Abstract
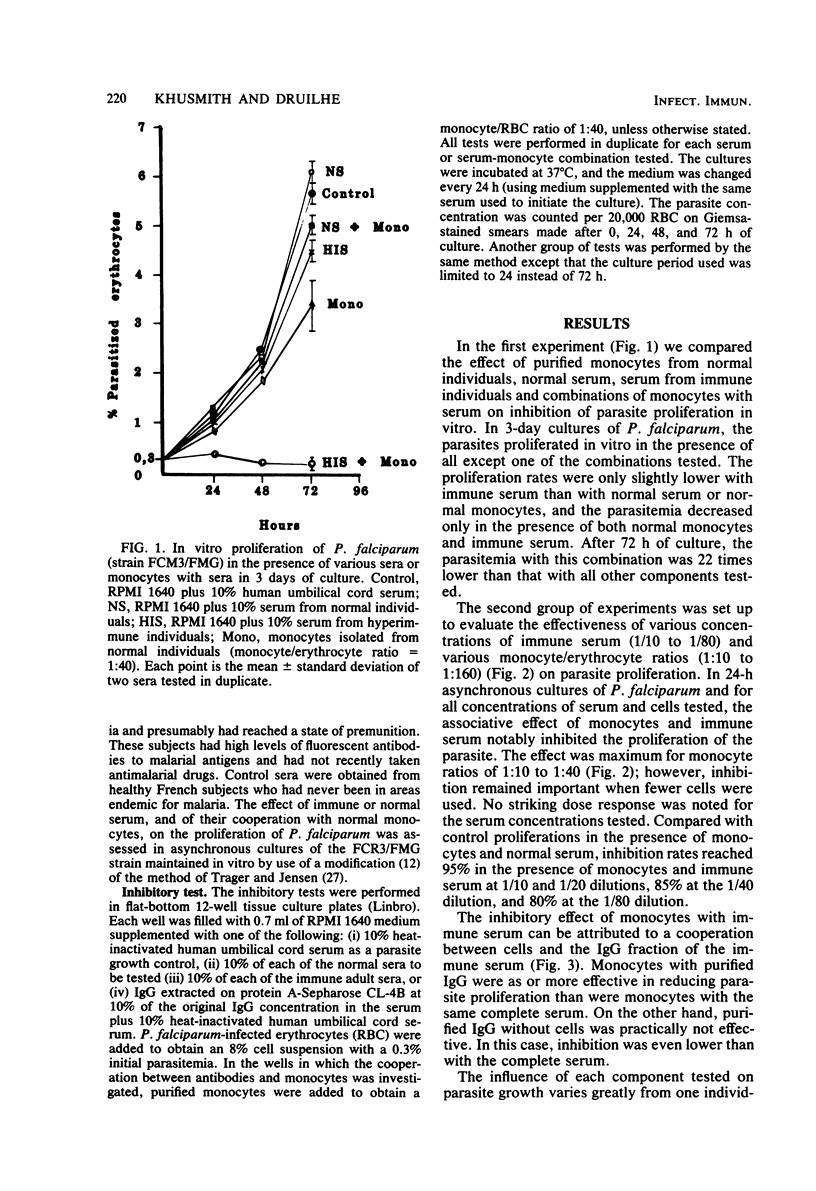
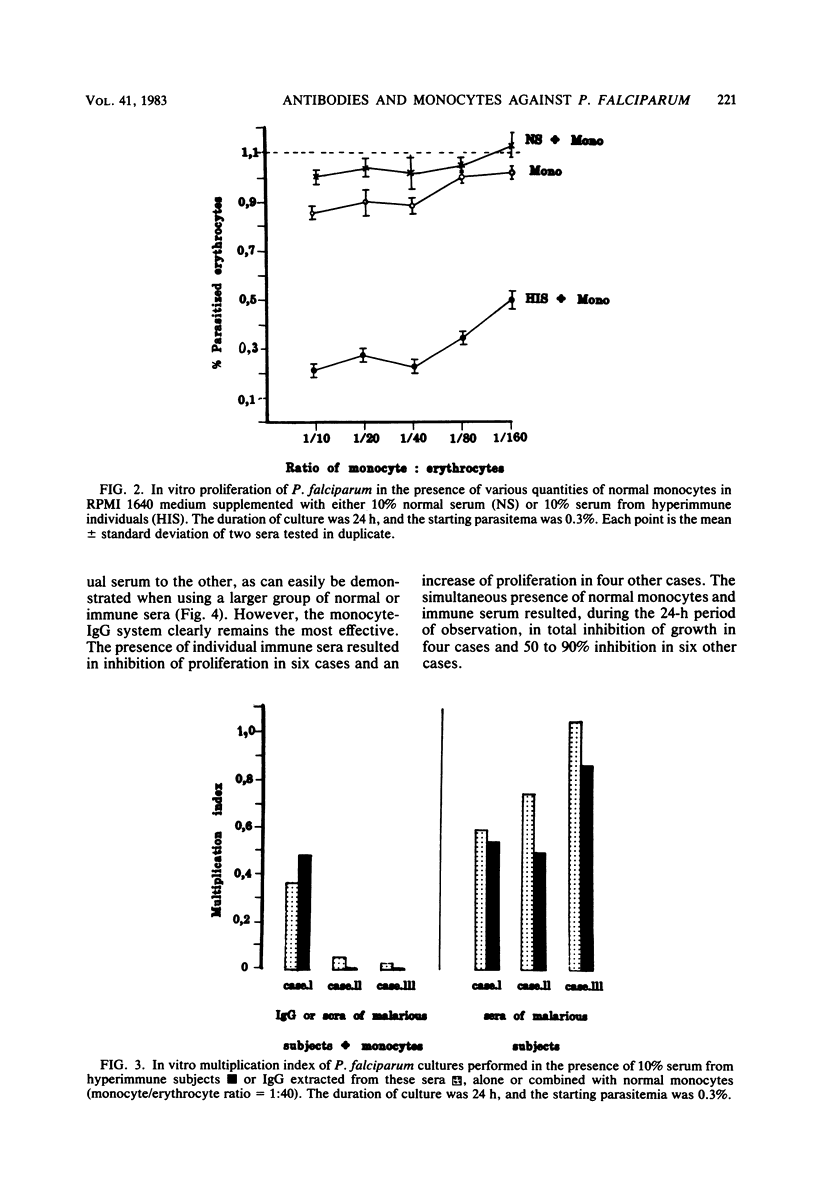
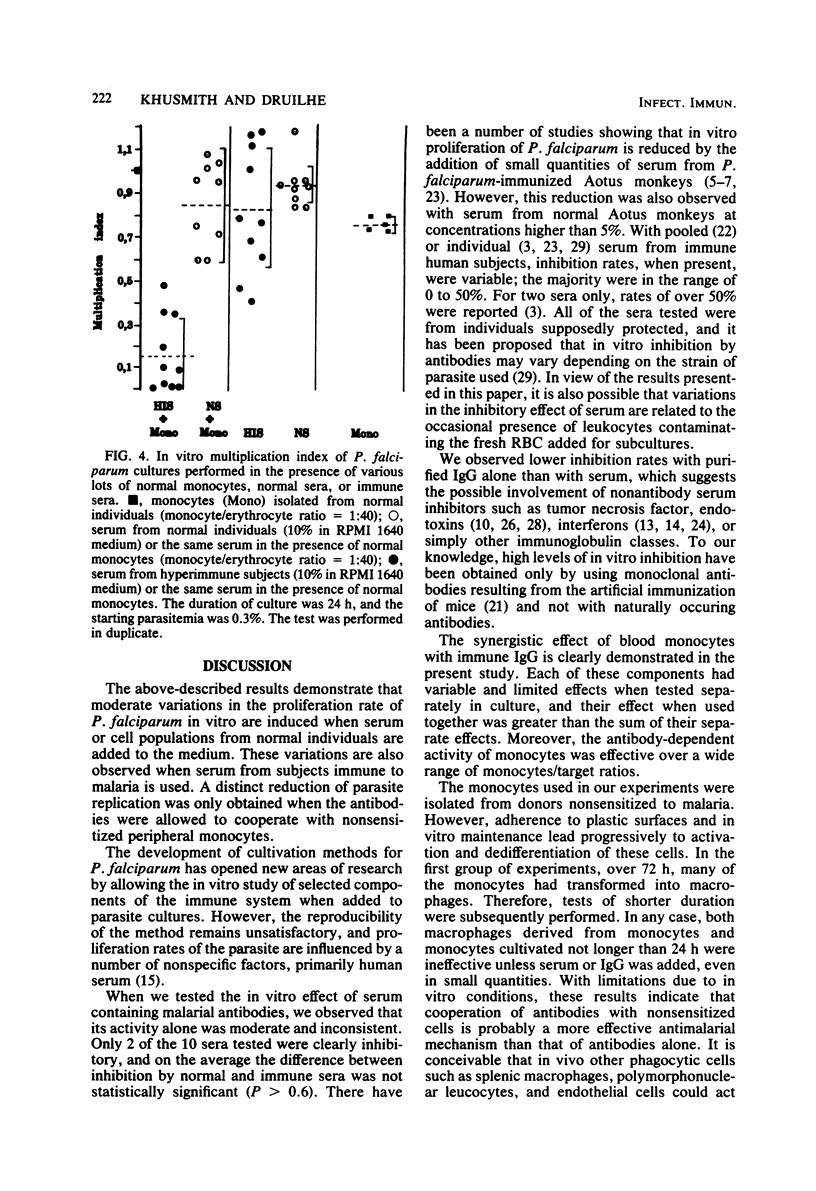
The cooperative effect between nonsensitized monocytes from normal individuals and malarial antibody in depressing parasite multiplication was investigated in an in vitro assay. The addition of purified normal monocytes to Plasmodium falciparum cultures in the presence of serum from immune individuals markedly inhibited the proliferation of the parasite in vitro: the parasitemia observed was about 22 times lower than that in the presence of immune serum alone. This cooperative effect was found to be effective over a wide range of monocyte/erythrocyte ratios (1:10 to 1:160) and serum concentrations (1/10 to 1/80). Immunoglobulin G extracted on protein A-Sepharose was as effective as total serum in this system. These data suggest that cooperation between nonsensitized cells and immunoglobulins could be an important effector mechanism against P. falciparum parasites in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. A radical interpretation of immunity to malaria parasites. Lancet. 1982 Dec 25;2(8313):1431–1433. doi: 10.1016/s0140-6736(82)91330-7. [DOI] [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Stace J. D., Alpers M. P., Mitchell G. F. Immunoprecipitation of biosynthetically-labelled proteins from different Papua New Guinea Plasmodium falciparum isolates by sera from individuals in the endemic area. Parasite Immunol. 