Abstract
Background
The upregulation of G protein–coupled receptor kinase 2 in failing myocardium appears to contribute to dysfunctional β-adrenergic receptor (βAR) signaling and cardiac function. The peptide βARKct, which can inhibit the activation of G protein–coupled receptor kinase 2 and improve βAR signaling, has been shown in transgenic models and short-term gene transfer experiments to rescue heart failure (HF). This study was designed to evaluate long-term βARKct expression in HF with the use of stable myocardial gene delivery with adeno-associated virus serotype 6 (AAV6).
Methods and Results
In HF rats, we delivered βARKct or green fluorescent protein as a control via AAV6-mediated direct intramyocardial injection. We also treated groups with concurrent administration of the β-blocker metoprolol. We found robust and long-term transgene expression in the left ventricle at least 12 weeks after delivery. βARKct significantly improved cardiac contractility and reversed left ventricular remodeling, which was accompanied by a normalization of the neurohormonal (catecholamines and aldosterone) status of the chronic HF animals, including normalization of cardiac βAR signaling. Addition of metoprolol neither enhanced nor decreased βARKct-mediated beneficial effects, although metoprolol alone, despite not improving contractility, prevented further deterioration of the left ventricle.
Conclusions
Long-term cardiac AAV6-βARKct gene therapy in HF results in sustained improvement of global cardiac function and reversal of remodeling at least in part as a result of a normalization of the neurohormonal signaling axis. In addition, βARKct alone improves outcomes more than a β-blocker alone, whereas both treatments are compatible. These findings show that βARKct gene therapy can be of long-term therapeutic value in HF.
Keywords: gene therapy; heart failure; neurohormones; cardiac remodeling, ventricular
Despite significant improvement in diagnosis and treatment of the post–myocardial infarction (MI) population, these patients ultimately develop heart failure (HF), which still carries high morbidity and mortality that render it the top-ranking cardiovascular cause of mortality in the Western world.1 Current HF management includes the goal of correcting molecular abnormalities that contribute to the pathophysiology of the disease2; to more specifically target these, viral-mediated gene transfer has become an attractive approach.3 Several potential gene therapy targets for HF have emerged over the last decade, primarily from transgenic mouse models and short-term proof-of-concept gene transfer experiments.3
Our group has extensively studied inhibition of G protein–coupled receptor kinase 2 (GRK2) activity as a molecular target for combating left ventricular (LV) dysfunction.4 GRK2 (also known as βARK1) is robustly expressed in myocardium, and it functions to phosphorylate activated G protein–coupled receptors, leading to their desensitization or signaling deactivation.5 A primary target for GRK2 in the heart is the β-adrenergic receptor (βAR), which is a critical receptor system in the heart controlling cardiac chronotropy, inotropy, and lusitropy.4,6 The βAR system in the failing heart has long been known to be dysfunctional as β1ARs and β2ARs are uncoupled and β1ARs are downregulated, leading to the loss of inotropic reserve.7,8 This promotes hyperactivity of the sympathetic nervous system in attempting to drive the failing pump; however, a vicious cycle is created because βARs do not respond properly. This is a basis for the use of βAR antagonists in HF because they can block the noxious effects of catecholamines on myocytes.9
Importantly, GRK2 levels are increased in failing myocardium,8,10 and studies have shown that not only does enhanced GRK2 activity lead to the aforementioned βAR changes in HF, but it also appears to contribute to HF progression.4 Moreover, levels of GRK2 can have a profound effect on the contractile function of the heart.11 Previously, we have shown that a peptide, βARKct, can inhibit the membrane translocation and activation of GRK2 against myocardial βARs.12 The βARKct has prevented or rescued several models of HF, with a contributing mechanism being improved βAR signaling.13-17 This may appear paradoxical to the use of β-blockers in HF; however, long-term βAR antagonism also can promote receptor upregulation and GRK2 downregulation,18 which can also improve βAR signaling.19 Thus, these therapeutic approaches may be more similar than they appear on the surface, and data suggest that GRK2 inhibition and βAR antagonism could be synergistic.14
Of note, all of the aforementioned studies showing beneficial effects of βARKct on the heart have utilized transgenic animals or adenoviral-mediated gene transfer.20 From a clinical point of view, however, it is clear that for chronic diseases such as HF, long-term and stable myocardial gene expression is needed, and the candidate vehicle that has emerged to accomplish this is recombinant adeno-associated viral (rAAV) vectors. rAAV vectors enable efficient and sustained expression without significant toxicity, properties that make them suitable for human use.3,21 In this study, we utilized a rAAV serotype 6 (rAAV6) vector to deliver βARKct in hearts of post-MI HF rats. This approach allowed us, for the first time, to directly investigate the therapeutic effects of long-term (up to 12 weeks) expression of βARKct in the failing heart. Our data demonstrate a sustained rescue of preexisting HF with βARKct delivery that, interestingly, appears to offer benefits beyond long-term β-blockade.
