Abstract
Apoptotic cells can be detected in the parenchyma and airways of patients with chronic obstructive pulmonary disease (COPD) in greater numbers than seen in normal lungs or those from smokers without COPD. Implications include more apoptosis and/or decreased clearance of apoptotic cells. Both epithelial and endothelial cells become apoptotic. What role does the apoptosis play in the emphysema or small airway alterations seen in COPD? In simple terms, loss of cells by apoptosis would be expected to accompany, or perhaps initiate, the overall tissue destruction normally believed responsible. Indeed, direct induction of apoptosis in pulmonary endothelial or epithelial cells in rodents is accompanied by emphysematous changes. On the other hand, apoptotic cells are normally removed from tissues rapidly with minimal tissue response, to be followed by cell replacement to maintain homeostasis. The presence of detectable apoptotic cells, therefore, may imply defects in these clearance mechanisms, and, in keeping with this hypothesis, there is increasing evidence for such defects in patients with COPD. Mice with abnormalities in apoptotic cell removal also tend to develop spontaneous “emphysema.” A reconciling hypothesis is that recognition of apoptotic cells not only leads to removal but also, normally, to signals for cell replacement. If this latter response is lacking in COPD-susceptible smokers, defects in normal alveolar or small airway repair could significantly contribute to the structural disruption. The concept puts emphasis on defective repair as well as initial injury (i.e., persistent alteration of dynamic tissue homeostasis, as a key contributor to COPD), with, it is hoped, additional approaches for mitigation.
Keywords: apoptosis, efferocytosis, growth factors, homeostasis
APOPTOSIS AND CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Examination of lung tissue from patients suffering from chronic obstructive pulmonary disease (COPD) reveals the presence of apoptotic cells in greater numbers than in control lungs or those from smokers without COPD (e.g., References 1–7). Apoptotic cells include alveolar and bronchial epithelial cells as well as endothelial cells in the parenchyma. Importantly, the apoptosis persists in patients with COPD after smoking has ceased. In rodents, deliberate induction of endothelial or epithelial apoptosis (8–10) is accompanied by loss of pulmonary alveoli and pathologic evidence of emphysematous changes.
These different types of observation raise the important question of a possible causal relationship between apoptosis (or response to this) and COPD. Simplistically, this could be no more than an indication of cell damage in the lung that would be expected within the hypothesis that emphysema is a consequence of alveolar destruction. The similar pathologic changes seen after targeting either the endothelium or epithelium could be readily reconciled by the demonstration (e.g., References 11 and 12) that there is significant interaction between the two cell layers of the alveolus so that severe damage to one could reasonably lead to disruption of the other, and presumably the matrix and interstitium as well. An alternative direction of effect in COPD could be induction of apoptosis subsequent to destruction of the supporting alveolar matrix—that is, a form of anoikis. The finding of more detectable apoptosis in smokers with COPD than in non-COPD smokers is intriguing, especially since it appears to persist after cessation of smoking in the former. The observations suggest that cigarette smoke itself is not the sole agent causing the apoptosis, or its ability to be detected, although COPD-prone individuals could conceivably be more susceptible to smoke-induced cell damage and apoptosis. An alternative explanation comes from considering the dynamics of apoptosis and cell turnover in normal tissues.
