Abstract
Classically, emphysema has been believed to develop when mediators of tissue injury exceed protective mechanisms within the lung. Evidence also supports the concept that tissue destruction represents a balance between tissue injury and tissue repair. In this context, cigarette smoke is directly toxic to cells within the lung and can impair the repair functions of fibroblasts, epithelial cells, and mesenchymal cells. This may occur in the absence of overt cytotoxicity and may result from alteration of selected biochemical pathways. A variety of repair functions can be affected, including chemotaxis, proliferation, production of extracellular matrix, and remodeling of extracellular matrix. Finally, cigarette smoke can damage DNA but can also compromise apoptosis. As a result, DNA repair mechanisms can be initiated, leading to recovery of cells that potentially contain somatic cell mutations. This pathway may contribute not only to the development of cancer but to the persistent abnormalities in tissue structure that characterize chronic obstructive pulmonary disease. Understanding the mechanisms that mediate normal tissue repair and understanding the bases for altered tissue repair in the face of cigarette smoking offer new opportunities designed to address the structural alterations that characterize chronic obstructive pulmonary disease.
Keywords: alveolarization, emphysema, remodeling, repair
Emphysema is characterized by enlargement of alveolar spaces together with destruction of alveolar walls in the absence of obvious fibrosis (1). Far and away, the most common cause of pulmonary emphysema is cigarette smoking. Cigarette smoke causes an inflammatory response in the lower respiratory tract characterized by the accumulation of pigment-laden alveolar macrophages together with recruitment of smaller numbers of neutrophils (1, 2). These activated inflammatory cells release a variety of mediators, including proteases, oxidants, and toxic peptides, which can damage lung structures and are believed to be a major cause of the tissue destruction by which emphysema is defined (1).
PATHOGENESIS OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
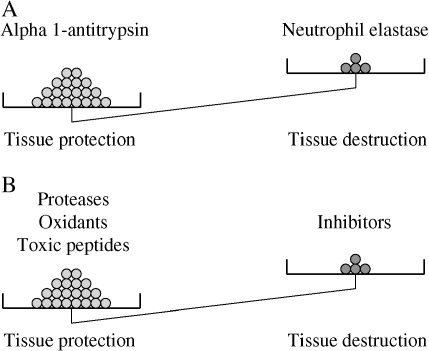
The “protease–antiprotease” hypothesis for the development of emphysema arose from the observation that patients with α1-antitrypsin deficiency are susceptible to the development of emphysema (3). This suggested the concept that tissue destruction results when protease burden—for example, from neutrophil elastase—exceeds the protective screen provided by antiproteases, such as α1-antitrysin. The concept has been expanded to include several classes of proteases and antiproteases as well as oxidants and antioxidants (Figure 1) (4, 5). A similar balance between toxic peptides, such as defensins and their inhibitors, may also play a role in chronic obstructive pulmonary disease (COPD), at least in bronchitis (6). Importantly, there are many interactions among these mediators suggesting integrated pathophysiologic roles. Although α1-antitrypsin deficiency remains the only well-characterized genetic disorder associated with COPD, a number of other candidates have been suggested (7). Among these are other antiproteases, antioxidants (7), and mutations in defensin genes (8), consistent with the concept of an imbalance between tissue injury and tissue protection.
Figure 1.
Emphysema represents an imbalance. (A) In the “classic” protease–antiprotease hypothesis of emphysema, tissue destruction resulted when neutrophil elastase overwhelmed the endogenous protection provided by α1-antitrypsin. This balance could be upset either by increasing the elastase burden (e.g., with inflammation from smoking) or by decreasing the antielastase protection (e.g., by genetic deficiency of α1-antitrypsin. (B) Expanded mediator of injury–inhibitor hypothesis. The classic concept has been expanded to include several classes of antiproteases, each with many members as well as other injurious mediators, including oxidants and toxic peptides. There is a corresponding set of inhibitors for each of these mediators, and the overall balance determines whether tissue injury results. (C) Injury repair hypothesis. Because tissues can repair injury, tissue integrity can be maintained in the face of injury if repair is adequate. Tissue destruction, therefore, represents an imbalance between tissue injury and repair.