Methods
Experimental Procedures
Experimental procedures were performed essentially as described previously.14,22 An expanded Methods section appears in the online-only Data Supplement.
Statistical Analysis
Data are summarized as mean±SEM. Comparisons were made with the use of t tests or ANOVA as appropriate. A Bonferroni correction was applied to the probability values whenever multiple comparisons arose. Values of P<0.05 were considered significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Study Design and Transgene Expression
At 12 weeks after cryoinfarction, a time point with established HF,22 rats were randomized to 5 different groups: 1 group receiving cardiac gene transfer of rAAV6-βARKct, 1 rAAV6–green fluorescent protein (GFP), and 1 vehicle (saline). In addition, 2 more HF groups received the β1AR-selective blocker metoprolol in conjunction with gene transfer of rAAV6-βARKct or rAAV6-GFP. One day before treatment was started, all groups were analyzed by echocardiography to confirm the presence of similar levels of LV dysfunction and HF before gene delivery. All groups were then studied over the course of 3 more months (6 months after MI) (Figure 1A), and all assays in the 5 HF groups were compared with a control sham-operated group that received neither MI nor gene transfer (6 experimental groups in total).
Figure 1.

A, Overall design of the 24-week study. Representative Western blot analysis of βARKct (B) and GFP (C) protein expression in rat tissues 12 weeks after intramyocardial delivery with AAV6/βARKct and AAV6/GFP, respectively. RV indicates right ventricle; SKM, skeletal muscle; +, positive controls. D, Representative GFP fluorescence microscopy (left), light microscopy (middle), and overlay of both (right) of LV myocardium 12 weeks after intramyocardial rAAV6-GFP delivery. Magnification ×4. Bar=0.5 mm.
At 12 weeks after gene delivery, both transgenes (βARKct and GFP) were robustly expressed specifically in the LV tissue of the respective groups, as assessed by Western blotting of cardiac protein extracts (Figure 1B and 1C). This expression was restricted to the LV because no expression was detected in the right ventricle, and limited expression (liver only) was found in extracardiac tissue (Figure 1B and 1C). GFP fluorescence in cardiac sections from rAAV6-GFP–treated rats at 12 weeks after gene delivery confirmed that a large area of the LV free wall was transduced, although the expression was not homogeneous (Figure 1D). βARKct immunofluorescence in cardiac sections from AAV6-βARKct–treated HF rats showed comparable areas of the LV transduced (Figure I in the online-only Data Supplement).
In Vivo Cardiac Function in All Groups at 12 Weeks After Gene Delivery
Echocardiography 12 weeks after MI revealed that LV ejection fraction (EF) and internal diameter at diastole were equivalent among all HF groups before the initiation of treatment (Figure 2A and 2D). EF was significantly decreased and LV diastolic diameter was significantly increased in all groups compared with sham, confirming a similar degree of HF (Figure 2A and 2D). These pre–gene delivery functional data are consistent with the poststudy analysis of LV infarct size after cryoinjury because we found similar infarct sizes (Figure II in the online-only Data Supplement).
Figure 2.
EF as measured by echocardiography 12 weeks after MI (A) and 12 weeks after in vivo rAAV6-βARKct gene delivery (B) with or without metoprolol (meto) treatment (and 24 weeks after MI). C, Percentage change in alteration of EF after the 12 weeks of treatment. LV internal diameter at diastole (LVIDd) measured by echocardiography 12 weeks after MI (D) and after 12 weeks of treatment (E) is shown. F, Heart rate (HR) at 24 weeks after MI. G, Representative raw tracings of M-mode echocardiography 12 weeks after gene delivery. Sham, n=11; HF/saline, n=14; HF/GFP, n=11; HF/βARKct, n=12; HF/GFP-metoprolol (Meto), n=11; and HF/βARKct-metoprolol, n=11. Data are presented as mean±SEM. Bar=8 mm. ^P<0.05 vs HF/saline, HF/GFP, or HF/GFP-metoprolol groups; #P<0.05 vs sham; *P<0.05 vs HF/saline or HF/GFP groups; &P<0.05 vs each non-metoprolol-treated group; ANOVA analysis and Bonferroni test among all groups.
