Abstract
OBJECTIVE—To estimate the prevalence and incidence of diabetes, clinical characteristics, and risk factors for chronic complications among Navajo youth, using data collected by the SEARCH for Diabetes in Youth Study (SEARCH study).
RESEARCH DESIGN AND METHODS—The SEARCH study identified all prevalent cases of diabetes in 2001 and all incident cases in 2002–2005 among Navajo youth. We estimated denominators with the user population for eligible health care facilities. Youth with diabetes also attended a research visit that included questionnaires, physical examination, blood and urine collection, and extended medical record abstraction.
RESULTS—Diabetes is infrequent among Navajo youth aged <10 years. However, both prevalence and incidence of diabetes are high in older youth. Among adolescents aged 15–19 years, 1 in 359 Navajo youth had diabetes in 2001 and 1 in 2,542 developed diabetes annually. The vast majority of diabetes among Navajo youth with diabetes is type 2, although type 1 diabetes is also present, especially among younger children. Navajo youth with either diabetes type were likely to have poor glycemic control, high prevalence of unhealthy behaviors, and evidence of severely depressed mood. Youth with type 2 diabetes had more metabolic factors associated with obesity and insulin resistance (abdominal fat deposition, dyslipidemia, and higher albumin-to-creatinine ratio) than youth with type 1 diabetes.
CONCLUSIONS—Our data provide evidence that diabetes is an important health problem for Navajo youth. Targeted efforts aimed at primary prevention of diabetes in Navajo youth and efforts to prevent or delay the development of chronic complications among those with diabetes are warranted.
Data on the burden of diabetes in U.S. Native American youth are limited, with the exception of the Pima Indians of Arizona (1,2). In adults, Pima Indians have the highest occurrence of type 2 diabetes in the world, and very high rates have been reported in many other U.S. Indian tribes (3). Although type 2 diabetes has traditionally been viewed as a disorder of adults, an alarming two- to threefold increase in the prevalence of type 2 diabetes has been documented over the last 30 years among Pima Indian youth, in parallel with changes in obesity (1).
The Navajo Nation is the largest land area assigned primarily to a Native American jurisdiction within the U.S., occupying all of northeastern Arizona and extending into Utah and New Mexico. Diabetes in Navajo people has been reported since the 1960s, with recent reports in adults suggesting estimates of type 2 diabetes two to four times those of non-Hispanic white (NHW) populations and rising over the past 20–30 years (4,5). However, there are no population-based studies of diabetes occurrence in Navajo youth, although earlier reports (6,7) suggested that type 2 diabetes in youth was a relatively rare condition. It has been assumed that diabetes in Native American youth is primarily type 2 diabetes (8). Therefore, the relative contribution of autoimmune-mediated type 1 diabetes to the overall burden of diabetes among Navajo youth has not been systematically studied, although type 1 diabetes has been clinically recognized. Finally, there are no published reports on clinical characteristics and risk factors for diabetes-related complications. We report here population-based estimates of prevalence and incidence of diabetes by type, clinical characteristics, and risk factors for chronic complications.
RESEARCH DESIGN AND METHODS
The SEARCH for Diabetes in Youth Study (SEARCH study) ascertained diabetes in youth aged <20 years in 2001 and continues to ascertain incident cases since 2002 (9) in geographically defined populations in Ohio, Washington, South Carolina, and Colorado and among health plan enrollees in Hawaii and southern California. Coordinated by the Colorado center, diabetes cases were also identified among Navajo youth.
The SEARCH-Navajo population
The SEARCH-Navajo study sought to identify all prevalent cases of diabetes in 2001 and all incident cases in 2002–2005. The study was approved by the Indian Health Services (IHS) directors, directors of tribally operated health care facilities, and the Navajo Nation Human Research Review Board (NNHRRB) and was Health Insurance Portability and Accountability Act compliant. The population denominator was defined as individuals aged <20 years who self-identified themselves as Navajo and had at least one visit in the preceding 3 years to an eligible health care facility. Health care facilities include six Navajo area HIS units (Chinle, Crownpoint, Fort Defiance, Gallup, Kayenta, and Shiprock) and two tribally operated facilities (Tuba City and Winslow).