In the face of injury, the lung, like most tissues, can initiate repair mechanisms. The net tissue destruction that characterizes emphysema represents an imbalance between tissue destruction and tissue repair processes, analogous to the protease–antiprotease balance (Figure 1). In this context, repair processes appear to be active even in the “normal” lung. The magnitude of tissue repair in the lung is suggested by the observation that as much as 5% of lung collagen can turn over in the adult animal on a daily basis (9). This suggests a rapidly exchanging pool of extracellular matrix collagen consistent with rapid tissue turnover. Maintenance of normal tissue structure requires that the amount of newly synthesized collagen balances the amount of degraded collagen, which is consistent with active repair of damaged or effete tissue in the normal lung.
PORES OF KOHN AS SITES OF INJURY AND REPAIR
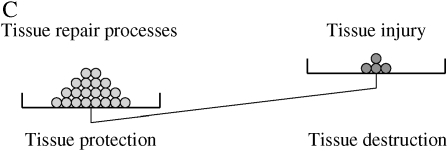
Pores of Kohn are holes in the alveolar wall that could provide channels between adjacent alveoli. Although traditionally regarded as pathways for collateral ventilation, the small size of pores of Kohn and the fact that, in most cases, they will be occluded with surfactant probably precludes them from functioning in this capacity (10, 11). Although not proven, it has been suggested that pores of Kohn represent the sites for this tissue injury/repair. The histologic description of a whole range of varying size holes with varying degrees of intact connective tissue supports the concept that these pores represent injuries that may have been repaired (12). Enlargement of pores of Kohn has also been suggested as a mechanism for cigarette smoke–induced emphysema (13, 14). Interestingly, as animals age, the number and size of the pores of Kohn increase (Figure 2) (11). This is consistent with an age-related attenuation of repair responses. Concurrent with the increase in number and size of alveolar pores, alveolar size also increases with age (15). Sometimes termed “senile emphysema,” the changes that occur in the aging lung resemble, in many respects, changes that are present in pulmonary emphysema. The degree to which these structural changes account for the physiologic changes associated with aging remains to be determined, but a relationship between elastic recoil and alveolar size has been described (16).
Figure 2.
Scanning electron micrograph of normal mouse lung. (A) Normal young adult (2 mo) C57/Bl6 mouse. (B) Normal old (24 mo) C57/Bl6 mouse. Alveolar pores can be readily observed in both preparations. They are increased in size and number in the old mouse lung. An increase in alveolar size and effacement of the alveolar walls can also be appreciated.
MESENCHYMAL CELLS
Although the cellular pathways and control mechanisms responsible for the maintenance of normal alveolar structure and for the repair of injuries, such as pores of Kohn, remain to be defined, it is likely that mesenchymal cells present in the alveolar walls play a key role. Signals derived from mesenchymal cells are crucial in the initial development and maintenance of lung branching (17, 18). In addition, retinoic acid, which is a major signal for the formation of secondary septation (19, 20), is stored in and released from alveolar mesenchymal cells (21). In addition, mesenchymal cells are believed to be the major source of extracellular matrix macromolecules both in the lung and in other organs.
INHIBITION OF MESENCHYMAL REPAIR BY CIGARETTE SMOKE
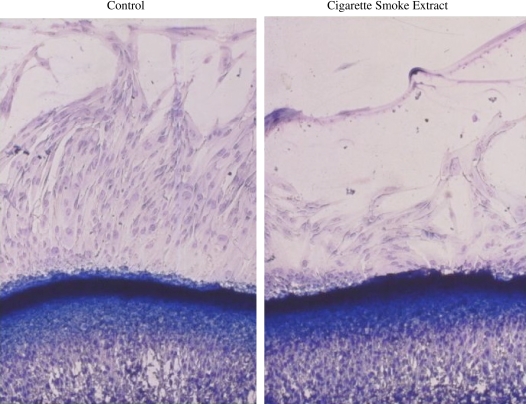
The ability of mesenchymal cells to support repair has been modeled in vitro in a number of bioassays. Importantly, cigarette smoke can inhibit these model repair responses. Cigarette smoke inhibits the ability of fibroblasts to migrate toward the chemoattractant fibronectin (Figure 3) (22). Inhibitory activity is contained in both the volatile and nonvolatile components of smoke. Two of the volatile components, acetaldehyde and acrolein, can both inhibit chemotaxis. Both acetaldehyde and acrolein individually can inhibit proliferation, as does cigarette smoke (22). Similarly, cigarette smoke inhibits the ability of fibroblasts to release the extracellular macromolecule fibronectin (23), which mediates many interactions between cells and the extracellular matrix.
Figure 3.
Cigarette smoke inhibition of fibroblast chemotaxis. Fibroblasts were plated in an agarose well and induced to migrate under the agarose toward a gradient of fibronectin contained in a separate well. Migrating cells can be recognized by the morphology as they stream along the surface of the culture dish beneath the agarose. The inhibitory effect of cigarette smoke extract is readily apparent.