Twelve weeks after gene delivery, all groups still had significantly impaired cardiac function compared with sham rats; however, EF was significantly increased in rats receiving βARKct, indicating improvement of cardiac function (Figure 2B and 2C). Of note, this improvement was observed regardless of concomitant metoprolol treatment (Figure 2B and 2C). Conversely, treatment with GFP or saline alone led to further deterioration of cardiac function after 12 weeks, which was prevented but not reversed by concurrent metoprolol treatment (Figure 2B and 2C). Adverse LV remodeling as measured by ventricular dilatation also progressed further in saline- and GFP-treated rats, and this was prevented by βARKct expression (Figure 2E). Representative M-mode echocardiography views demonstrating these results on cardiac function are shown in Figure 2G. In contrast to cardiac function, LV dilatation improvement was equal in βARKct and metoprolol groups, and the 2 treatment modalities together also provided a similar beneficial effect (Figure 2E). Importantly, βARKct expression in the LV did not have any effect on heart rate, in contrast to metoprolol treatment, which significantly decreased heart rate as expected (Figure 2F).
LV catheterization and hemodynamic analysis in rats 12 weeks after gene delivery showed significant decreases in measurements of LV contractility and relaxation in saline and GFP groups compared with the sham group, indicating HF (Table and Figure 3). Moreover, LV systolic pressure was significantly reduced in the saline and GFP groups, whereas LV end-diastolic pressure was significantly increased compared with sham (Table). Representative in vivo LV dP/dt tracings from all groups are shown in Figure 3A. Cardiac βARKct expression significantly improved LV contractility and relaxation 12 weeks after treatment (Table and Figure 3B and 3C). Metoprolol treatment (HF/GFP-metoprolol) significantly reduced LV diastolic pressure, as did βARKct alone and βARKct plus metoprolol; however, LV contractility and relaxation indices were similar between GFP-metoprolol and the saline or GFP groups (Table and Figure 3B and 3C). βARKct gene delivery improved all measures of cardiac function, including increased LV systolic pressure and decreased LV end-diastolic pressure (Table). βARKct gene therapy actually led to restoration of normal contractile function of failing hearts because basal LV+dP/dtmax (but not LV−dP/dtmin) of either group that received rAAV6-βARKct was indistinguishable from that of the sham group (Table and Figure 3B and 3C). Importantly, on infusion of a maximal dose of isoproterenol, LV βARKct expression resulted in marked improvement of all parameters of cardiac performance (Table and Figure 3B and 3C). βARKct plus metoprolol also showed similar improvements with the exception of LV systolic pressure, which was not increased compared with saline or GFP groups (Table).
Table.
In Vivo Functional Parameters After AAV6-Mediated Cardiac Gene Delivery
| Sham | HF/Saline | HF/GFP | HF/βARKct | HF/GFP- Metoprolol |
HF/βARKct- Metoprolol |
||
|---|---|---|---|---|---|---|---|
| LV catheterization, basal | |||||||
| HR, bpm | 303±5 | 296±6 | 305±6 | 307±4 | 264±9* | 270±6* | |
| LV dP/dt, mm Hg/s | 6621±275 | 4557±276† | 4697±338† | 5939±297‡ | 4331±244† | 5793±289‡ | |
| LV −dP/dt, mm Hg/s | 6904±343 | 3681±237† | 3722±254† | 4886±202†‡ | 3386±193† | 4828±210†‡ | |
| LVEDP, mm Hg | 2.5±0.3 | 11.3±1.8† | 12.8±1.5† | 3.5±0.5§ | 5.3±1.1†§ | 4.1±0.7§ | |
| LVESP, mm Hg | 124.2±4.1 | 100.5±3.7† | 98.9±4.7† | 117.5±3.7‡ | 101.7±4.1† | 109.9±4.5† | |
| Isoproterenol (333 ng/kg BW) | |||||||
| Heart rate, bpm | 362±3 | 353±4 | 354±5 | 362±6 | 315±8* | 324±6* | |
| LV dP/dt, mm Hg/s | 15553±348 | 8278±439† | 7458±534† | 11107±294†‡ | 7709±541† | 10440±523†‡ | |
| LV −dP/dt, mm Hg/s | 8407±368 | 4935±210† | 4620±333† | 6410±272†‡ | 4571±313† | 6107±245†‡ | |
| LVEDP, mm Hg | 1.6±0.4 | 8.6±1.6† | 10.7±1.4† | 2.0±0.4‡ | 5.0±1.2†§ | 2.5±0.4§ | |
| LVESP, mm Hg | 124.4±3.4 | 98.0±3.2† | 100.6±4.4† | 116.2±3.8‡ | 98.9±4.0† | 108.0±4.4† | |
| BW, kg | 0.467±0.011 | 0.462±0.010 | 0.461±0.008 | 0.473±0.007 | 0.470±0.011 | 0.469±0.011 | |
| HW/BW ratio, g/kg | 2.53±0.06 | 3.14±0.08† | 3.10±0.09† | 2.74±0.08§ | 2.879±0.09§ | 2.75±0.08§ | |
| LW/BW ratio, g/kg | 3.39±0.13 | 5.05±0.53† | 5.48±0.32† | 3.60±0.38§ | 3.62±0.26§ | 3.97±0.29§ | |
Data are presented as mean±SEM. Effect of βARKct gene therapy on LV function evaluated at 12 weeks after gene delivery is shown. In vivo LV +dP/dt, −dP/dt, end-diastolic pressure (EDP), end-systolic pressure (ESP), and heart rate were assessed in sham (n=11), HF/saline (n=13), HF/GFP (n=12), HF/βARKct (n=12), HF/GFP-metoprolol (n=13), and HF/βARKct-metoprolol (n=12) rats under basal conditions and after maximal isoproterenol stimulation. Ratios of heart weight to body weight and lung weight to body weight (LW/BW) were also measured in all groups. ANOVA analysis and Bonferroni test were used between all groups.