RECOGNITION AND REMOVAL OF APOPTOTIC CELLS
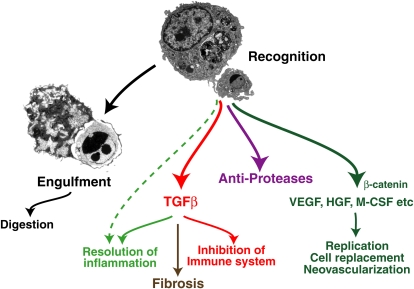
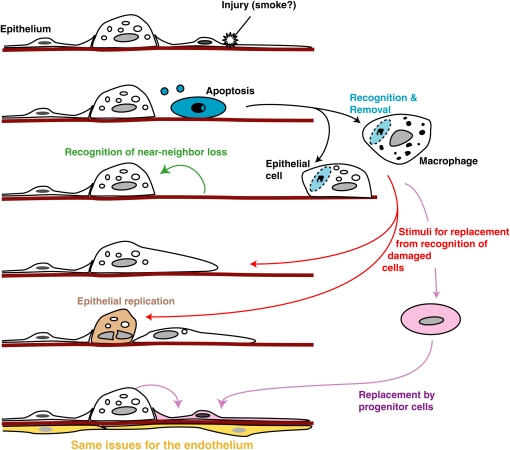
Programmed cell death, of which apoptosis is one example, is a physiologic mechanism for cell deletion. Various forms of damage, or potential damage, to cells lead to DNA fragmentation and destruction of the replicative potential of the cell as well as surface alterations that allow its recognition as “foreign.” This in turn results in uptake and subsequent digestion of the apoptotic cell by phagocytic processes with, usually, minimal release of intracellular contents from the dying cell. Cells undergoing programmed cell death can be removed rapidly and efficiently in situ with minimal tissue response by not only professional phagocytes of the mononuclear phagocyte system but also by a wide variety of tissue cell types, including fibroblasts and endothelial, epithelial, smooth muscle, and stromal cells. The uptake process is highly evolutionarily conserved, uses unique receptors and signaling pathways and has been termed “efferocytosis” (13–15). Importantly, recognition of apoptotic cells does not only lead to their removal but also initiates antiinflammatory and antiimmunogenic responses, leading to quiet removal in situ with minimal effects on the tissues (16) (Figure 1). Induction of antiproteases, such as secretory leukocyte protease inhibitor (SLPI), has also been reported (17). More recently, it is becoming clear that apoptotic cell recognition can also induce production of tissue cell growth factors or other stimuli that may, in the normal circumstance, contribute to replenishment of the damaged cell(s) (e.g., References 18 and 19). The underlying context is one of tissue homeostasis with sporadic apoptosis, leading not only to cell deletion but also providing signals for replacement. It has been suggested that most cells in the body turn over, although at different rates according to cell type. It seems likely that this is also true for the lung, especially given its exposure to the external environment, and there is increasing evidence for constituitive levels of cell death and replication in the normal adult lung (e.g., References 1 and 4) as well as substantial cell replication after specific injury (e.g., see References 20–22). The new, exciting paradigm is for some of the replacement to come from local or hematogenous “progenitor” cells (see Reference 23 and articles by Krause and Randell in this issue) and one might wonder here whether apoptotic cell recognition could also provide signals for recruitment or stimulation of such cells (Figure 2).
Figure 1.
Recognition of apoptotic cells can initiate not only uptake and digestion of the target but also production of a variety of molecules involved in regulation of inflammation and immunity as well as of potential replacement of the damaged cells. We suggest here that defects in these processes may contribute to the pathogenesis of chronic obstructive pulmonary disease. HGF = hepatocyte growth factor; M-CSF = macrophage-colony stimulating factor; TGFβ = transforming growth factor β; VEGF = vascular endothelial growth factor. Reprinted by permission from Reference 15.
Figure 2.
Possible participation of apoptotic cell recognition processes and consequences in maintenance of normal tissue homeostasis. Damage to an epithelial (or endothelial) cell leads to its becoming apoptotic. Near-neighbor loss of the cell can lead to spreading and replacement from local progenitor cells. However, recognition of the apoptotic cell by either macrophages or local tissue cells may also result in growth or attraction factors to recruit or stimulate cell replacement. We suggest here that defects in these processes may contribute to the pathogenesis of chronic obstructive pulmonary disease.
APOPTOTIC CELL CLEARANCE IN COPD
Finding apoptotic cells in histologic sections of COPD lungs, therefore, must be considered as a snapshot of dynamic processes representing a balance between a likely increased induction of cell death and a possible decrease in normal cell removal. In fact, there is increasing evidence that apoptotic clearance mechanisms are less effective in COPD lungs and that macrophages from such lungs show a defect in recognizing and ingesting such targets (e.g., References 2 and 16, T.K. Finney-Hayward and P.J. Barnes, American Thoracic Society [ATS International Conference], 2005; R.W. Vandivier and colleagues, unpublished observations). There are many potential mechanisms that could account for such a defect from alterations in apoptotic cell recognition to defects in the efferocytosis signaling pathways (15). For example, neutrophil elastase and matrix metalloproteinase 12 can both inhibit recognition of apoptotic cells in vitro by cleaving the responsible receptors on the macrophages, and the former has been implicated in a defect in apoptotic cell clearance seen in cystic fibrosis (7). This effect could tie the protease balance hypothesis for COPD into the effect on apoptotic cell uptake. The lung collectins, surfactant protein (SP)-A and SP-D, have both been shown to enhance uptake of apoptotic cells, and SP-D−/− mice exhibit a lung clearance defect in vivo (24). Decreased levels of SP-A and SP-D have been reported in smokers' lungs (25, 26) (see also Reference 27) and could therefore contribute to the observed effects on apoptotic cell removal. A third example comes from the observation that tumor necrosis factor α can inhibit apoptotic cell uptake by macrophages in vitro and clearance of apoptotic cells from the lung in vivo (K. McPhillips and colleagues, unpublished observations). Increased levels of this mediator have been reported in COPD (28–31). Cigarette smoke can directly decrease apoptotic cell uptake in vitro, and mice exposed to smoke also show clearance defects in the lungs (R.W. Vandivier and R. Keith, unpublished observations). At this point, all of these observations would, per se, do no more than suggest an association. However, important to our thesis, all of these disparate conditions of defective apoptotic cell clearance are also accompanied by evidence of emphysematous changes in mice. Thus, in addition to the well-known proemphysematous effects of proteases and cigarette smoke, SP-D−/− mice also develop spontaneous alveolar enlargement (32) as do mice overexpressing tumor necrosis factor α in the lung (33). This does not prove cause but does suggest a possible functional association and raises questions of how defects in apoptotic cell recognition and clearance might contribute to COPD pathogenesis.