A characteristic feature that may be particularly important in closing gaps such as pores of Kohn is the ability of mesenchymal cells to contract. This is a characteristic feature of mesenchymal cells in healing wounds (24). As a result of their contractile activity, mesenchymal cells cause scars to contract. Similarly, fibrotic tissues, which may be thought of as an example of excess repair, are shrunken. The ability of mesenchymal cells to contract, however, might be highly functional if it results in closure of defects in the alveolar wall, such as alveolar pores. The ability of mesenchymal cells to contract has been modeled in vitro by the culture of fibroblasts/myofibroblasts in three-dimensional collagen gels (25–27). Volatile components of cigarette smoke also inhibit this function of mesenchymal cells, and this effect is also due to volatile components of the smoke (23). Importantly, the inhibitory activities of cigarette smoke are not due to nonspecific cytotoxicity. The inhibition of fibroblast-mediated contraction of collagen gels, for example, is due, in large part, to the ability of smoke to inhibit fibronectin release. The addition of exogenous fibronectin can restore fibroblast contractility even in the presence of cigarette smoke (23).
Cigarette smoke also inhibits lung repair in animal models of emphysema. Intratracheal elastase is a widely used injury that leads to the development of emphysema in many species (28). In the hamster, the injury induced by elastase is associated with a rapid loss of lung elastin and collagen (29, 30). This is followed over the next days to weeks by the de novo synthesis and tissue accumulation of both elastin and collagen. Interestingly, the elastin content per lung returns very close to normal and the collagen content exceeds normal. This injury-induced induction of elastin synthesis contrasts markedly with the turnover of elastin in the lungs of normal animals. In support of this, Shapiro and colleagues estimated that, in the normal human lung, elastin molecules have a half-life greater than the life expectancy of the individual, suggesting very little turnover (31). The new synthesis in the face of injury, however, suggests that lung repair mechanisms can be activated. Injury-induced synthesis of elastin is also inhibited by cigarette smoke. After intratracheal elastase in the hamster, new elastin synthesis is markedly inhibited by concurrent smoke exposure (32). Moreover, the concurrent exposure of elastase and smoke results in much more severe emphysema than elastase alone, consistent with the concept that repair processes serve to mitigate the severity of the injury.
Consistent with the concept of elastic fiber repair, in vitro studies have suggested that damaged elastic fibers can be “repaired” by fibroblast activity (33, 34). Maturation of elastic fibers, and likely their repair, requires cross-linking of elastin monomers, a process dependent on the activity of the enzyme lysyl oxidase (35). This enzyme is inhibited by smoke (36), suggesting that smoke can inhibit both the polymerization and maturation of elastin as well as its production.
STARVATION AND EMPHYSEMA
Starvation has also been associated with the development of emphysema in animal models (37–39) and in humans (40, 41). Studies in the mouse suggest that lung cell apoptosis driven by T-cell–derived factors may play a role in starvation-induced emphysema (39). Refeeding is associated with reversibility of this lesion. Importantly, starvation also exacerbates the emphysema that follows intratracheal elastase infusion (42). This is consistent with a repair mechanism serving to mitigate the elastase-induced injury and that, in the face of starvation, this mechanism is unable to compensate, thus resulting in worse emphysema.
EPITHELIAL AND ENDOTHELIAL CELLS
The epithelial cells and endothelial cells present in the lung undoubtedly also participate in repair responses. In this context, cigarette smoke has well-defined toxic effects on these cells as well. Cigarette smoke is toxic to both endothelial cells (43, 44) and their circulating precursors (45). Consistent with these observations, smokers have reduced circulating vascular endothelial cell precursors (46, 47). Because death of endothelial cells can cause emphysema (48), smoke-mediated endothelial damage or impairment of endothelial cell maintenance could lead to emphysema.
