Abstract
OBJECTIVE—To report the prevalence and incidence of type 1 and type 2 diabetes among African American youth and to describe demographic, clinical, and behavioral characteristics.
RESEARCH DESIGN AND METHODS—Data from the SEARCH for Diabetes in Youth Study, a population-based, multicenter observational study of youth with clinically diagnosed diabetes aged 0–19 years, were used to estimate the prevalence for calendar year 2001 (692 cases) and incidence based on 748 African American case subjects diagnosed in 2002–2005. Characteristics of these youth were obtained during a research visit for 436 African American youth with type 1 diabetes and 212 African American youth with type 2 diabetes.
RESULTS—Among African American youth aged 0–9 years, prevalence (per 1,000) of type 1 diabetes was 0.57 (95% CI 0.47–0.69) and for those aged 10–19 years 2.04 (1.85–2.26). Among African American youth aged 0–9 years, annual type 1 diabetes incidence (per 100,000) was 15.7 (13.7–17.9) and for those aged 10–19 years 15.7 (13.8–17.8). A1C was ≥9.5% among 50% of youth with type 1 diabetes aged ≥15 years. Across age-groups and sex, 44.7% of African American youth with type 1 diabetes were overweight or obese. Among African American youth aged 10–19 years, prevalence (per 1,000) of type 2 diabetes was 1.06 (0.93–1.22) and annual incidence (per 100,000) was 19.0 (16.9–21.3). About 60% of African American youth with type 2 diabetes had an annual household income of <$25,000. Among those aged ≥15 years, 27.5% had an A1C ≥9.5%, 22.5% had high blood pressure, and, across subgroups of age and sex, >90% were overweight or obese.
CONCLUSIONS—Type 1 diabetes presents a serious burden among African American youth aged <10 years, and African American adolescents are impacted substantially by both type 1 and type 2 diabetes.
Consistent with pattern of disease occurrence in adults, type 2 diabetes in youth is more common among nonwhite populations, including African Americans, than among non-Hispanic white (NHW) populations (1). The burden of type 1 diabetes in African American youth has been less emphasized in the literature than that of type 2 diabetes. The SEARCH for Diabetes in Youth Study (SEARCH study) (2) reported that among youth aged 10–19 years, the estimated prevalence of type 1 diabetes in 2001 among African American youth was 2.07 per 1,000 compared with 1.05 per 1,000 for type 2 diabetes, thus demonstrating that type 1 diabetes is an important contributor to the overall health status of the population of African American youth.
African American individuals with diabetes have a higher risk for the chronic complications of diabetes than NHW Americans (3), particularly for diabetic nephropathy (4). In 1998, Harris et al. (5) reported an increased prevalence of diabetic retinopathy among African American compared with NHW subjects that was explained in part by higher A1C in African American participants. A recent meta-analysis confirmed systematically higher A1C among African American individuals with diabetes compared with white counterparts (6), which may contribute to ongoing racial disparities in the occurrence of diabetes complications. The SEARCH study provides the opportunity to estimate the prevalence and incidence of type 1 and type 2 diabetes in African American youth and to describe, in detail, important clinical and behavioral characteristics that will accrue over their lifetime and that may contribute to risk for the many complications of diabetes.
RESEARCH DESIGN AND METHODS
Data for these analyses derive from the SEARCH study. A detailed description of SEARCH study methods has been published elsewhere (7). The SEARCH study is a multicenter observational study that began conducting population-based ascertainment of cases of nongestational diabetes in youth aged <20 years beginning in 2001 and continuing through the present. The SEARCH study has six clinical centers located in Ohio, Colorado, Washington, South Carolina, Hawaii, and California. Youth with diabetes were identified in geographically defined populations in Ohio (eight urban and suburban counties encompassing and surrounding Cincinnati), Washington (five urban counties encompassing and surrounding Seattle), South Carolina, and Colorado (selected counties in 2001, all counties in subsequent years); among managed health care plan enrollees in Hawaii and southern California; and among Indian Health Service beneficiaries in four American Indian populations.