The apoptotic cell clearance defect is not (only) driven by direct effects of cigarette smoke on the lung phagocytes because it persists long after smoking has been stopped (2, 5). It also appears selective for apoptotic cells (i.e., the process of efferocytosis) compared with uptake of polystyrene beads (2). Although there are a number of possible explanations for these observations, genetic alterations in cellular response to apoptotic cells seems a likely candidate, and certainly worth investigation. Along these lines, preliminary data have been reported for defective phagocytosis in monocyte-derived macrophages from patients with COPD (T.K. Finney-Hayward, L. Donnelly, and P.J. Barnes, ATS International Conference, 2005), and in our unpublished collaborative studies with Donnelly and colleagues, this may extend to uptake of apoptotic cells. If confirmed, the defect must extend through maturation of blood monocytes in vitro in the presence of non-COPD serum and suggests some primary effect on the mononuclear phagocytes.
Clearly, parenchymal cell death itself would be expected to alter alveolar and small airway structure and function. Second, an inability to effectively remove apoptotic cells would be expected to result in their post-apoptotic cytolysis (secondary necrosis) with its associated release of constituents having proinflammatory, proteolytic, and even proimmunogenic potential. Third, an inability to clear damaged endothelial or epithelial cells from their in situ position might significantly impede normal cell replacement (20). However, we are also intrigued by the possibility that there is normally a positive feedback from recognition and removal of apoptotic cells to mechanisms for replacement of such cells (Figure 1). Thus, as noted, recognition of apoptotic cells has been shown to initiate production of growth/maintenance factors for both epithelial and endothelial cells as well as to stimulate endothelial angiogenic responses by more direct effects (34). For pulmonary epithelial cells, hepatocyte growth factor (HGF) can be used as an example. This growth factor is found to be reduced in lungs from patients with COPD (35) and can restore lung structure when given to animals with alveolar loss induced by proteases (36). In the context of apoptotic cells, their recognition by macrophages leads to production of HGF (19) and so, we would predict, abnormal recognition could result in reduced local generation of this replacement factor. Feedback from recognition of apoptotic circulating leukocytes to regulation of their production in the bone marrow has also been reported (37). This observation raises the further possibility that signals from apoptotic cell recognition could contribute to stimulation, release, and/or attraction of progenitor cells for tissue replenishment (Figure 2). Such observations support the above-mentioned concept of feedback stimulation to maintain homeostasis. Other relevant molecules known to be produced in response to apoptotic cells include transforming growth factor β, which is likely the major contributor to the antiinflammatory and antiimmunogenic effects as well as to potential fibrotic changes in the small airways.
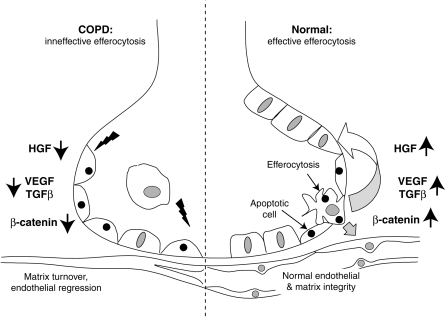
Disruption of all these effects because of defective apoptotic cell recognition would, therefore, be expected to have many consequences that impinge on the pathogenesis of COPD (Figure 3), from increased inflammation to loss of cellular repair and replenishment to increased proteases, and in some locations, fibrosis. An additional intriguing observation is the possible association between the numbers of detectable apoptotic cells in COPD lungs and the increased number of T lymphocytes (e.g., Reference 5). Whether this represents a causal, or merely associative, relationship is by no means clear. However, the possibility that reversal of a normally immunosuppressive environment in the lung due to ineffective apoptotic cell recognition (38) might contribute to local lymphocyte accumulation and immune responses seen in severe COPD, although speculative, is intriguing.