Similarly, smoke inhibits airway epithelial cell chemotaxis and proliferation (49–51). When cultured on the surface of three-dimensional collagen gels, alveolar epithelial cells are capable of mediating gel contraction (52). This suggests that epithelial cells could also participate in the closure of alveolar pores. Smoke can inhibit this function of alveolar epithelial cells (53), but significantly higher concentrations of smoke are required than those that inhibit fibroblasts. Interestingly, if a fibroblast-populated three-dimensional collagen gel is floated above a monolayer of alveolar epithelial cells, the alveolar cells are able to partially protect the fibroblasts from cigarette smoke–mediated inhibition (54). This appears to be due, at least in part, to the production of glutathione by the alveolar epithelial cells (55). In this regard, alveolar epithelial cells produce relatively large amounts of glutathione, which they secrete into the extracellular space (54, 56). Although the mechanism by which cigarette smoke impairs mesenchymal cell–mediated contraction is incompletely defined, available data support an oxidant-mediated pathway (55). The ability of alveolar epithelial cells to produce and secrete glutathione could provide a means by which alveolar cells could protect fibroblast repair responses from inhibition by cigarette smoke.
FIBROSIS AND REPAIR
COPD is characterized not only by the development of emphysema but also by the development of peribronchiolar fibrosis (1, 57). Like all fibrotic lesions, the fibrosis that develops in the small airways is characterized by accumulations of fibroblasts and myofibroblasts together with the extracellular matrix they produce—largely, fibrillar collagen. This collagen is contracted and, as a result, the small airways are narrowed (58), resulting in reduction in airflow. This raises an interesting paradox: How could cigarette smoke, which inhibits fibroblast-mediated repair responses, also be associated with peribronchiolar fibrosis? Interestingly, the effect of cigarette smoke on repair may be dependent on cell density.
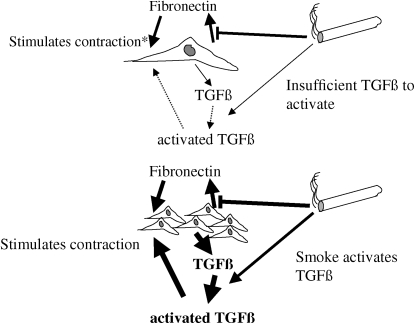
Wang and colleagues evaluated the effect of cigarette smoke on fibroblast contraction of three-dimensional collagen gels as a function of cell density (59). At low density, cigarette smoke inhibited contraction of three-dimensional collagen gels. When the gels were populated by fibroblasts at a higher density, however, cigarette smoke stimulated collagen gel contraction, although sufficiently high concentrations of cigarette smoke could inhibit contraction. Wang and colleagues demonstrated that, at high density, cigarette smoke results in activation of latent transforming growth factor (TGF)-β. The activated TGF-β subsequently stimulates fibroblast contraction of three-dimensional collagen gels.
The importance of cell density suggests a principle that may be key to repair and remodeling responses—namely, that regulation through paracrine mechanisms may be key in coordinating cell activity. In this context, the ability of fibroblasts to respond to an injury through the production or inhibition of secondary mediators such as TGF-β, fibronectin or prostaglandin E may in fact determine the nature of the repair response. The concentration of these secondary mediators will be a function of cell density. By directly inhibiting repair responses—for example, by inhibiting fibronectin production and hence contraction—while simultaneously activating TGF-β, cigarette smoke could lead to inhibition of repair at low cell density but stimulation of fibrosis at higher densities (Figure 4).
Figure 4.
Cigarette smoke modulation of repair by paracrine mechanisms: effect of cell density. (Upper panel) At low cell density, cigarette smoke inhibits fibronectin production and because fibronectin stimulates contraction, smoke inhibits contraction as well. Any effect of smoke in activating transforming growth factor (TGF)-β is inconsequential as the concentrations of TGF-β are too low to elicit a response. *Loss of stimulation causes inhibition in the presence of smoke. (Lower panel) At higher cell density, sufficient TGF-β is produced by the many cells in the local milieu to elicit a response. Smoke-induced activation of TGF-β results in augmented contraction, even in the face of direct inhibition of fibronectin production. Other paracrine factors (e.g., prostaglandin E or matrix metalloproteinases) could also interact and would also have effects that would depend on cell density.
REPAIR FUNCTIONS IN CELLS IN PATIENTS WITH COPD
Abnormal mesenchymal repair functions have been observed in fibroblasts obtained from emphysematous human lung. Holz and colleagues have demonstrated that fibroblasts cultured from lungs of patients with emphysema proliferate more slowly than do fibroblasts obtained from the lungs of similarly aged individuals without emphysema (60). Togo and Holz have extended these results to demonstrate that fibroblasts from emphysematous individuals are also less capable of responding to a chemotactic stimulus and are less potent in contracting three-dimensional collagen gels (S. Togo and O. Holz, unpublished observations).