Estimation of prevalence and incidence
The 2001 prevalence denominator included 98,014 users of health care facilities. Potential case subjects had one or more outpatient visits or hospitalizations coded for diabetes (ICD-9 codes 250.0–250.9) and were aged <20 years on 31 December 2001. For 2002–2005 incidence estimates, the denominator included eligible youth with at least one visit in the preceding 3 calendar years. The sum of the annual denominators was used as average total denominator (n = 380,633 person-years) over the 4-year period. Potential case subjects had one or more diabetes-related ICD-9 codes, were diagnosed in 2002–2005, and were aged <20 years on 31 December of the year of diagnosis.
Case validation and collection of core variables
All eligible potential case subjects were validated using medical records. Less than 50% of case subjects identified using the IHS database had diabetes; miscoding was the most common reason for invalid case reports. For all validated cases, core demographic and diagnostic information was obtained from medical records. The clinical diabetes type assigned by the health care provider was categorized as type 1 (combining types 1, 1a, and 1b) and type 2 diabetes. Youth with other types (including hybrid, secondary diabetes, unknown, and missing) were excluded (n = 3).
Additional data collection
Youth were asked to participate in a research visit and they and/or their parent provided written informed consent according to the guidelines established by the NNHRRB. Questionnaires collected socioeconomic and medical history. Information about dietary intake, physical activity, smoking, and depressive symptoms was collected from participants aged ≥10 years. Diet was assessed by a food frequency questionnaire modified for administration in youth and capturing culturally specific foods (10). Physical activity questions were from the Youth Risk Behavioral Surveillance System questionnaire (11). Depressive symptoms were assessed using the Center for Epidemiologic Studies–Depression Scale (CES-D) (12). A CES-D score ≥24 was used as evidence of severely depressed mood (13).
Blood was drawn after an overnight fast under conditions of metabolic stability (14) for measurement of GAD65 antibodies and fasting and stimulated C-peptide (mixed-meal challenge), which were used to further characterize the provider-assigned diabetes type. A1C, fasting glucose, and lipids (total and HDL and LDL cholesterol, triglycerides, apolipoprotein B, and LDL particle density) were also measured (9). Glycemic control was categorized according to American Diabetes Association guidelines (15) as adequate when A1C <8% and poor when A1C ≥9.5%. A spot-urine sample was collected in the morning, and urinary albumin and creatinine were measured. An albumin-to-creatinine ratio ≥30 μg/mg (16) was considered elevated.
For youth aged ≥3 years, a physical examination included height, weight, and waist circumference; evaluation for acanthosis nigricans; and measurement of systolic and diastolic blood pressure (9). Obesity was defined as a BMI ≥95th percentile, and overweight was a BMI in the 85th to <95th percentile (17). Weight and height were compared with U.S. standards (17) to calculate normalized z-scores. Waist circumference was measured using the National Health and Nutrition Examination Survey protocol (17). Elevated blood pressure levels were present if systolic or diastolic blood pressure were ≥95th percentile for age, sex, and height. Hypertension was estimated based on elevated blood pressure levels or antihypertensive medication use (18).
Statistical analyses
Comparisons were made between subgroups of diabetes types (type 1 and type 2 diabetes) to determine whether characteristics differed. Fisher's exact tests for categorical variables and ANOVA for continuous variables were used. Linear or logistic regression was used to adjust for differences in age and duration of diabetes between type 1 and type 2 diabetic patients. Variables that were not normally distributed were logarithmically transformed. Given the descriptive nature of these analyses, we used an α level of 0.05 and did not correct for multiple comparisons.
RESULTS
Table 1 shows the number of Navajo youth with prevalent (2001) and incident diabetes (2002–2005), prevalence and incidence rates, and their distribution by age-group. A total of 92 youth aged <20 years had diabetes in 2001, and 66 were newly diagnosed in 2002–2005. The prevalence of diabetes in Navajo youth was low under the age of 10 years (0.11 per 1,000) and high (2.78 per 1,000) in youth aged 15–19 years. Similarly, the incidence of diabetes was low in children aged <10 years (2.24 per 100,000 per year) but high in adolescents aged 15–19 years (39.34 per 100,000 per year).
Table 1.