P<0.05 vs each non–metoprolol-treated group
P<0.05 vs sham
P<0.05 vs HF/saline, HF/GFP, or HF/GFP-metoprolol groups
P<0.05 vs HF/saline or HF/GFP.
Figure 3.
A, Representative raw tracings of LV dP/dt values 24 weeks after MI in all 6 experimental groups. B, Average LV +dP/dt and LV −dP/dt values (C) in the 6 experimental groups (sham, n=11; HF/saline, n=13; HF/GFP, n=12; HF/βARKct, n=12; HF/GFP-metoprolol (meto), n=13; and HF/βARKct-metoprolol, n=12) evaluated under basal conditions and after maximal isoproterenol stimulation. ANOVA analysis and Bonferroni test were used among all groups. Data are presented as mean±SEM. #P<0.05 vs sham at basal; †P<0.05 vs sham after maximal isoproterenol stimulation; ^P<0.05 vs HF/saline, HF/GFP, or HF/GFP-metoprolol groups at basal; §P<0.05 vs HF/saline, HF/GFP, or HF/GFP-metoprolol groups after maximal isoproterenol stimulation.
Myocardial β-Adrenergic Status After In Vivo Gene Delivery
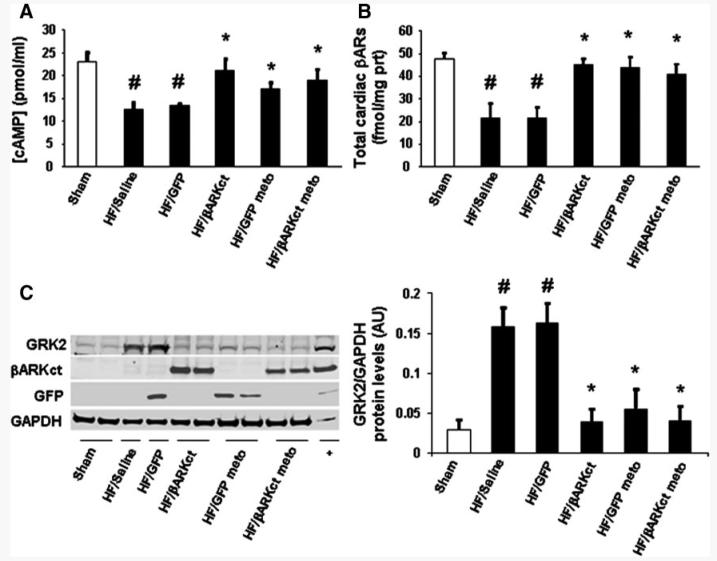
At the end of the 24-week study, hearts from all groups were excised to examine the effects of the various treatments on cardiac βAR signaling at the molecular level. We first measured steady state levels of cAMP in myocardium to determine whether this βAR-mediated second messenger was altered in our different experimental groups. As shown in Figure 4A, myocardial cAMP levels in HF/saline and HF/GFP hearts were significantly reduced compared with sham hearts, consistent with impaired βAR signaling in HF. Cardiac βARKct expression with or without metoprolol as well as metoprolol alone led to restored steady state cardiac cAMP levels in the HF rats because cardiac cAMP concentrations found in these groups were indistinguishable from that of sham hearts (Figure 4A). This probably reflects increased adenylate cyclase activity because cAMP-specific phosphodiesterase activity was equal among all HF experimental groups (Figure III in the online-only Date Supplement). Levels of cAMP with βARKct expression were not elevated or enhanced over sham (nonfailing) levels (Figure 4A).