Figure 3.
A model for possible roles for defective efferocytosis and apoptotic cell recognition in generation of emphysematous alterations to the alveolus. COPD = chronic obstructive pulmonary disease.
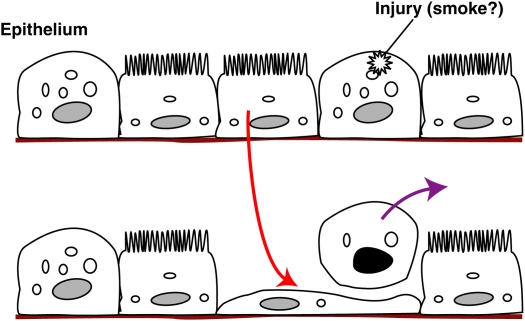
Most of the data and concepts discussed have been directed more to the lung parenchyma and emphysema than to the all-important small airway changes of COPD. Although one can speculate about similar processes and alterations participating in the latter, direct evidence of such effects is meager, but certainly worthy of future investigation. A recent study by Park and coworkers (22) suggests that damage to small airway epithelial cells is in part repaired by redifferentiation of ciliated epithelial cells, with an intermediate stage in a squamous state in which the cell underlies the damaged cell as it is extruded from the monolayer (Figure 4). This is also in keeping with the exciting evidence of manipulable transdifferentiation in the airway epithelium shown by Tyner and colleagues (35). Might there be feedback signals from the damaged and apoptotic cells for these effects? The old concept of a relationship between cellular repair and embryonic tissue plasticity may also have value here, especially as COPD tends to be a disease of the aging, and less regenerative, lung, at least in terms of the normal lifespan during most of human evolution.
Figure 4.
Alterations in small airway epithelial cells after injury and apoptosis leading to de-differentiation of ciliated cells to maintain epithelial integrity during extrusion of the damaged cells. Adapted from Reference 15.
Supported by NIH HL68864, HL81151, GM61031 (P.M.H.), HL072018 (R.W.V.), and HL070940 (I.S.D.).
Conflict of Interest Statement: P.M.H. participated as a speaker in this symposium (at which the present article was presented), which was supported by AstraZeneca. He also has received financial support for COPD studies from GlaxoSmithKline (GSK) in 2005. R.W.V. is the recipient of an ARA grant from Pfizer totaling $100,000 and a grant from GSK totaling $100,000. I.S.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 2.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 2003;81:289–296. [DOI] [PubMed] [Google Scholar]
- 3.Imai K, Mercer BA, Schulman LL, Sonett JR, D'Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J 2005;25:250–258. [DOI] [PubMed] [Google Scholar]
- 4.Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest 2004;125:626–632. [DOI] [PubMed] [Google Scholar]
- 5.Hodge S, Hodge G, Holmes M, Reynolds PN. Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur Respir J 2005;25:447–454. [DOI] [PubMed] [Google Scholar]
- 6.Majo J, Ghezzo H, Cosio MG. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur Respir J 2001;17:946–953. [DOI] [PubMed] [Google Scholar]
- 7.Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest 2002;109:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 2004;97:1559–1566. [Discussion, 1549.] [DOI] [PubMed] [Google Scholar]
- 10.Aoshiba K, Nagai A. Differences in airway remodeling between asthma and chronic obstructive pulmonary disease. Clin Rev Allergy Immunol 2004;27:35–43. [DOI] [PubMed] [Google Scholar]
- 11.Sirianni FE, Chu FS, Walker DC. Human alveolar wall fibroblasts directly link epithelial type 2 cells to capillary endothelium. Am J Respir Crit Care Med 2003;168:1532–1537. [DOI] [PubMed] [Google Scholar]
- 12.Sirianni FE, Milaninezhad A, Chu FS, Walker DC. Alteration of fibroblast architecture and loss of basal lamina apertures in human emphysematous lung. Am J Respir Crit Care Med 2006;173:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem 2003;39:105–117. [DOI] [PubMed] [Google Scholar]
- 14.Gardai SJ, McPhillips KA, Frasch C, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg P-A, Michalak M, Henson PM. Cell surface calreticulin initiates clearance of viable or apoptotic cells through trans activation of LRP on the phagocyte. Cell 2006;123:321–324. [DOI] [PubMed] [Google Scholar]
- 15.Gardai SJ, Bratton DL, Ogden CA, Henson PM. Recognition ligands on apoptotic cells: a perspective. J Leukoc Biol 2006;79:896–903. [DOI] [PubMed] [Google Scholar]