Prevalence and incidence of diabetes among Navajo youth
| Charcteristics | |||
|---|---|---|---|
| Age (years) | 0–9 | 10–14 | 15–19 |
| Prevalence (2001) | |||
| Population | 47,553 | 27,107 | 23,354 |
| Cases | 5 | 22 | 65 |
| Prevalence per 1,000 | 0.11 | 0.81 | 2.78 |
| 95% CI* | 0.04–0.25 | 0.54–1.23 | 2.18–3.55 |
| Population/case | 9,510 | 1,232 | 359 |
| Incidence (2002–2005) | |||
| Person-years | 178,793 | 102,693 | 99,147 |
| Cases | 4 | 23 | 39 |
| Rate per 100,000 person-years | 2.24 | 22.40 | 39.34 |
| 95% CI* | 0.87–5.75 | 14.93–33.61 | 28.78–53.76 |
| Population/case | 44,698 | 4,465 | 2,542 |
95% CIs are calculated using an inverted-score test from the binomial distribution (24).
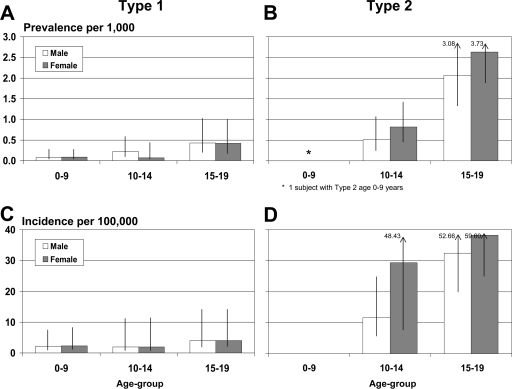
Overall, 18 prevalent case subjects had type 1 (19.6%) and 74 had type 2 (80.4%) diabetes. There were 10 type 1 (15.2%) and 56 type 2 (84.8%) diabetic patients incident in 2002–2005. Figure 1 shows type-specific estimates of prevalence (upper panel) and incidence (lower panel), according to age-group and sex. Estimates of type 1 diabetes prevalence and incidence are low and vary little with age and sex. These data indicate that type 1 diabetes is present in all age-groups and both sexes but is a rare condition among Navajo children and adolescents. In contrast, type 2 diabetes is exceptionally rare in youth aged <10 years (only one individual with type 2 diabetes) but very common in adolescents aged 15–19 years. Among adolescents aged 15–19 years, the prevalence of type 2 diabetes was 2.63 and 2.07 per 1,000. In the same age-group, the incidence rates of type 2 diabetes were 38.16 and 32.42 per 100,000 per year in female and male subjects, respectively. Incidence rates of type 2 diabetes increased with age (P = 0.04 for youth aged 15–19 years vs. youth aged 10–14 years) and female subjects aged 10–14 years were more likely to have type 2 diabetes than male subjects (P = 0.04).
Figure 1.
Prevalence (per 1,000, upper panels) and incidence (per 100,000 per year, lower panels) of diabetes among Navajo Nation youth, by diabetes type, age-group, and sex.
Table 2 presents characteristics associated with provider-defined diabetes type. Of 158 registered patients, 83 participated in the research visit. Youth with type 2 diabetes were older and had a shorter duration of diabetes than those with type 1 diabetes. Almost 50% of youth with clinical type 1 diabetes had positive GAD65 antibody titers, while only 20% of those with type 2 diabetes had such antibodies (P = 0.05). Mean fasting and stimulated C-peptide levels were significantly higher in youth with type 2 diabetes, even after adjusting for differences in age and diabetes duration, reflecting important differences in residual insulin secretory capacity and likely different pathophysiologic mechanisms in youth with type 2 versus type 1 diabetes. As expected, youth with type 2 diabetes had significantly higher BMI (33.6 vs. 23.5 kg/m2) and BMI z scores (1.8 vs. 0.7), as well as higher prevalence of obesity (67.7 vs. 12.5%). More youth with type 2 diabetes had evidence of insulin resistance, as manifested by presence of acanthosis nigricans (64.1 vs. 17.7%), than youth with type 1 diabetes. Youth with both type 2 and type 1 diabetes had a very strong family history of diabetes (92.2 and 76.5%, respectively). The presence of diabetic ketoacidosis at diabetes onset was nonsignificantly greater among youth with type 1 diabetes (40 vs. 14.3% in type 2 diabetes), suggesting a more acute onset possibly caused by more aggressive etiopathogenesis. While all but one youth with type 1 diabetes were on insulin therapy at study visit, only 26.4% of those with type 2 diabetes were treated with insulin (nine patients with insulin only and another five with insulin and metformin). Most patients with type 2 diabetes were treated with metformin (overall 67.9%); however, 15.1% had no pharmacologic treatment for diabetes (on average 3.5 years after diagnosis).