Figure 4.
A, Average steady state cAMP production and total βAR density (B) in cardiac homogenates purified from hearts of all 6 experimental groups at 24 weeks after MI (n=6 for each group). Representative Western blot (C, left panel) and average densitometric quantitative analysis (C, right panel) from blots showing the ratio of GRK2 to GAPDH and expression of βARKct or GFP transgenes in LV homogenates (n=8 for each group). meto indicates metoprolol. Data are presented as mean±SEM. *P<0.05 vs HF/saline or HF/GFP groups; #P<0.05 vs sham; ANOVA analysis and Bonferroni test among all groups.
We measured total βAR density on plasma membranes isolated from all groups of rat hearts and, as expected, found cardiac βARs to be significantly reduced in HF/saline- and HF/GFP-treated rats compared with sham (Figure 4B). However, the groups that received treatment (βARKct and/or metoprolol) had significantly increased cardiac βAR density, which was equivalent to normal levels (Figure 4B).
Finally, we measured myocardial GRK2 levels and found that GRK2 was significantly increased in the HF groups that received only saline of rAAV6-GFP (Figure 4C). βARKct alone or in combination with metoprolol as well as metoprolol alone significantly lowered cardiac GRK2 expression after 12 weeks in these failing rat hearts to levels close to sham values (Figure 4C). Taken together, these results suggest that long-term cardiac βARKct expression with or without concomitant β-blocker treatment significantly ameliorates βAR signaling of the failing heart.
Cardiac Remodeling and Functional Biomarkers After In Vivo Gene Delivery
To analyze morphological and molecular aspects of adverse LV remodeling, we determined the ratios of heart weight to body weight (HW/BW ratios) in the various groups of rats at the end of the 24-week study and also assessed mRNA expression of specific genes known to play a role in HF pathogenesis. The HW/BW ratio was significantly increased in control HF groups compared with sham rats, consistent with a HF phenotype, whereas cardiac mass was significantly decreased in all 3 HF treatment groups (Table). For molecular LV remodeling and hypertrophy at 12 weeks after gene delivery, we measured the mRNA levels of collagen 1, transforming growth factor-β1, and atrial natriuretic factor in the LV of all groups via real-time polymerase chain reaction. Consistent with the echocardiographic data that demonstrated LV remodeling (Figure 2), collagen 1, transforming growth factor-β1, and atrial natriuretic factor mRNA levels were markedly increased in HF/saline and HF/GFP groups compared with sham rats at 24 weeks after MI, and levels of these detrimental cardiac markers were significantly reduced with long-term βARKct expression and/or metoprolol treatment (Figure 5A, 5B, 5C). These results demonstrate that long-term βARKct expression and/or metoprolol treatment is able to attenuate the HF-related remodeling. Finally, mRNA levels of brain natriuretic peptide (BNP), a fetal gene marker of cardiac hypertrophy, HF, and volume overload, were measured. Levels of BNP were significantly elevated in HF/saline and HF/GFP groups, whereas long-term βARKct expression with or without metoprolol significantly lowered cardiac BNP expression; however, metoprolol alone did not decrease BNP in HF (Figure 5D). These results are consistent with our aforementioned hemodynamic analysis data (Figure 3 and Table).
Figure 5.
Heart mRNA levels of collagen I (Col1) (A); transforming growth factor β1 (TGFβ1) (B); atrial natriuretic factor (ANF) (C) in all experimental groups at 24 weeks after MI; and BNP (D). All values were standardized to amplified 28S rRNA. Data are presented as mean±SEM and plotted as fold over sham values. *P<0.05 vs HF/saline or HF/GFP groups; #P<0.05 vs sham; ^P<0.05 vs HF/saline, HF/GFP, or HF/GFP-metoprolol groups; ANOVA analysis and Bonferroni test among all groups.
Neurohormonal Status of HF After In Vivo Gene Therapy
Chronic HF is associated with elevated circulating levels of various neurohormones, most prominently catecholamines (norepinephrine and epinephrine) as well as aldosterone, which are all known to adversely affect HF progression and cardiac function.23,24 Moreover, in the case of catecholamines and the sympathetic nervous system, the poor contractile performance of the failing heart and desensitization of the cardiac βAR system can continually feed into this vicious cycle of stimulation. Thus, our hypothesis, which has not been previously examined directly, was that as a result of the improvement in contractile function, βAR signaling, and reverse LV remodeling after long-term myocardial βARKct expression and GRK2 inhibition, there would be novel feedback on the aforementioned neurohormonal systems that would contribute to the beneficial outcomes seen in our βARKct- and metoprolol-treated HF rats. Accordingly, we measured circulating plasma catecholamine and aldosterone levels in all groups at 24 weeks after MI (Figure 6). As expected, HF (saline- or GFP-treated) was associated with significantly elevated plasma levels of epinephrine, norepinephrine, and aldosterone compared with sham (Figure 6). In contrast, long-term cardiac βARKct expression with or without metoprolol treatment, as well as metoprolol treatment alone, led to a significant reduction of all 3 of these plasma neurohormones (Figure 6), demonstrating for the first time how this may play a role in βARKct-mediated rescue of HF.