The SEARCH study sought to identify all existing (prevalent) cases of diabetes in 2001 and all newly diagnosed (incident) cases in subsequent years. Diabetes cases were considered valid if diagnosed by a health care provider. Analyses herein include prevalent (2001) and incident cases (2002–2005). Before implementation of the protocol, the study was reviewed and approved by the local institutional review board(s) that had jurisdiction over the local study population, and compliance with the Health Insurance Portability and Accountability regulations was ensured. All study personnel were trained in study procedures before initiation of data collection and then recertified annually.
Data collection
Youth with diabetes or their parent/guardian were asked to complete a short initial survey that collected information on race and ethnicity and diabetes-related factors. Self-reported race and ethnicity were collected using the 2000 U.S. Census questions (8). All youth who replied to the initial survey, excluding those whose diabetes was secondary to other conditions, were invited to a study visit.
Written informed consent and assent was obtained according to the guidelines established by the local institutional review board at the beginning of the study visit. During this visit, additional survey information was collected, including symptoms at presentation, medications, medical care utilization, perceptions of care, and family history. Information about dietary intake, physical activity and other health behaviors, and depressive symptoms was collected from participants aged ≥10 years. This information was not shared with the parent/legal guardian in accordance with written consent by the parent/legal guardian. Dietary intake was assessed by a food frequency instrument modified for administration in youth and designed to capture regionally and culturally specific foods in the SEARCH study population, as previously described (9). Physical activity questions were derived from the Youth Risk Behavioral Surveillance System questionnaire (10). Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) score, as previously reported (11,12).
For all participants, blood was drawn for measurement of diabetes autoantibodies, A1C, fasting glucose, C-peptide, and lipids. Specific laboratory methods for these tests have been previously described (7,13). For youth aged ≥3 years, a brief physical examination included height, weight, and waist circumference; evaluation for acanthosis nigricans; and measurement of systolic and diastolic blood pressure.
Categorization of key variables
Diabetes type was reported by the health care professional or abstracted from the medical records as type 1, type 1a, type 1b, type 2, maturity-onset diabetes of the young, hybrid, or other type. For this report, we have restricted our analyses to youth with type 1 (including type 1a and type 1b) or type 2 diabetes. Cases with maturity-onset diabetes of the young, hybrid, other types, or missing type were excluded from the present analyses (2.8% of registered African American case subjects).
Race/ethnicity was categorized somewhat differently for the prevalence and incidence estimates using all registered youth than it was in the analysis of respondent characteristics based only on those who had a study visit. For both analyses, all participants who reported “Hispanic” ethnicity were categorized as “Hispanic,” regardless of race. For the prevalence and incidence estimates, participants who reported multiple race categories were race bridged using methods developed by the National Center for Health Statistics (8). Participants with missing race and ethnicity data or those classified as “other race” were race bridged or geocoded (7.6% of registered case subjects were race bridged or geocoded). For analyses of characteristics of youth with diabetes, among non-Hispanics, those who reported more than one race were placed into a single race category using the National Center for Health Statistics plurality approach (8). Subjects who could not be classified to one race group using the plurality approach (0.5% of case subjects with a study visit) and those with missing data (0.02% of those with a study visit) were excluded.
A1C was categorized using the American Diabetes Association guidelines as good (<8.0%), marginal (8.0–9.4%), or poor (≥9.5%) (14). Values to define high triglycerides and low HDL cholesterol were based on age-appropriate definitions of components of the metabolic syndrome (15) used previously by the SEARCH study (16). High LDL cholesterol, high apolipoprotein B, and high albumin-to-creatinine ratio were based on published clinical practice guidelines (17,18). High blood pressure was defined based on either systolic or diastolic blood pressure and age-, height-, and sex-specific 95th percentile (19). Other survey-based information related to blood pressure was also considered, including self-report of having been told by a health care provider of a diagnosis of high blood pressure and, based on a medication inventory, self-report of taking medicine known to lower blood pressure. Diabetic ketoacidosis (DKA) at diagnosis was reported for incident cases only and is based on having at least one of the following criteria noted in the medical record: 1) blood bicarbonate <15 mmol/l or pH <7.25 (venous) or <7.30 (arterial or capillary), 2) ICD-9 code 250.1 at discharge, or 3) diagnosis of DKA mentioned in the medical records (20). Depressive symptoms were categorized based on an approach developed by Rushton et al. (21) for use with children and adolescents as minimally (0–15), mildly (16–23), and moderately/severely (24–60) depressed mood.