Table 2.
Characteristics of diabetes type among Navajo youth with diabetes (2001 prevalent and 2002–2005 incident cases combined)
| Variable | Type 1 diabetes | Type 2 diabetes | P |
|---|---|---|---|
| n | 17 | 66 | |
| Current age (years) (means ± SD) | 15.3 ± 4.5 | 18.0 ± 2.8 | 0.03 |
| Diabetes duration (years) (means ± SD) | 6.0 ± 4.7 | 3.5 ± 2.2 | 0.047 |
| GAD antibody positive [n (%)] | 7 (46.7) | 12 (20.3) | 0.05 |
| Fasting C-peptide (ng/ml) | |||
| Unadjusted (means ± SD) | 0.7 ± 0.9 | 3.8 ± 2.3 | <0.0001 |
| Adjusted (means ± SE)* | 1.3 ± 0.6 | 3.6 ± 0.3 | 0.002 |
| Stimulated C-peptide (area under the curve) (ng/ml) | |||
| Unadjusted (means ± SD) | 2.0 ± 3.2 | 11.6 ± 7.0 | <0.0001 |
| Adjusted (means ± SE)* | 4.4 ± 1.9 | 11.0 ± 0.8 | 0.003 |
| BMI (means ± SD) | 23.5 ± 5.3 | 33.6 ± 9.1 | <0.0001 |
| BMI z score (means ± SD) | 0.7 ± 0.9 | 1.8 ± 1.1 | 0.0004 |
| Weight [n (%)] | |||
| Underweight | 1 (6.3) | 1 (1.6) | |
| Normal | 9 (56.2) | 9 (14.5) | <0.0001 |
| Overweight 85th to 95th percentile | 4 (25.0) | 10 (16.1) | |
| Obese ≥95th percentile | 2 (12.5) | 42 (67.7) | |
| Acanthosis Nigricans [n (%)] | 3 (17.7) | 41 (64.1) | |
| Diabetes therapy (current) [n (%)] | |||
| Insulin | 15 (88.2) | 9 (17.0) | |
| Metformin | 0 (0.0) | 31 (58.5) | <0.0001 |
| Both | 1 (5.9) | 5 (9.4) | |
| None | 1 (5.9) | 8 (15.1) | |
| Diabetic ketoacidosis at onset [n (%)]† | 2 (40.0) | 3 (14.3) | 0.2 |
| Family history of diabetes [n (%)] | |||
| Yes | 13 (76.5) | 59 (92.2) | 0.087 |
| No | 4 (23.5) | 5 (7.8) |
Family history of diabetes = mother, father, sibling, or grandparent with positive family history.
Adjusted for age and duration.
Medical record evidence of hyperglycemia and one or more of the following: 1) blood bicarbonate <15 mmol/l or pH <7.25 (venous) or <7.30 (arterial or capillary), 2) ICD-9 code 250.1 at discharge, and 3) a diagnosis of diabetic ketoacidosis in the medical records.
Table 3 presents risk factors for chronic complications among Navajo youth by diabetes type. No major differences between type 1 and type 2 diabetes youth were noted in terms of glycemic control, health-related behaviors (smoking, physical activity, total fat, and fruit and vegetable intake) and socioeconomic status (family income and education). Nevertheless, Navajo youth with both diabetes types were very likely to have poor glycemic control (53.3 and 43.3% of youth with type 1 and type 2 diabetes, respectively, had A1C levels ≥9.5%) and a high prevalence of unhealthy behaviors (smoking, physical inactivity, and high dietary fat). In addition, 14.3% of type 1 and 21.9% of type 2 diabetic youth had evidence of severely depressed mood. Youth with type 2 diabetes had more metabolic factors associated with obesity and insulin resistance (higher abdominal fat deposition, dyslipidemia [higher triglycerides, low HDL cholesterol, and more small dense LDL cholesterol particles], and higher albumin-to-creatinine ratio). Although mean blood pressure levels and the prevalence of elevated blood pressure were similar in type 1 and type 2 diabetes, more patients with type 2 diabetes were on antihypertensive medication (ACE inhibitors), resulting in a nonsignificantly higher prevalence of hypertension (35.3 vs. 12.5%; P = 0.1).