Figure 6.
Plasma norepinephrine (A), plasma epinephrine (B), and plasma aldosterone (C) levels in the 6 groups at 24 weeks after MI. Data are presented as mean±SEM. *P<0.05 vs HF/saline or HF/GFP groups; #P<0.05 vs sham; ANOVA analysis and Bonferroni test among all groups.
Discussion
A number of previous studies have suggested that cardiac GRK2 inhibition, via in vivo ventricular βARKct gene delivery, could be a potential novel treatment for chronic HF.3,15-17 However, these studies have utilized adenoviral-mediated gene delivery, which only allows for short-term transgene expression. Thus, no clinically relevant conclusions about the long-term efficacy and adverse effects of βARKct gene therapy could be drawn. The present study has now addressed these limitations and has examined the long-term nature of βARKct-mediated HF rescue. This was made possible by taking advantage of rAAV6, which has high natural tropism for myocardium25 and can support long-term and stable gene expression with minimal immunogenic and inflammatory host responses.3,21 Our present study demonstrates that βARKct delivery via rAAV6 into already failing myocardium results in robust expression in the LV of HF rats for >3 months, and this leads to a long-term rescue of LV contractile dysfunction and remodeling. Mechanistically, this appears to be mediated in part by a normalization of cardiac βAR signaling, including a lowering of GRK2 expression/activity, as well as silencing of detrimental genes associated with adverse LV remodeling. Moreover, we have uncovered a novel finding that long-term myocardial βARKct expression can lead to neurohormonal feedback decreasing the hyperactivity of the sympathetic nervous system and renin-angiotensin-aldosterone signaling axis in HF.
To achieve βARKct transgene expression in the LV, we chose direct intramyocardial injection of rAAV6 because this technique is feasible and is applicable in the clinical setting.3,21 Interestingly, we found that temporarily stopping the heart after injection of virus led to significantly improved efficiency of gene delivery (Figure IV in the online-only Data Supplement). This modification has not been reported previously, and it led to robust transgene expression in the anterior, posterior, and lateral walls of the LV. The only limitation of this technique appears to be that only minor transgene expression was found in the septum (data not shown). However, as our functional and molecular data reveal, gene delivery of the βARKct to the LV was sufficient to mediate significant improvement in cardiac function and reverse remodeling in our post-MI rat HF model. To make our study clinically relevant, we did not apply any treatment until 12 weeks after cryoinfarct in rats to determine whether βARKct can therapeutically rescue existing HF. Indeed, before our therapeutic interventions, our animal population showed a significant decrease of LV contractile function and an increase in LV chamber dimensions. Importantly, LV dysfunction and remodeling were similar in all randomized treatment groups. To increase translational relevance, we also treated HF rats with the clinically used βAR blocker metoprolol alone (+AAV6-GFP) or in combination with βARKct expression.
Our present results add significantly to previous studies using short-term adenoviral-mediated βARKct expression in the failing heart15-17 because long-term AAV-mediated βARKct expression in the failing heart led to rescue of global heart function. At 3 months after gene transfer, we found significantly increased LV EF, dP/dt, and systolic blood pressure with long-term βARKct expression, whereas LV end-diastolic diameter and pressure were decreased. The beneficial effects of βARKct gene therapy were also evident on acute βAR challenge, in which case maximal inotropic reserve was almost completely restored. This degree of rescue was remarkable because control HF rats treated with intramyocardial injections of only saline or rAAV6-GFP demonstrated significant progression of HF as all parameters measured showed further deterioration over the 3-month treatment period. Interestingly, although metoprolol alone produced structural changes similar to those of βARKct expression in the failing LVs, including a reduction in dilatation and hypertrophy, long-term βAR blockade did not improve cardiac contractile functional performance over the control untreated groups but only prevented further deterioration. Long-term βARKct expression actually increased global heart function and significantly ameliorated all parameters of cardiac performance over the entire treatment period.