It is unclear if the differences between the fibroblasts obtained from normal and emphysematous individuals represent underlying genetic differences and hence susceptibility to developing emphysema, or if they are an acquired defect. It is possible that cigarette smoke can contribute to acquired genetic abnormalities. The acquisition of somatic cell mutations has been believed to be a major mechanism in the pathways leading to cigarette smoke–induced cancer. The accumulation of these mutations in lung structural cells has also been suggested to contribute to COPD (61). A well-recognized toxicity of cigarette smoke is its ability to damage DNA by several mechanisms (62). In the face of this damage, however, cigarette smoke can also inhibit apoptosis (51, 63). As a result, when fibroblasts are injured by cigarette smoke, rather than undergoing programmed cell death that can protect the organism from a population of cells with a potentially altered genome, DNA repair can be initiated followed by cell recovery and subsequent proliferation. Such a mechanism could lead to the accumulation of cells within the lung of smokers that have altered gene function and thus impaired repair responses.
RESTORATION OF NORMAL TISSUE IN EMPHYSEMA
The landmark studies by Massaro and Massaro (64) clearly established that the adult mammalian lung is able to form new alveolar wall. Following the development of emphysema after elastase, treatment with all-trans-retinoic acid induced new alveolar wall formation in adult rats. These results have been repeated by other investigators (65) and in mice (66), but have been difficult to repeat in other species (67). Limited studies of all-trans-retinoic acid in human volunteers with emphysema have demonstrated safety, but have shown no evidence of alveolar repair (68). There are a number of mechanisms that could account for the difference in response to all-trans-retinoic acid in mice, rats, and humans. One of these mechanisms might be that the repair responses of the adult human lung may be relatively deficient. In this context, the ability to transplant stem cells, which can subsequently engraft into the lung (69–70), offers a potential means to restore repair functions to the lung. Limited studies of alveolar repair after elastase-induced emphysema suggest that transplanted stem cells can contribute to alveolar repair (71, 72). Such strategies could represent novel approaches designed to restore repair responses and hence to restore lung function in human emphysema.
CONCLUSIONS
In summary, pulmonary emphysema represents a balance between tissue destruction and tissue repair. It is likely that cigarette smoke causes tissue destruction through several direct and indirect pathways. Similarly, it is likely that cigarette smoke impairs repair responses. The ability to target repair responses will create novel opportunities to slow the progression of emphysema and, potentially, to restore function in the damaged lung.
Supported by NIH grant RO1 HL64088 (S.I.R.), Cytokine Modulation of Matrix Remodeling.
Conflict of Interest Statement: S.I.R. has participated as a speaker in scientific meetings and courses under the sponsorship of AstraZeneca and GlaxoSmithKline. He serves on advisory boards for Altana, AstraZeneca, Dey, GlaxoSmithKline, and Inspire. He has conducted clinical trials for AstraZeneca, Centocor, GlaxoSmithKline, Pfizer, Roche, and Sanofi. He has served as a consultant for AstraZeneca, GlaxoSmithKline, Novartis, Pfizer, and Roche. A patent is pending on the use of PDE4 inhibitors in repair; he is a coinventor of the patent owned by the University of Nebraska Medical Center. S.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. O.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Shapiro SD, Snider GL, Rennard SI. Chronic bronchitis and emphysema. In: Mason RJ, Broadus VC, Murray JF, Nadel JA, editors. Textbook of respiratory medicine. Vol. 1. Philadelphia: Elsevier; 2005. pp. 1115–1167.