Table 3.
Clinical, metabolic, behavioral, and sociodemographic risk factors for chronic complications among Navajo youth with diabetes, by diabetes type (2001 prevalent and 2002–2005 incident cases combined)
| Variable | Clinical type 1 diabetes | Clinical type 2 diabetes | P |
|---|---|---|---|
| n | 17 | 66 | |
| A1C (%) (means ± SD) | 9.8 ± 2.3 | 9.0 ± 2.8 | 0.3 |
| Glycemic control (A1C) [n (%)] | |||
| <8% | 3 (20.0) | 26 (43.3) | |
| 8–9.5% | 4 (26.7) | 8 (13.3) | 0.197 |
| ≥9.5% | 8 (53.3) | 26 (43.3) | |
| Waist circumference | |||
| Unadjusted (means ± SD) | 88.4 ± 16.3 | 110.7 ± 24.2 | 0.0008 |
| Adjusted (means ± SE)* | 94.9 ± 6.6 | 109.0 ± 3.0 | 0.07 |
| High waist circumference [n (%)] | |||
| ≥90th percentile for age and sex | 4 (25.0) | 52 (82.5) | <0.0001 |
| Systolic blood pressure (means ± SD) | 114.5 ± 10.6 | 118.2 ± 12.5 | 0.3 |
| Diastolic blood pressure (means ± SD) | 70.3 ± 10.4 | 73.3 ± 9.2 | 0.2 |
| Elevated blood pressure [n (%)]† | 2 (12.5) | 9 (14.5) | 0.8 |
| Taking antihypertensive medication [n (%)]‡ | 0 (0.0) | 14 (26.4) | 0.016 |
| Hypertension prevalence [n (%)]§ | 2 (12.5) | 22 (35.5) | 0.1 |
| Triglycerides (mg/dl) | |||
| Unadjusted [median (interquartile range)] | 109.5 (78.0) | 147.0 (137.0) | 0.03 |
| Adjusted [median (interquartile range)]* | 99.2 (25.9) | 162.3 (24.3) | 0.06 |
| High triglycerides (≥110 mg/dl) [n (%)] | |||
| Unadjusted | 7 (50.0) | 42 (73.7) | 0.1 |
| Adjusted* | (48.7) | (74.1) | 0.1 |
| HDL cholesterol (mg/dl) | |||
| Unadjusted (means ± SD) | 55.9 ± 16.3 | 41.7 ± 10.4 | 0.005 |
| Adjusted (means ± SE)* | 53.2 ± 3.4 | 42.3 ± 1.5 | 0.008 |
| Low HDL cholesterol (≤40 mg/dl) [n (%)] | |||
| Unadjusted (means ± SD) | 2 (13.3) | 26 (44.1) | 0.04 |
| Adjusted* | (15.4) | (41.1) | 0.1 |
| LDL cholesterol (mg/dl) | |||
| Unadjusted (means ± SD) | 109.9 ± 38.8 | 101.4 ± 35.1 | 0.4 |
| Adjusted (means ± SE)* | 110.6 ± 10.9 | 101.2 ± 4.8 | 0.5 |
| High LDL cholesterol (≥100 mg/dl) [n (%)] | |||
| Unadjusted | 6 (42.9) | 26 (45.6) | 0.9 |
| Adjusted* | (46.6) | (43.7) | 0.9 |
| LDL cholesterol density (Rf) | |||
| Unadjusted (means ± SD) | 0.27 ± 0.02 | 0.25 ± 0.03 | 0.02 |
| Adjusted (means ± SE)* | 0.27 ± 0.01 | 0.25 ± 0.005 | 0.07 |
| Apolipoprotein B (mg/dl) | |||
| Unadjusted [median (interquartile range)] | 94.0 (39.0) | 84.0 (57.0) | 0.9 |
| Adjusted [median (interquartile range)]* | 87.6 (23.7) | 87.9 (13.9) | 0.7 |
| High albumin-to-creatinine ratio (≥30 μg/mg) [n (%)] | |||
| Unadjusted | 2 (13.3) | 17 (28.8) | 0.3 |
| Adjusted (means ± SE)* | (5.6) | (31.5) | 0.05 |
| Smoking [n (%)] | |||
| Never | 10 (66.7) | 26 (39.4) | |
| Former | 2 (13.3) | 23 (34.8) | 0.15 |
| Current | 3 (20.0) | 17 (25.8) | |
| Physical activity (means ± SD) | |||
| Average number of days per week | 3.7 ± 2.5 | 3.0 ± 2.0 | 0.2 |
| Physically active [n (%)] | |||
| 0–2 days/week | 5 (33.3) | 33 (50.0) | |
| 3–7 days/week | 10 (66.7) | 33 (50.0) | 0.3 |
| Percent calories from fat (means ± SD) | |||
| Total fat | 36.3 ± 7.7 | 36.4 ± 6.9 | 0.9 |
| Saturated fat | 12.5 ± 2.7 | 12.5 ± 2.9 | 0.9 |
| Fruits and vegetables (servings/day) (means ± SD) | 2.8 ± 2.2 | 4.0 ± 2.5 | 0.1 |