1981 Winter;3(4):283–298. doi: 10.1111/j.1365-3024.1981.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Brown J., Smalley M. E. Specific antibody-dependent cellular cytotoxicity in human malaria. Clin Exp Immunol. 1980 Sep;41(3):423–429. [PMC free article] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Campbell G. H., Mrema J. E., O'Leary T. R., Jost R. C., Rieckmann K. H. In vitro inhibition of the growth of Plasmodium falciparum by Aotus serum. Bull World Health Organ. 1979;57 (Suppl 1):219–225. [PMC free article] [PubMed] [Google Scholar]
- Chulay J. D., Aikawa M., Diggs C., Haynes J. D. Inhibitory effects of immune monkey serum on synchronized Plasmodium falciparum cultures. Am J Trop Med Hyg. 1981 Jan;30(1):12–19. doi: 10.4269/ajtmh.1981.30.12. [DOI] [PubMed] [Google Scholar]
- Chulay J. D., Haynes J. D., Diggs C. L. Inhibition of in vitro growth of Plasmodium falciparum by immune serum from monkeys. J Infect Dis. 1981 Sep;144(3):270–278. doi: 10.1093/infdis/144.3.270. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A. Free oxygen radical generators as antimalarial drugs. Lancet. 1983 Jan 29;1(8318):234–234. doi: 10.1016/s0140-6736(83)92603-x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druilhe P., Jacquier P., Lambert D., Gentilini M. Plasmodium falciparum in vitro culture: improvements using umbilical cord serum and medium modifications. Tropenmed Parasitol. 1980 Dec;31(4):409–413. [PubMed] [Google Scholar]