Of note, long-term βAR blockade in our model produced effects consistent with its well-documented role in post-MI HF therapy as it significantly attenuated HF-associated cardiac remodeling and hypertrophy, lowered end-diastolic pressure, and prevented further deterioration of cardiac function.9,26 Interestingly, we found that all of the beneficial effects of βARKct expression in HF, including increased βAR inotropic reserve, were still present when metoprolol was administered concomitantly, which suggests that these 2 therapeutic modalities are completely compatible and adds to the potential future clinical application of βARKct gene therapy in HF.
The results found with βARKct and metoprolol alone and together are interesting and add to the apparent paradox of understanding how blocking βAR activation versus blocking receptor desensitization can both lead to therapeutic effects in HF. The βARKct has potential additive mechanisms of action because GRK2 phosphorylates other receptors in the heart besides βARs, and thus improved signaling through other systems could be part of the mechanism of GRK2 inhibition. As a result of comprehensive molecular data we have uncovered demonstrating that both βARKct expression and βAR blockade lead to similar effects in chronic HF, perhaps this apparent paradox should be revisited. Indeed, both modalities reversed the abnormalities found within the βAR system of failing myocardium, including normalized βAR number, decreased upregulation of GRK2, and significantly improved steady state whole LV cAMP accumulation compared with untreated HF controls. We believe that this demonstrates that these changes in βARs in failing myocardium are indeed maladaptive and that normalization or “molecular remodeling” of this important inotropic system is critical for halting HF progression.
Consistent with the aforementioned hypothesis, we have previously shown, using short-term βARKct gene delivery to rabbits at the time of MI, that if βAR desensitization and downregulation are prevented from the onset of myocardial injury, HF can be prevented.15 Moreover, this is also consistent with recent results in conditional, cardiac-specific GRK2 knockout mice in which βAR signaling abnormalities were prevented and LV function was improved after MI.27 Therefore, results with the βARKct in acute and chronic HF coupled with the almost identical changes seen in this study with long-term metoprolol treatment in HF are consistent with the hypothesis that uncoupled βARs are maladaptive in the injured heart. The correction of βAR signaling could also be an indirect effect of improved cardiac function, although the βARKct and metoprolol did not produce identical functional changes. Thus, long-term βAR blockade alone did not improve cardiac contractile function compared with βARKct expression, and this could be because the normalized βAR signaling is still being blocked in real time; however, in the rats treated with βARKct and metoprolol together, this improved contractile function was still present, as well as restored inotropic reserve.
Long-term βARKct expression in the failing heart also had significant effects on cardiac post-MI adverse remodeling. Of note, the expression of various fibrosis-related genes and hypertrophic markers was markedly decreased by βARKct expression, indicating significant reversal of LV remodeling and maladaptive hypertrophy. Several possible mechanistic explanations could be suggested for these findings. First, there might be an indirect effect resulting from enhanced contractile function of the heart reducing biomechanical overload, which results in reverse remodeling. Additionally, this finding might reflect decreased apoptosis and altered gene expression patterns that increase cardiomyocyte survival and proliferation. In fact, β2AR signaling has been reported to be antiapoptotic in the myocardium, whereas β1AR signaling increases myocardial apoptosis,28 and we have recently shown that β2AR signaling is enhanced after GRK2 lowering in the failing heart.27 Therefore, βARKct might alter the balance of the signaling cascade elicited by these 2 βAR subtypes in favor of less apoptosis and increased survival and proliferation. Moreover, we cannot exclude the possible effect of GRK2 inhibition on cardiac fibroblasts, endothelial cells, and/or smooth muscle cells, where we also found βARKct to be expressed 12 weeks after gene delivery (Figure V in the online-only Data Supplement). This finding of transgene expression in cardiac cells other than myocytes warrants further mechanistic investigation. Nevertheless, it is evident that, regardless of the mechanism, long-term cardiac βARKct overexpression beneficially affects cardiac post-MI remodeling. Metoprolol also led to reverse remodeling in our model, which adds to the aforementioned apparent paradox and also may indicate that increased β2AR signals are part of the mechanism because of the use of selective β1AR blockade.
Another interesting finding of the present study is that long-term βARKct expression in the heart is also accompanied by significant amelioration of the HF-related neurohormonal status. Specifically, circulating levels of both catecholamines (norepinephrine and epinephrine) as well as aldosterone, hormones that are elevated during chronic HF progression and significantly enhance morbidity and mortality,23,24 were markedly lowered in the βARKct-treated animals. This was also the case when metoprolol was given to the HF rats. This most certainly could also be part of the mechanism inducing the substantial reverse LV remodeling that was seen with βARKct (and βAR blockade) treatment. Moreover, this could be part of a unique mechanism for the βARKct and GRK2 inhibition for reversing HF in general. Of importance, this is the first study, to our knowledge, to examine the effects of cardiac βARKct (or any other) gene therapy on the neurohormonal status of chronic HF and shows that GRK2 inhibition in the myocardium lowers circulating levels of these pro-HF neurohormones and probably represents a negative feedback phenomenon, in which improved cardiac output indirectly attenuates the level of cardiac neurohormonal drive. Thus, we believe this is a key new finding from this study, which could also directly lead to the improved signaling status of cardiac βARs and contribute to improved contractile function.