| High CES-D score (≥24) [n (%)] | 2 (14.3) | 14 (21.9) | 0.7 |
| Annual family income [n (%)] | |||
| <$25,000 | 6 (46.2) | 33 (67.3) | |
| $25,000 to $49,000 | 6 (46.2) | 12 (24.5) | 0.3 |
| ≥$50,000 | 1 (7.7) | 4 (8.2) | |
| Parental education [n (%)] | |||
| Less than high school | 3 (18.8) | 15 (23.8) | 0.7 |
| High school graduate or higher | 13 (81.2) | 48 (76.2) |
Adjusted for age and duration.
High blood pressure at study visit = systolic or diastolic blood pressure ≥95th percentile for age, sex, and height.
On antihypertensive medication: ACE inhibitors (n = 13), calcium channel blockers (n = 0), and diuretics (n = 0).
Hypertension = systolic or diastolic blood pressure ≥95th percentile for age, sex, and height or on antihypertensive medications.
CONCLUSIONS
The SEARCH-Navajo study is the first population-based study to provide estimates of diabetes prevalence and incidence among Navajo youth. We found that diabetes was infrequent in youth aged <10 years; however, both prevalence and incidence of diabetes were high in older youth, especially adolescents aged 15–19 years. In this age-group, 1 in 359 Navajo youth had diabetes in 2001 and 1 in 2,542 developed diabetes annually. The majority of diabetes among Navajo youth was type 2 diabetes, although type 1 (autoimmune) diabetes was present, especially among younger children. Of all racial/ethnic groups in the SEARCH study, Navajo youth aged 15–19 years had the greatest risk of type 2 diabetes (14). In contrast, the rates of type 1 diabetes in Navajo youth were the lowest of all SEARCH study racial/ethnic groups (14).
Data on prevalence of diabetes in Navajo youth are minimal. There are virtually no data on diabetes incidence, and no differentiation according to diabetes type was published. An earlier study (7) of 160 Navajo youth aged 12–19 years found that the prevalence of impaired glucose tolerance or undiagnosed diabetes was 8%. In 1999, Kim et al. (6) screened 234 high school student volunteers with oral glucose tolerance tests. One student was found to have diabetes (0.4%) and 3% had impaired glucose tolerance or impaired fasting glucose. Recent efforts by Navajo area IHS investigators are focused on a more comprehensive assessment of the current burden of diabetes in Navajo youth (C.A., personal communication). The majority of data on diabetes among American Indian youth come from population-screening studies, such as the Pima Indian study (1). The prevalence of type 2 diabetes among Pima Indians of Arizona is much higher than reported here for Navajo youth (37.8 and 53.1 per 1,000 among Pima male and female subjects aged 15–19 years, respectively, vs. 2.07 and 2.63 per 1,000 in Navajo male and female subjects aged 15–19 years, respectively). Similarly, the incidence rates of type 2 diabetes are >10-fold higher among Pima youth aged 5–14 years (331.9 per 100,000 per year) than among Navajo youth (19). These data may reflect population differences in risk for type 2 diabetes but are also partly due to systematic screening for case ascertainment among the Pima Indians.