- Druilhe P., Rhodes-Feuillette A., Canivet M., Gentilini M., Periês J. Circulating interferon in patients with Plasmodium falciparum, P. ovale and P. vivax malaria. Trans R Soc Trop Med Hyg. 1982;76(3):422–423. doi: 10.1016/0035-9203(82)90207-3. [DOI] [PubMed] [Google Scholar]
- Jahiel R. I., Vilcek J., Nussenzweig R. S. Exogenous interferon protects mice against Plasmodium berghei malaria. Nature. 1970 Sep 26;227(5265):1350–1351. doi: 10.1038/2271350a0. [DOI] [PubMed] [Google Scholar]
- Jensen J. B. Some aspects of serum requirements for continuous cultivation of Plasmodium falciparum. Bull World Health Organ. 1979;57 (Suppl 1):27–31. [PMC free article] [PubMed] [Google Scholar]
- Khusmith S., Druilhe P., Gentilini M. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infect Immun. 1982 Mar;35(3):874–879. doi: 10.1128/iai.35.3.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusmith S., Druilhe P. Specific arming of monocytes by cytophilic IgG promotes Plasmodium falciparum merozoite ingestion. Trans R Soc Trop Med Hyg. 1982;76(3):423–424. doi: 10.1016/0035-9203(82)90208-5. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- MCGREGOR I. A. THE PASSIVE TRANSFER OF HUMAN MALARIAL IMMUNITY. Am J Trop Med Hyg. 1964 Jan;13:SUPPL–239. doi: 10.4269/ajtmh.1964.13.237. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Ramirez E., Lambert P. H., Miescher P. A. Inhibition of P. falciparum growth in human erythrocytes by monoclonal antibodies. Nature. 1981 Jan 22;289(5795):301–303. doi: 10.1038/289301a0. [DOI] [PubMed] [Google Scholar]