- 16.Henson PM, Cosgrove GP, Vandivier RW. Apoptosis and cell homeostasis in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odaka C, Mizuochi T, Yang J, Ding A. Murine macrophages produce secretory leukocyte protease inhibitor during clearance of apoptotic cells: implications for resolution of the inflammatory response. J Immunol 2003;171:1507–1514. [DOI] [PubMed] [Google Scholar]
- 18.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 2004;18:1716–1718. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, Nagatake T. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 2001;24:608–615. [DOI] [PubMed] [Google Scholar]
- 20.Hyde DM, Miller LA, McDonald RJ, Stovall MY, Wong V, Pinkerton KE, Wegner CD, Rothlein R, Plopper CG. Neutrophils enhance clearance of necrotic epithelial cells in ozone-induced lung injury in rhesus monkeys. Am J Physiol 1999;277:L1190–L1198. [DOI] [PubMed] [Google Scholar]
- 21.Adamson IY, Young L, Bowden DH. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol 1988;130:377–383. [PMC free article] [PubMed] [Google Scholar]
- 22.Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 2006;34:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore KA, Lemischka IR. Stem cells and their niches. Science 2006;311: 1880–1885. [DOI] [PubMed] [Google Scholar]
- 24.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol 2002;169:3978–3986. [DOI] [PubMed] [Google Scholar]
- 25.Betsuyaku T, Kuroki Y, Nagai K, Nasuhara Y, Nishimura M. Effects of ageing and smoking on SP-A and SP-D levels in bronchoalveolar lavage fluid. Eur Respir J 2004;24:964–970. [DOI] [PubMed] [Google Scholar]
- 26.Honda Y, Takahashi H, Kuroki Y, Akino T, Abe S. Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest 1996;109:1006–1009. [DOI] [PubMed] [Google Scholar]
- 27.Guo X, Lin HM, Lin Z, Montano M, Sansores R, Wang G, DiAngelo S, Pardo A, Selman M, Floros J. Surfactant protein gene A, B, and D marker alleles in chronic obstructive pulmonary disease of a Mexican population. Eur Respir J 2001;18:482–490. [DOI] [PubMed] [Google Scholar]
- 28.Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-α levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;150:1453–1455. [DOI] [PubMed] [Google Scholar]
- 29.Pitsiou G, Kyriazis G, Hatzizisi O, Argyropoulou P, Mavrofridis E, Patakas D. Tumor necrosis factor-alpha serum levels, weight loss and tissue oxygenation in chronic obstructive pulmonary disease. Respir Med 2002;96:594–598. [DOI] [PubMed] [Google Scholar]
- 30.Vernooy JH, Kucukaycan M, Jacobs JA, Chavannes NH, Buurman WA, Dentener MA, Wouters EF. Local and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors are increased in sputum. Am J Respir Crit Care Med 2002;166:1218–1224. [DOI] [PubMed] [Google Scholar]
- 31.Calikoglu M, Sahin G, Unlu A, Ozturk C, Tamer L, Ercan B, Kanik A, Atik U. Leptin and TNF-alpha levels in patients with chronic obstructive pulmonary disease and their relationship to nutritional parameters. Respiration (Herrlisheim) 2004;71:45–50. [DOI] [PubMed] [Google Scholar]
- 32.Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci USA 2000;97:5972–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, Mason RJ. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2001;280:L39–L49. [DOI] [PubMed] [Google Scholar]
- 34.Weihua Z, Tsan R, Schroit AJ, Fidler IJ. Apoptotic cells initiate endothelial cell sprouting via electrostatic signaling. Cancer Res 2005;65:11529–11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 2006;116:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shigemura N, Sawa Y, Mizuno S, Ono M, Ohta M, Nakamura T, Kaneda Y, Matsuda H. Amelioration of pulmonary emphysema by in vivo gene transfection with hepatocyte growth factor in rats. Circulation 2005;111:1407–1414. [DOI] [PubMed] [Google Scholar]
- 37.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005;22:285–294. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, Marrack P, Henson PM, Fadok VA. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol 2005;174:1393–1404. [DOI] [PubMed] [Google Scholar]