Using the IHS database, Acton et al. (20) recently found that the prevalence of all diabetes among American Indian/Alaska Native youth aged 15–19 years was 5.4 per 1,000, a figure much closer to that reported here (2.78 per 1,000), although this study did not conduct case validation through medical record abstraction. Our experience is that ∼50% of cases identified through the Navajo IHS database in youth aged <20 years were not valid diagnoses of diabetes, due largely to miscoding. Another recent study (21) conducted between 1999 and 2001 among American Indian youth aged <20 years in Montana and Wyoming used methods similar to the SEARCH study (case identification through the IHS database followed by medical record validation). In these populations, the prevalence and incidence of diabetes were similar to those reported for Navajo Indians (prevalence estimates were 0.7 and 1.3 per 1,000 and incidence rates were 5.8 and 23.3 per 100,000 per year for type 1 and type 2 diabetes, respectively).
Our study also provides important descriptive information on characteristics of diabetes type, as well as risk factors for chronic complications. Disturbingly, 40–50% of youth with diabetes had poor glycemic control. In addition, there was a high prevalence of obesity and related cardiometabolic disturbances (central fat deposition, dyslipidemia, and hypertension), especially among type 2 diabetic youth (Table 3). No such information was previously available in youth with diabetes, although one of the earliest studies (6) of nondiabetic Navajo youth found them to have higher BMI levels, with higher triglyceride and lower HDL cholesterol levels than NHW youth. Coupled with high prevalence of unhealthy behaviors (smoking, high-fat diets, and sedentary lifestyles) and lower socioeconomic status (Table 3), these findings may translate to an increased prevalence of cardiovascular disease in the future as these youth mature.
There are a several potential limitations to this study. We did not include Navajo Indians who received services from non-IHS providers. Because the IHS facilities provide universal health care coverage to all American Indian/Alaskan Native people, we do not believe that our ascertainment approach excluded an important number of youth with diabetes. We did not attempt to assess how much undiagnosed diabetes existed through screening. We may, therefore, have underestimated the true risk of type 2 diabetes in Navajo youth. Similar to other population-based studies (1,22), our analyses utilized diabetes type assignments made by health care providers. Diabetes type was further characterized in a subset of Navajo youth participating in the research visit. With a clinical diagnosis of type 1 diabetes, GAD65 antibody positivity was present in almost 50% of individuals (Table 2), similar to other racial/ethnic groups participating in the SEARCH study (14). The GAD65-negative participants with type 1 diabetes may have lost GAD positivity, may be positive for other autoantibodies, or may have other causes of insulin deficiency. With a clinical diagnosis of type 2 diabetes, 20.3% of Navajo participants had positive GAD65 antibodies, which is similar to other racial/ethnic groups participating in the SEARCH study (14), as well as other smaller U.S. studies (23). The role of autoantibody positivity in the etiology and natural evolution of diabetes among youth with a clinical phenotype of type 2 diabetes requires further exploration.
Our data provide strong evidence that diabetes is an important health problem for Navajo youth. Because young people with diabetes will have more years of disease burden and an increased risk of developing diabetes-related complications early in life, targeted efforts aimed at primary prevention of diabetes in Navajo youth and efforts to prevent or delay the development of chronic complications among those who already developed diabetes are warranted.
Acknowledgments
The study was conducted under a Memorandum of Understanding (MOU) between the Navajo Nation, the Navajo Area Indian Health Service, and the University of Colorado Denver SEARCH investigative team and funded by the Centers for Disease Control and Prevention and the National Institutes of Diabetes, Digestive and Kidney Diseases under cooperative agreement U01 DP000247.
No potential conflicts of interest relevant to this article were reported.