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Workshop Report: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Update 2005. Available from: www.goldcopd.com [DOI] [PubMed]
- 3.Laurell CB, Eriksson S. The electrophoretic alpha 1-globulin pattern of serum in alpha 1-antitrypsin deficiency. Scand J Clin Lab Invest 1963;15:132–140. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro SD. The pathogenesis of emphysema: the elastase:antielastase hypothesis 30 years later. Proc Assoc Am Physicians 1995;107:346–352. [PubMed] [Google Scholar]
- 5.Seagrave J. Oxidative mechanisms in tobacco smoke-induced emphysema. J Toxicol Environ Health A 2000;61:69–78. [DOI] [PubMed] [Google Scholar]
- 6.Hiemstra PS, van Wetering S, Stolk J. Neutrophil serine proteinases and defensins in chronic obstructive pulmonary disease: effects on pulmonary epithelium. Eur Respir J 1998;12:1200–1208. [DOI] [PubMed] [Google Scholar]
- 7.Sandford AJ, Joos L, Pare PD. Genetic risk factors for chronic obstructive pulmonary disease. Curr Opin Pulm Med 2002;8:87–94. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita I, Hasegawa K, Nakata K, Yasuda K, Tokunaga K, Keicho N. Genetic variants of human beta-defensin-1 and chronic obstructive pulmonary disease. Biochem Biophys Res Commun 2002;291:17–22. [DOI] [PubMed] [Google Scholar]
- 9.Laurent GJ. Rates of collagen synthesis in lung, skin and muscle obtained in vivo by a simplified method using [3H]proline. Biochem J 1982;206: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzone RW, Kornblau S. Size of pores of Kohn: influence of transpulmonary and vascular pressures. J Appl Physiol 1981;51:739–745. [DOI] [PubMed] [Google Scholar]
- 11.Gillett NA, Gerlach RF, Muggenburg BA, Harkema JR, Griffith WC, Mauderly JL. Relationship between collateral flow resistance and alveolar pores in the aging beagle dog. Exp Lung Res 1989;15:709–719. [DOI] [PubMed] [Google Scholar]
- 12.Weiss MJ, Burri PH. Formation of interalveolar pores in the rat lung. Anat Rec 1996;244:481–489. [DOI] [PubMed] [Google Scholar]
- 13.Wright JL. The importance of ultramicroscopic emphysema in cigarette smoke-induced lung disease. Lung 2001;179:71–81. [DOI] [PubMed] [Google Scholar]
- 14.Lu DF, Stanley C, Nunez G, Frazer D. A mathematical description of pressures in alveolar pores of Kohn. J Biomech Eng 1991;113:104–107. [DOI] [PubMed] [Google Scholar]
- 15.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung: comparison with normal and emphysematous lungs. 1. Structural aspects. Chest 1992;101:793–799. [DOI] [PubMed] [Google Scholar]
- 16.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung: comparison with normal and emphysematous lungs. 2. Functional aspects. Chest 1992;101:800–809. [DOI] [PubMed] [Google Scholar]
- 17.Kim N, Vu TH. Parabronchial smooth muscle cells and alveolar myofibroblasts in lung development. Birth Defects Res C: Embryo Today 2006;78:80–89. [DOI] [PubMed] [Google Scholar]
- 18.Warburton D, Bellusci S, De Langhe S, Del Moral PM, Fleury V, Mailleux A, Tefft D, Unbekandt M, Wang K, Shi W. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res 2005;57:26R–37R. [DOI] [PubMed] [Google Scholar]
- 19.Maden M, Hind M. Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci 2004;359:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massaro D, Massaro GD. Retinoids, alveolus formation, and alveolar deficiency: clinical implications. Am J Respir Cell Mol Biol 2003;28: 271–274. [DOI] [PubMed] [Google Scholar]
- 21.Dirami G, Massaro GD, Clerch LB, Ryan US, Reczek PR, Massaro D. Lung retinol storing cells synthesize and secrete retinoic acid, an inducer of alveolus formation. Am J Physiol Lung Cell Mol Physiol 2004;286:L249–L256. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y, Romberger DJ, Tate L, Ertl RF, Kawamoto M, Adachi Y, Mio T, Sisson JH, Spurzem JR, Rennard SI. Cigarette smoke inhibits lung fibroblast proliferation and chemotaxis. Am J Respir Crit Care Med 1995;151:1497–1503. [DOI] [PubMed] [Google Scholar]
- 23.Carnevali S, Nakamura Y, Mio T, Liu X, Takigawa K, Romberger DJ, Spurzem JR, Rennard SI. Cigarette smoke extract inhibits fibroblast-mediated collagen gel contraction. Am J Physiol Lung Cell Mol Physiol 1998;274:L591–L598. [DOI] [PubMed] [Google Scholar]
- 24.Grinnell F. Fibroblasts, myofibroblasts and wound contraction. J Cell Biol 1994;124:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 1979;76:1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasek JJ, Haaksma CJ, Eddy RJ, Vaughan MB. Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat Rec 1992;232:359–368. [DOI] [PubMed] [Google Scholar]
- 27.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 2003;13:264–269. [DOI] [PubMed] [Google Scholar]
- 28.Snider GL, Martorana PA, Lucey EC, Lungarella G. Animal models of emphysema. In: Voelkel NF, MacNee W, editors. Chronic obstructive lung diseases. Vol. 1. Hamilton, B.C., Canada: B.C. Decker; 2002. pp. 237–256.