In summary, the present study reports that rAAV6-mediated βARKct gene therapy reverses contractile dysfunction, attenuates LV remodeling, and restores the abnormalities of βAR signaling in a rat model of post-MI chronic HF. As a contributing mechanism, we found that myocardial GRK2 inhibition can lead to significantly lower catecholamine and aldosterone levels, and lower circulating amounts of these neurohormones probably account, in part, for the reversal of HF. Importantly, the beneficial effects of GRK2 inhibition are still evident at 3 months after in vivo gene delivery, providing evidence that this therapeutic approach is strongly effective long-term. The true clinical potential of rAAV6-βARKct gene therapy for a place in the HF therapeutic armamentarium awaits further validation in larger animal models and ultimately, of course, in patients.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported in part by National Institutes of Health grants HL056205, HL061690, HL085503, and HL075443 (Project 2) and P01-HL091799 to Dr Koch and by postdoctoral fellowships to Drs Rengo and Lymperopoulos from the American Heart Association (Great Rivers Affiliate). This work was also supported in part by grant A75301 from the Commonwealth of Pennsylvania Department of Health.
Footnotes
Disclosures
None.
References
- 1.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 2.Harnad E, Mather PJ, Srinivasan S, Rubin S, Whellan DJ, Feldman AM. Pharmacologic therapy of chronic heart failure. Am J Cardiovasc Drugs. 2007;7:235–248. doi: 10.2165/00129784-200707040-00002. [DOI] [PubMed] [Google Scholar]
- 3.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102:1458–1470. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 5.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 6.Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651–690. [PubMed] [Google Scholar]
- 7.Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: β-adrenergic receptors in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med. 2005;2:475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- 8.Tilley DG, Rockman HA. Role of β-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Exp Rev Cardiovasc Ther. 2006;4:417–432. doi: 10.1586/14779072.4.3.417. [DOI] [PubMed] [Google Scholar]
- 9.Bristow MR. Mechanistic and clinical rationales for using b-blockers in heart failure. J Card Fail. 2000;6:8–14. [PubMed] [Google Scholar]
- 10.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 11.Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ. Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. J Biol Chem. 1998;273:18180–18184. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- 12.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 13.Rockman HA, Chien KR, Choi D-J, Iaccarino G, Hunter JJ, Ross J, Jr, Lefkowitz RJ, Koch WJ. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of heart failure in gene targeted mice. Proc Natl Acad Sci U S A. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding V, Jones L, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac βARK1 inhibition prolongs survival and augments β blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci U S A. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial β-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5428–5433. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AS, White DC, Emani S, Kypson AP, Lilly RE, Wilson K, Glower DD, Lefkowitz RJ, Koch WJ. In vivo ventricular gene delivery of a β-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation. 2001;103:1311–1316. doi: 10.1161/01.cir.103.9.1311. [DOI] [PubMed] [Google Scholar]
- 17.Tevaearai HT, Eckhart AD, Shotwell KF, Wilson K, Koch WJ. Ventricular dysfunction following cardioplegic arrest is improved after myocardial gene transfer of a β-adrenergic receptor kinase inhibitor. Circulation. 2001;104:2069–2074. doi: 10.1161/hc4201.097188. [DOI] [PubMed] [Google Scholar]
- 18.Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by β-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert EM, Abraham WT, Olsen S, Hattler B, White M, Mealy P, Larrabee P, Bristow MR. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996;94:2817–2825. doi: 10.1161/01.cir.94.11.2817. [DOI] [PubMed] [Google Scholar]
- 20.Williams ML, Koch WJ. Viral-based myocardial gene therapy approaches to alter cardiac function. Annu Rev Physiol. 2004;66:49–75. doi: 10.1146/annurev.physiol.66.032102.141555. [DOI] [PubMed] [Google Scholar]
- 21.Müller OJ, Katus HA, Bekeredjian R. Targeting the heart with gene therapy-optimized gene delivery methods. Cardiovasc Res. 2007;73:453–462. doi: 10.1016/j.cardiores.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 23.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 24.Güder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Störk S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964. [DOI] [PubMed] [Google Scholar]
- 25.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 26.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of β-adreno-receptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–1090. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 27.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW, II, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–22. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The β2-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through Gi-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.