Estimation of prevalence
Methods for estimating diabetes prevalence in 2001 have been previously reported (2). The numerator for this analysis included all case subjects with nongestational diabetes prevalent in 2001 that were aged <20 years on 31 December 2001 and a resident of the defined population in 2001 (geographically based centers) or a member of the participating health plan in 2001 (membership-based centers). Age was based on the subject's age on 31 December 2001. The denominators included youth aged <20 years who were civilian residents of the study areas covered by the geographic centers or were members of the specific health plans in 2001. The prevalence of diabetes was expressed as cases per 1,000 youth using data pooled across all SEARCH study centers, with the 95% CIs calculated by using an inverted-score test from the binomial distribution (22). While prevalence has been previously reported in 10-year age categories (2), we present data in four age categories (0–4, 5–9, 10–14, and 15–19 years) for youth with type 1 diabetes and in two age categories (10–14 and 15–19 years) for youth with type 2 diabetes.
Estimation of incidence rates
Annual incidence rates for 2002 and 2003 were published previously (23). Here, we present more detailed, race/ethnic-specific incidence rates using diabetic case subjects ascertained with newly diagnosed diabetes over a 4-year period (2002–2005). Because the 2000 U.S. Census projections for youth residing in the participating areas were similar in 2002 and 2003 (−0.2% change overall), for simplicity, the 2002 denominator was multiplied by four and used as the total denominator for case subjects ascertained over the 4-year period of 2002–2005. The larger numbers made available for numerators and denominators by this approach allowed greater stability of the rate estimation within subgroups of race/ethnicity, age, sex, and clinically diagnosed diabetes type. Sensitivity analyses were conducted that demonstrated that this approach was unlikely to result in any quantitatively meaningful bias, even if the true denominator increased by as much as 5% per year, representing a cumulative change up to 16% over 4 years. Current census reports provide very little evidence that any race/ethnic or other subgroup studied in the SEARCH study would have shown such a large change. If such a dramatic change did occur, the impact on the estimation of annual incidence rates (per 100,000) would be <2.1 per 100,000 for high incidence rates (30/100,000) to <0.3 per 100,000 for low incidence rates (<5/100,000). The study covered 20,063,776 person-years at risk.
We assumed that with only 4 calendar years of data, we would not be able to reliably detect change over time in diabetes incidence, and we therefore did not evaluate the data for trends. Annual incidence rates were estimated per 100,000 youth, and 95% CIs were calculated by using an inverted score test from the binomial distribution (22).
Statistical testing was conducted across subgroups of interest using χ2 tests for categorical variables, t tests for two-group comparisons, or ANOVA models, as appropriate. Linear or logistic regression was used to adjust for differences in diabetes duration between age categories for continuous and dichotomous outcomes, respectively. Variables that were adjusted for diabetes duration were adjusted using the overall SEARCH study population. Despite the number of comparisons made, given the descriptive and hypothesis-generating nature of these analyses, we retained use of the traditional α of 0.05 to declare statistical significance. For efficiency, data tables and figures show results for both type 1 and type 2 diabetes; however, results are described first for type 1 diabetes, including prevalence, incidence, and characteristics, and then for type 2 diabetes.