- 29.Kuhn C, Yu SY, Chraplyvy M. The induction of emphysema with elastase: II. Changes in connective tissue. Lab Invest 1976;34:372–380. [PubMed] [Google Scholar]
- 30.Karlinsky JB, Fredette J, Davidovits G. The balance of lung connective tissue elements in elastase-induced emphysema. J Lab Clin Med 1983;102:151–162. [PubMed] [Google Scholar]
- 31.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 1991;87:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osman M. Cigarette smoke impairs elastin resynthesis in lungs of hamsters with elastase-induced emphysema. Am Rev Respir Dis 1985;132: 640–643. [DOI] [PubMed] [Google Scholar]
- 33.Stone PJ, Morris SM, Griffin S, Mithieux S, Weiss AS. Building elastin: incorporation of recombinant human tropoelastin into extracellular matrices using nonelastogenic rat-1 fibroblasts as a source for lysyl oxidase. Am J Respir Cell Mol Biol 2001;24:733–739. [DOI] [PubMed] [Google Scholar]
- 34.Stone PJ, Morris SM, Thomas KM, Schuhwerk K, Mitchelson A. Repair of elastase-digested elastic fibers in acellular matrices by replating with neonatal rat-lung lipid interstitial fibroblasts or other elastogenic cell types. Am J Respir Cell Mol Biol 1997;17:289–301. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J 1993;7:1208–1218. [PubMed] [Google Scholar]
- 36.Laurent P, Janoff A, Kagan HM. Cigarette smoke blocks cross-linking of elastin in vitro. Am Rev Respir Dis 1983;127:189–192. [DOI] [PubMed] [Google Scholar]
- 37.Sahebjami H. Lung mechanics and ultrastructure in prolonged starvation. Am Rev Respir Dis 1978;117:77. [DOI] [PubMed] [Google Scholar]
- 38.Sahebjami H, Wirman JA. Emphysema-like changes in the lungs of starved rats. Am Rev Respir Dis 1981;124:619–624. [DOI] [PubMed] [Google Scholar]
- 39.Massaro D, Massaro GD, Baras A, Hoffman EP, Clerch LB. Calorie-related rapid onset of alveolar loss, regeneration, and changes in mouse lung gene expression. Am J Physiol Lung Cell Mol Physiol 2004;286: L896–L906. [DOI] [PubMed] [Google Scholar]
- 40.Stein J, Fenigstein H. Anatomie pathologique de la maladie de famine. In: Apfelbaum E, editor. Maladie de famine. Warsaw, Poland: American Joint Distribution Committee; 1946. pp. 21–27.
- 41.Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med 2004;170:748–752. [DOI] [PubMed] [Google Scholar]
- 42.Sahebjami H, Vassallo CL. Influence of starvation on enzyme-induced emphysema. J Appl Physiol 1980;48:284–288. [DOI] [PubMed] [Google Scholar]
- 43.Hoshino S, Yoshida M, Inoue K, Yano Y, Yanagita M, Mawatari H, Yamane H, Kijima T, Kumagai T, Osaki T, et al. Cigarette smoke extract induces endothelial cell injury via JNK pathway. Biochem Biophys Res Commun 2005;329:58–63. [DOI] [PubMed] [Google Scholar]
- 44.Nagy J, Demaster EG, Wittmann I, Shultz P, Raij L. Induction of endothelial cell injury by cigarette smoke. Endothelium 1997;5:251–263. [DOI] [PubMed] [Google Scholar]
- 45.van Grevenynghe J, Monteiro P, Gilot D, Fest T, Fardel O. Human endothelial progenitors constitute targets for environmental atherogenic polycyclic aromatic hydrocarbons. Biochem Biophys Res Commun 2006;341:763–769. [DOI] [PubMed] [Google Scholar]
- 46.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol 2004;24:1442–1447. [DOI] [PubMed] [Google Scholar]
- 47.Palange P, Testa U, Huertas A, Calabro L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J 2006;27:529–541. [DOI] [PubMed] [Google Scholar]
- 48.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantral DE, Sisson JH, Veys T, Rennard SI, Spurzem JR. Effects of cigarette smoke extract on bovine bronchial epithelial cell attachment and migration. Am J Physiol 1995;268:L723–L728. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Liu X, Umino R, Skold CM, Zhu Y, Kohyama T, Spurzem JR, Romberger DJ, Rennard SI. Cigarette smoke inhibits human bronchial epithelial cell repair processes. Am J Respir Cell Mol Biol 2001;25:772–779. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Conner H, Kobayashi T, Kim H, Wen F, Abe S, Fang Q, Wang X, Hashimoto M, Bitterman P, et al. Cigarette smoke extract induces DNA damage but not apoptosis in human bronchial epithelial cells. Am J Respir Cell Mol Biol 2005;33:121–129. [DOI] [PubMed] [Google Scholar]