We thank the participants and their families, the Navajo Tribal Council, the Navajo Nation Division of Health, the Navajo Nation Human Research Review Board (NNHRRB), Tribal diabetes program staff, and healthcare providers of the Indian Health Service Navajo Area for their participation, support, and oversight of this study.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
References
- 1.Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ: Increasing prevalence of Type II diabetes in American Indian children. Diabetologia 41 :904 –910,1998 [DOI] [PubMed] [Google Scholar]
- 2.Fagot-Campagna A, Burrows NR, Williamson DF: The public health epidemiology of type 2 diabetes in children and adolescents: a case study of American Indian adolescents in the Southwestern United States. Clin Chim Acta 286 :81 –95,1999 [DOI] [PubMed] [Google Scholar]
- 3.Gohdes D, Kaufman S, Valway S: Diabetes in American Indians. Diabetes Care 16 :239 –243,1993 [DOI] [PubMed] [Google Scholar]
- 4.Sugarman JR, Gilbert TJ, Weiss NS: Prevalence of diabetes and impaired glucose tolerance among Navajo Indians. Diabetes Care 15 :114 –120,1992 [DOI] [PubMed] [Google Scholar]
- 5.Will JC, Strauss KF, Mendlein JM, Ballew C, White LL, Peter DG: Diabetes mellitus among Navajo Indians: findings from the Navajo Health and Nutrition Survey. J Nutr 127 (Suppl. 10):2106S –2113S,1997 [DOI] [PubMed] [Google Scholar]
- 6.Kim C, McHugh C, Kwok Y, Smith A: Type 2 diabetes mellitus in Navajo adolescents. West J Med 170 :210 –213,1999 [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman DS, Serdula MK, Percy CA, Ballew C, White L: Obesity, levels of lipids and glucose, and smoking among Navajo adolescents. J Nutr 127 (Suppl. 10):2120S –2127S,1997 [DOI] [PubMed] [Google Scholar]
- 8.Dabelea D, Pettitt DJ, Jones KL, Arslanian SA: Type 2 diabetes mellitus in minority children and adolescents: an emerging problem. Endocrinol Metab Clin North Am 28 :709 –729 [DOI] [PubMed]
- 9.The SEARCH Study Group: SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 458 –471,2004 [DOI] [PubMed]
- 10.Mayer-Davis EJ, Nichols M, Liese AD, Bell RA, Dabelea DM, Johansen JM, Pihoker C, Rodriguez BL, Thomas J, Williams D: Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Am Diet Assoc 106 :689 –697,2006 [DOI] [PubMed] [Google Scholar]
- 11.Brener ND, Kann L, Kinchen SA, Grunbaum JA, Whalen L, Eaton D, Hawkins J, Ross JG: Methodology of the youth risk behavior surveillance system. MMWR Recomm Rep 53 :1 –13,2004 [PubMed] [Google Scholar]
- 12.Radloff LS: The CES-D scale: a self-report depression scale for research in the general population. App Psych Measure 1 :385 –401,1977 [Google Scholar]
- 13.Lawrence JM, Standiford DA, Loots B, Klingensmith GJ, Williams DE, Ruggiero A, Liese AD, Bell RA, Waitzfelder BE, McKeown RE: Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics 117 :1348 –1358,2006 [DOI] [PubMed] [Google Scholar]
- 14.Writing Group for the SEARCH for Diabetes in Youth Study Group, Dabelea D, Bell RA, D'Agostino RB Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B: Incidence of diabetes in youth in the United States. JAMA 297 :2716 –2724,2007 [DOI] [PubMed] [Google Scholar]
- 15.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Deeb L, Grey M, Anderson B, Holzmeister LA, Clark N: Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 28 :186 –212,2005 [DOI] [PubMed] [Google Scholar]
- 16.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH: Diabetic nephropathy. Diabetes Care 26 (Suppl. 1):S94 –S98,2003 [DOI] [PubMed] [Google Scholar]
- 17.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL: CDC Growth Charts for the United States: methods and development. Vital Health Stat 111 –190:2002,2000 [PubMed]
- 18.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents: The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114 :555 –576,2004 [PubMed] [Google Scholar]
- 19.Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG: Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care 30 :1758 –1763,2007 [DOI] [PubMed] [Google Scholar]
- 20.Acton KJ, Burrows NR, Moore K, Querec L, Geiss LS, Engelgau MM: Trends in diabetes prevalence among American Indian and Alaska native children, adolescents, and young adults. Am J Public Health 92 :1485 –1490,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore KR, Harwell TS, McDowall JM, Helgerson SD, and Gohdes D: Three-year prevalence and incidence of diabetes among American Indian youth in Montana and Wyoming, 1999–2001. J Pediatr 143 :368 –371,2002 [DOI] [PubMed] [Google Scholar]
- 22.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J: Incidence of childhood type 1 diabetes worldwide: Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 23 :1516 –1526,2000 [DOI] [PubMed] [Google Scholar]
- 23.Brooks-Vorrell BM, Greenbaum CJ, Palmer JP, and Pihoker C: Autoimmunity to islet proteins in children diagnosed with new-onset diabetes. J Clin Endocrinol Metab 89 :2222 –2227,2004 [DOI] [PubMed] [Google Scholar]
- 24.Agresti A, Coull BA: Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 52 :119 –126,1998 [Google Scholar]