suppression of expression of potent MMPs, and susceptibility to airspace enlargement and emphysema. Other members of the Smad pathway may be involved, but because Smad2 null mice do not survive birth, one can only speculate about the role for this factor in adult lung disease. These data from animal models lead us to speculate on the potential role for deficiencies (genetic or acquired) in human fibrotic lung disease. There may be a few individuals with single gene modifications to Smad pathways, other TGF-β pathways, or other receptor systems, but most likely it will be a combination of genetic and environmental factors that then contribute to progressive fibrosis and/or emphysema. Whether such mutations or polymorphisms could account for the presence of both pathologic changes in the same lung will remain speculative until true animal models of such alterations can be examined.
Figure 4.
Bleomycin and IL-1β induces expression of TGF-β, which after activation, binds to its receptor and transphosphorylates Smad2 and Smad3, inducing a fibrotic response in experimental models. Animals deficient in Smad 3 are protected from fibrosis but susceptible to emphysema. MMP = matrix metalloproteinase.
FIBROSIS AND COPD: TWO DISEASES BUT SOME COMMON PATHWAYS
Inflammation is a critical element of the host response to infection or injury, which provides a protective, but sometimes pathologic, outcome. The Smad pathway for signal transduction is a critical element involved in this response. The pathway (and its primary ligand, TGF-β) mediates the switch from inflammation to chronic progressive fibrosis, whereas impaired Smad function could lead to chronic progressive emphysema. It will be of interest to examine allelic variation in aspects of the Smad signaling cascade to see if relationships exist in either fibrosis or emphysema.
With new approaches to small-animal imaging and functional measures of pulmonary physiology in rodents, we are now able to follow these progressive changes in a noninvasive manner and correlate the quantitative changes seen on morphologic examination with functional and image analysis. This will help correlate rodent models of disease with human disorders and allow us to further investigate the relationship between these disorders that appear at first to be unrelated, but on examination of processes as outlined above may be highly related, and discover alternate targets for therapeutic intervention in both diseases.
Supported by research grants from the Canadian Institutes for Health Research and NIH NHLBI PO1 HL60231 (D.W.).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 2006;35:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomperts BN, Belperio JA, Rao PN, Randell SH, Fishbein MC, Burdick MD, Strieter RM. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol 2006;176: 1916–1927. [DOI] [PubMed] [Google Scholar]
- 5.Albera C, Polak JM, Janes S, Griffiths MJ, Alison MR, Wright NA, Navaratnarasah S, Poulsom R, Jeffery R, Fisher C, et al. Repopulation of human pulmonary epithelium by bone marrow cells: a potential means to promote repair. Tissue Eng 2005;11:1115–1121. [DOI] [PubMed] [Google Scholar]
- 6.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 2001;345:517–525. [DOI] [PubMed] [Google Scholar]
- 7.Kapanci Y, Desmouliere A, Pache JC, Redard M, Gabbiani G. Cytoskeletal protein modulation in pulmonary alveolar myofibroblasts during idiopathic pulmonary fibrosis: possible role of transforming growth factor β and tumor necrosis factor α. Am J Respir Crit Care Med 1995;152:2163–2169. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society/European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2001;165:277–304. [DOI] [PubMed] [Google Scholar]
- 9.Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004;55:395–417. [DOI] [PubMed] [Google Scholar]
- 10.Keane MP, Strieter RM, Belperio JA. Mechanisms and mediators of pulmonary fibrosis. Crit Rev Immunol 2005;25:429–463. [DOI] [PubMed] [Google Scholar]
- 11.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol 2005;33:9–13. [DOI] [PubMed] [Google Scholar]
- 12.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998;157: 1301–1315. [DOI] [PubMed] [Google Scholar]
- 13.King TE Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001;164:1025–1032. [DOI] [PubMed] [Google Scholar]
- 14.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 2005;166:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res 2005;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005;26:586–593. [DOI] [PubMed] [Google Scholar]
- 17.Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, Colby TV, du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003;167:962–969. [DOI] [PubMed] [Google Scholar]
- 18.Wiggins J, Strickland B, Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution computed tomography in assessment. Respir Med 1990;84:365–369. [DOI] [PubMed] [Google Scholar]
- 19.Mura M, Zompatori M, Pacilli AM, Fasano L, Schiavina M, Fabbri M. The presence of emphysema further impairs physiologic function in patients with idiopathic pulmonary fibrosis. Respir Care 2006;51:257–265. [PubMed] [Google Scholar]
- 20.Lang MR, Fiaux GW, Gillooly M, Stewart JA, Hulmes DJ, Lamb D. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax 1994;49:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spira A, Beane J, Pinto-Plata V, Kadar A, Liu G, Shah V, Celli B, Brody JS. Gene expression profiling of human lung tissue from smokers with severe emphysema. Am J Respir Cell Mol Biol 2004;31:601–610. [DOI] [PubMed] [Google Scholar]