- 52.Umino T, Wang H, Zhu Y, Liu X, Manouilova SL, Spurzem JR, Leuschen MP, Rennard SI. Modification of type I collagenous gels by alveolar epithelial cells. Am J Respir Cell Molec Biol 2000;22:702–707. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Umino T, Liu XD, Zhu YK, Leuschen MP, Spurzem JR, Romgerger DJ, Rennard SI. Glutathione can protect A549 from smoke induced inhibition of collagen gel contraction. Chest 2000;117:248S–249S. [PubMed] [Google Scholar]
- 54.Wang H, Umino T, Liu X, Zhu Y, Leuschen M, Rennard SI. A549 epithelial cells can protect fibroblasts from smoke induced inhibition of collagen gel contraction [abstract]. Am J Respir Crit Care Med 1999; 159:A182. [Google Scholar]
- 55.Kim HJ, Liu X, Wang H, Kohyama T, Kobayashi T, Wen FQ, Romberger DJ, Abe S, MacNee W, Rahman I, et al. Glutathione prevents inhibition of fibroblast-mediated collagen gel contraction by cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2002;283:L409–L417. [DOI] [PubMed] [Google Scholar]
- 56.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol 1987;63:152–157. [DOI] [PubMed] [Google Scholar]
- 57.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 58.Kuwano K, Bosken CH, Pare PD, Bai TR, Wiggs BR, Hogg JC. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis 1993;148:1220–1225. [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Liu X, Umino T, Kohyama T, Zhu YK, Wen FQ, Spurzem JR, Romberger DJ, Kim HJ, Rennard SI. Effect of cigarette smoke on fibroblast-mediated gel contraction is dependent on cell density. Am J Physiol Lung Cell Mol Physiol 2003;284:L205–L213. [DOI] [PubMed] [Google Scholar]
- 60.Holz O, Zuhlke I, Jaksztat E, Muller KC, Welker L, Nakashima M, Diemel KD, Branscheid D, Magnussen H, Jorres RA. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J 2004;24:575–579. [DOI] [PubMed] [Google Scholar]
- 61.Anderson GP, Bozinovski S. Acquired somatic mutations in the molecular pathogenesis of COPD. Trends Pharmacol Sci 2003;24:71–76. [DOI] [PubMed] [Google Scholar]
- 62.Rennard SI, Hepp L. Cigarette smoke induced disease. In: Stockley, RA, editor. Chronic obstructive pulmonary disease Oxford, U.K.: Blackwell. (In press)
- 63.Kim HJ, Liu XD, Kobayashi T, Conner H, Wen FQ, Kohyama T, Fang QH, Abe S, Bitterman PB, Rennard SI. Cigarette smoke induces reversible DNA damage in human fetal lung fibroblast cultured in three-dimensional collagen gels. Am J Respir Crit Care Med 2003;167: A489. [Google Scholar]
- 64.Massaro G, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med 1997;3:675–677. [DOI] [PubMed] [Google Scholar]
- 65.Belloni PN, Garvin L, Mao CP, Bailey-Healy I, Leaffer D. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest 2000;117: 235S–241S. [DOI] [PubMed] [Google Scholar]
- 66.Hind M, Maden M. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur Respir J 2004;23:20–27. [DOI] [PubMed] [Google Scholar]
- 67.Meshi B, Vitalis TZ, Ionescu D, Elliott WM, Liu C, Wang XD, Hayashi S, Hogg JC. Emphysematous lung destruction by cigarette smoke: the effects of latent adenoviral infection on the lung inflammatory response. Am J Respir Cell Mol Biol 2002;26:52–57. [DOI] [PubMed] [Google Scholar]
- 68.Mao JT, Goldin JG, Dermand J, Ibrahim G, Brown MS, Emerick A, McNitt-Gray MF, Gjertson DW, Estrada F, Tashkin DP, et al. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med 2002;165:718–723. [DOI] [PubMed] [Google Scholar]
- 69.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 70.Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS. Human pulmonary chimerism following hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:318–322. [DOI] [PubMed] [Google Scholar]
- 71.Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, Wang X, Hashimoto M, Sharp JG, Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med 2004;170:1158–1163. [DOI] [PubMed] [Google Scholar]
- 72.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 2004;556:249–252. [DOI] [PubMed] [Google Scholar]