RESULTS
Type 1 diabetes
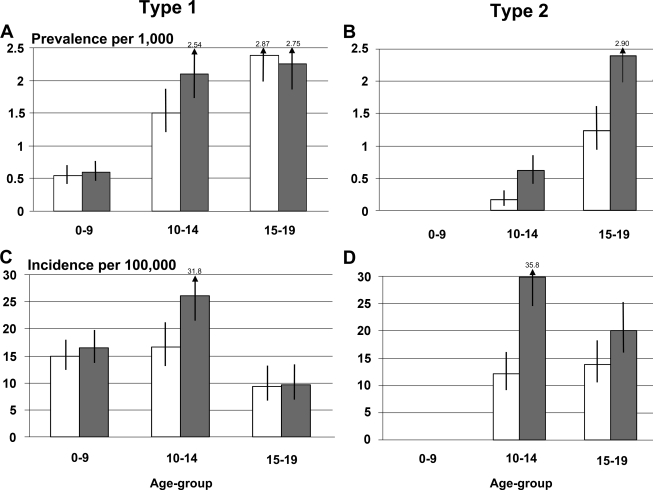
Estimates of prevalence and incidence of type 1 diabetes among African American boys and girls are given in Fig. 1A and C. An online table (available at http://dx.doi.org/10.2337/dc09-s203) shows details of numerators, denominators, and prevalence and incidence rates with 95% CIs. For African American girls aged 10–14 years, prevalence and incidence of type 1 diabetes exceeded that of boys the same age.
Figure 1.
Prevalence (2001) and incidence (2002–2005) of type 1 and type 2 diabetes among African American youth according to age and sex: the SEARCH study. □, male subjects;
 , female subjects.
, female subjects.
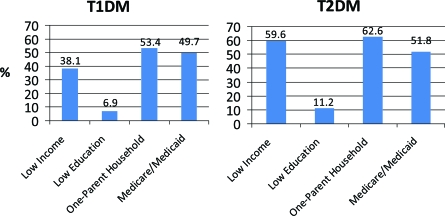
Socioeconomic and family characteristics are shown in Fig. 2. More than one-third of African American youth with type 1 diabetes had a household annual income of <$25,000, and >50% lived in one-parent households.
Figure 2.
Markers of socioeconomic status among African American youth with type 1 and type 2 diabetes: the SEARCH study. Less income is <$25,000 annual household income. Low education is less than high school diploma. Medicare/Medicade is insurance reported by participants from the four geographic-based sites only (health plan sites excluded).
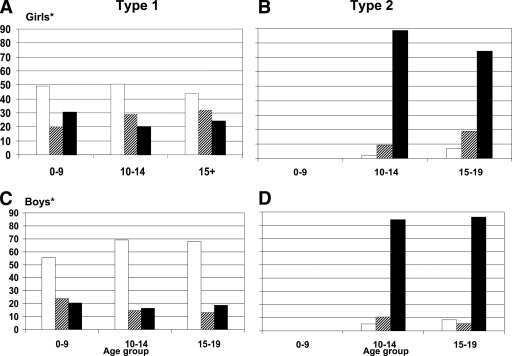
Figure 3 displays the prevalence of overweight and obesity. There was a significant association between the age-groups and BMI categories, where girls with type 1 diabetes had higher percentages of overweight and obese than boys. Among those aged ≥15 years, 24% of girls and 18.9% of boys were classified as obese.
Figure 3.
Overweight and obesity among African American youth with type 1 and type 2 diabetes according to age and sex: the SEARCH study. *Difference in weight status between type 1 diabetic female and male subjects is significant (P < 0.01). Age represents age at time of SEARCH study visit. Weight categories are defined based on Centers for Disease Control and Prevention definitions of weight status. □, underweight/normal;
 , overweight; ▪, obese.
, overweight; ▪, obese.
Clinical characteristics are presented in Table 1. A substantial proportion of incident case subjects aged <14 years at the study visit presented with DKA at diagnosis (30.8% of youth aged 0–9 years and 25.9% of youth aged 10–14 years). Glycemic control was significantly associated with age, with 20.6% of youth with type 1 diabetes aged 0–9 years having A1C ≥9.5 compared with ∼50% of youth aged ≥15 years. Results were similar after adjustment for duration of diabetes. About 25% of African American youth aged ≥15 years had acanthosis nigricans.
Table 1.