- 22.Sheppard D. Roger S. Mitchell lecture: uses of expression microarrays in studies of pulmonary fibrosis, asthma, acute lung injury, and emphysema. Chest 2002;121:21S–25S. [DOI] [PubMed] [Google Scholar]
- 23.Erickson AM, de la Monte SM, Moore GW, Hutchins GM. The progression of morphologic changes in bronchopulmonary dysplasia. Am J Pathol 1987;127:474–484. [PMC free article] [PubMed] [Google Scholar]
- 24.Lucattelli M, Bartalesi B, Cavarra E, Fineschi S, Lunghi B, Martorana PA, Lungarella G. Is neutrophil elastase the missing link between emphysema and fibrosis? Evidence from two mouse models. Respir Res 2005;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med 2005;171:1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 2003;163:2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 2004;31:650–656. [DOI] [PubMed] [Google Scholar]
- 28.Palange P, Testa U, Huertas A, Calabro L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J 2006;27:529–541. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz DA, Merchant RK, Helmers RA, Gilbert SR, Dayton CS, Hunninghake GW. The influence of cigarette smoking on lung function in patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis 1991;144:504–506. [DOI] [PubMed] [Google Scholar]
- 30.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 1991;88:6642–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalil N, O'Connor RN, Flanders KC, Unruh H. TGF-β1, but not TGF-β2 or TGF-β3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol 1996;14:131–138. [DOI] [PubMed] [Google Scholar]
- 32.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis: a combined immunohistochemical and in situ hybridization study. Am J Pathol 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997;100: 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly M, Kolb M, Bonniaud P, Gauldie J. Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des 2003;9:39–49. [DOI] [PubMed] [Google Scholar]
- 36.Gauldie J, Kolb M, Sime PJ. A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respir Res 2002;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubois CM, Blanchette F, Laprise MH, Leduc R, Grondin F, Seidah NG. Evidence that furin is an authentic transforming growth factor-beta1-converting enzyme. Am J Pathol 2001;158:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nunes I, Gleizes PJ, Metz CN, Rifkin DB. Latent transforming growth factor-beta binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-beta. J Cell Biol 1997;136:1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taipale J, Koli K, Keski-Oja J. Release of transforming growth factor-beta 1 from the pericellular matrix of cultured fibroblasts and fibrosarcoma cells by plasmin and thrombin. J Biol Chem 1992;267:25378–25384. [PubMed] [Google Scholar]
- 40.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003;116:217–224. [DOI] [PubMed] [Google Scholar]
- 41.Khalil N, Parekh TV, O'Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax 2001;56:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem 1999;274:13586–13593. [DOI] [PubMed] [Google Scholar]
- 43.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol 1990;110:1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 45.Khalil N, Corne S, Whitman C, Yacyshyn H. Plasmin regulates the activation of cell-associated latent TGF-beta 1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am J Respir Cell Mol Biol 1996;15:252–259. [DOI] [PubMed] [Google Scholar]
- 46.Strieter RM. Pathogenesis and natural history of usual interstitial pneumonia: the whole story or the last chapter of a long novel. Chest 2005;128:526S–532S. [DOI] [PubMed] [Google Scholar]
- 47.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. [DOI] [PubMed] [Google Scholar]
- 48.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 2001;107:1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997;390:465–471. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- 51.Mehra A, Wrana JL. TGF-beta and the Smad signal transduction pathway. I. 2002;80:605–622. [DOI] [PubMed]
- 52.Karlsson G, Liu Y, Larsson J, Goumans MJ, Lee JS, Thorgeirsson SS, Ringner M, Karlsson S. Gene expression profiling demonstrates that TGF-beta1 signals exclusively through receptor complexes involving Alk5 and identifies targets of TGF-beta signaling. Physiol Genomics 2005;21:396–403. [DOI] [PubMed] [Google Scholar]
- 53.Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, et al. Progressive transforming growth factor β1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med 2005;171:889–898. [DOI] [PubMed] [Google Scholar]
- 54.Zhao J, Shi W, Wang YL, Chen H, Bringas P Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2002;282:L585–L593. [DOI] [PubMed] [Google Scholar]
- 55.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol 2004;173:2099–2108. [DOI] [PubMed] [Google Scholar]
- 56.Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol 2005;175:5390–5395. [DOI] [PubMed] [Google Scholar]
- 57.Chen H, Ye H, Zhen Y, Zhang Z, Cai Q, Chen Q. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am J Physiol Lung Cell Mol Physiol 2005;288:L683–L691. [DOI] [PubMed] [Google Scholar]
- 58.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 2003; 422:169–173. [DOI] [PubMed] [Google Scholar]
- 59.Smart RC, Hodgson E, editors. Introduction to molecular and biochemical toxicology, 4th ed. New York: Wiley (In press)