- Phillips R. S., Trigg P. I., Scott-Finnigan T. J., Bartholomew R. K. Culture of Plasmodium falciparum in vitro: a subculture technique used for demonstrating antiplasmodial activity in serum from some Gambians, resident in an endemic malarious area. Parasitology. 1972 Dec;65(3):525–535. doi: 10.1017/s0031182000044139. [DOI] [PubMed] [Google Scholar]
- Reese R. T., Motyl M. R., Hofer-Warbinek R. Reaction of immune sera with components of the human malarial parasite, Plasmodium falciparum. Am J Trop Med Hyg. 1981 Nov;30(6):1168–1178. doi: 10.4269/ajtmh.1981.30.1168. [DOI] [PubMed] [Google Scholar]
- Schultz W. W., Huang K. Y., Gordon F. B. Role of interferon in experimental mouse malaria. Nature. 1968 Nov 16;220(5168):709–710. doi: 10.1038/220709a0. [DOI] [PubMed] [Google Scholar]
- Taverne J., Dockrell H. M., Playfair J. H. Endotoxin-induced serum factor kills malarial parasites in vitro. Infect Immun. 1981 Jul;33(1):83–89. doi: 10.1128/iai.33.1.83-89.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Tubbs H. Endotoxin in human and murine malaria. Trans R Soc Trop Med Hyg. 1980;74(1):121–123. doi: 10.1016/0035-9203(80)90026-7. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., Phillips R. S. Method to test inhibitory antibodies in human sera to wild populations of Plasmodium falciparum. Nature. 1976 Sep 9;263(5573):132–134. doi: 10.1038/263132a0. [DOI] [PubMed] [Google Scholar]