Clinical characteristics of African American youth with diabetes, according to current age: the SEARCH study, cases from 2001 (prevalent) and 2002–2005 (incident)
| Type 1 diabetes |
Type 2 diabetes |
||||||
|---|---|---|---|---|---|---|---|
| 0–9 years | 10–14 years | ≥15 years | P*†‡ | 10–14 years | ≥15 years | P*†‡ | |
| n (%)§ | 161 (37) | 162 (37) | 113 (26) | 0.0045 | 81 (38) | 131 (62) | 0.0006 |
| Diabetes diagnosis | |||||||
| Age at diagnosis (years) (means ± SD) | 4.7 ± 2.4 | 9.1 ± 3.1 | 12.2 ± 4.4 | <0.0001 | 11.7 ± 1 | 15.1 ± 1.9 | <0.0001 |
| Symptoms present (% yes) | 93.1 | 88.1 | 86.4 | 0.1556 | 68.4 | 69.3 | 0.8876 |
| Diagnosis during routine check-up (% yes) | 9.9 | 5.7 | 15.5 | 0.03 | 41.3 | 34.4 | 0.3178 |
| DKA present at diagnosis (% yes)‖ | 30.8 | 25.9 | 16.7 | 0.2401 | 10.9 | 13.1 | 0.7081 |
| Diabetes duration (months) (means ± SD) | 19.5 ± 19.4 | 35.6 ± 36.7 | 60.9 ± 56.7 | <0.0001 | 13.5 ± 8.8 | 31.6 ± 24.8 | <0.0001 |
| Diabetes duration categories (%) | <0.0001 | <0.0001 | |||||
| <6 months | 20.5 | 11.7 | 9.7 | 18.5 | 9.2 | ||
| 6–12 months | 29.2 | 19.8 | 10.6 | 29.6 | 13.7 | ||
| >12 months | 50.3 | 68.5 | 79.7 | 51.9 | 77.1 | ||
| Family history of diabetes (% yes)¶ | 57.5 | 71.3 | 76.1 | 0.0023 | 95.0 | 90.6 | 0.2493 |
| Current medication | |||||||
| Insulin (% yes) | 98.8 | 96.8 | 86.5 | 0.0004 | 18 | 27.5 | 0.1087 |
| Metformin (% yes) | 0.6 | 0 | 1.8 | 51.3 | 38.3 | ||
| Both insulin and metformin (% yes) | 0 | 3.2 | 9.9 | 25.6 | 22.5 | ||
| Recent emergency room or hospitalization** | |||||||
| DKA (% yes) | 8.7 | 10.1 | 9.7 | 0.9107 | 6.2 | 6.2 | 0.9955 |
| Hypoglycemia (% yes) | 6.8 | 3.8 | 5.3 | 0.4752 | 1.2 | 0 | 0.2059 |
| Current glycemic control | |||||||
| A1C <8.0% | 39.7 | 32.8 | 29.6 | 0.0001 | 74.2 | 59.6 | 0.0854 |
| 8 ≤ A1C < 9.5% | 39.7 | 28.2 | 20.4 | 12.1 | 12.8 | ||
| A1C ≥9.5% | 20.6 | 38.9 | 50 | 13.6 | 27.5 | ||
| Current glycemic control (adjusted for duration) | |||||||
| A1C <8.0% | 27.1 | 26.8 | 30.8 | 0.7975 | 69.3 | 62.3 | 0.3972 |
| 8 ≤ A1C < 9.5% | 43 | 28.8 | 18.2 | 0.0016 | 13.6 | 12 | 0.7840 |
| A1C ≥9.5% | 24.5 | 40.2 | 46 | 0.0070 | 16.1 | 25.3 | 0.2079 |
| GAD65 positive (% yes) | 52.3 | 64.3 | 51.6 | 0.0783 | 17.2 | 18.1 | 0.8809 |
| GAD65 positive (adjusted for duration) (% yes) | 47.4 | 63.3 | 55.5 | 0.0456 | 16.2 | 18.4 | 0.7325 |
| Fasting C-peptide (ng/ml) (adjusted for duration) (means ± SE) | 0.4 ± 0.07 | 0.6 ± 0.06 | 0.8 ± 0.07 | 0.0023 | 3.1 ± 0.2 | 2.8 ± 0.1 | 0.3159 |
| Acanthosis (% yes) | 11.7 | 14.6 | 25.3 | 0.0163 | 69.4 | 79.8 | 0.1113 |
| Blood pressure | |||||||
| Systolic blood pressure (mmHg) (means ± SD) | 92.7 ± 13.2 | 105 ± 11.5 | 112.4 ± 11.4 | <0.0001 | 116 ± 12.9 | 118.6 ± 12.6 | 0.1714 |
| Diastolic blood pressure (mmHg) (means ± SD) | 58.3 ± 11.4 | 65.5 ± 10.8 | 73.1 ± 10.7 | <0.0001 | 71.7 ± 10.5 | 74.6 ± 10.1 | 0.0633 |
| High blood pressure (% yes)†† | 6.1 | 7.8 | 9.8 | 0.5779 | 24.7 | 22.5 | 0.7378 |
| Ever told by provider they had high blood pressure (% yes) | 2.5 | 5.1 | 12.4 | 0.0026 | 22.2 | 23.3 | 0.8622 |
| Blood pressure (adjusted for duration) | |||||||
| Systolic blood pressure (mmHg) (means ± SE) | 93 ± 1.1 | 105.2 ± 1 | 112.1 ± 1.2 | <0.0001 | 115.5 ± 1.6 | 118.8 ± 1.2 | 0.1137 |
| Diastolic blood pressure (mmHg) (means ± SE) | 59.3 ± 1.0 | 65.8 ± 0.9 | 72.4 ± 1.1 | <0.0001 | 72.4 ± 1.3 | 74.3 ± 1.0 | 0.2534 |
| High blood pressure (% yes)†† | 6.7 | 8 | 8.9 | 0.8465 | 23.4 | 22.9 | 0.9375 |
| Ever told by provider they had high blood pressure (% yes) | 3.1 | 5.1 | 9.3 | 0.1456 | 22 | 23.4 | 0.8255 |
| High albumin-to-creatinine ratio (% yes)‡‡ | 6.4 | 11 | 11.5 | 0.3272 | 15.2 | 13.4 | 0.7528 |
| High albumin-to-creatinine ratio (adjusted for duration) | 7.5 | 11.2 | 8.9 | 0.6187 | 16.9 | 12.5 | 0.4867 |
| LDL cholesterol (mg/dl) (means ± SD) | 103.3 ± 26.4 | 97.4 ± 28.7 | 112.4 ± 35.8 | 0.0024 | 103.3 ± 34.3 | 112.8 ± 31.7 | 0.0803 |
| LDL cholesterol (mg/dl) (adjusted for duration) (means ± SE) | 106.5 ± 3.2 | 98.2 ± 2.7 | 110.1 ± 3.3 | 0.0149 | 106.7 ± 4.5 | 111.4 ± 3.3 | 0.4147 |
| LDL cholesterol | |||||||
| Percent with LDL cholesterol ≥100 mg/dl§§ | 57 | 39.2 | 65.5 | 0.0005 | 58.6 | 62.4 | 0.6402 |
| Percent with LDL cholesterol ≥100 mg/dl (adjusted for duration) | 61.4 | 40.2 | 63.1 | 0.0012 | 65.2 | 60.8 | 0.6029 |
| Percent with LDL cholesterol ≥70 mg/dl§§ | 90.0 | 87.5 | 91.7 | 0.6204 | 77.6 | 95.1 | 0.0008 |
| Percent with LDL cholesterol ≥70 mg/dl (adjusted for duration) | 91.1 | 88 | 91.2 | 0.6687 | 85.8 | 95.6 | 0.0259 |
| Triglycerides (mg/dl) (geometric means ± SD) | 50.1 ± 1.5 | 57.5 ± 1.5 | 73.9 ± 1.8 | <0.0001 | 85.3 ± 1.7 | 101.5 ± 1.9 | 0.0732 |
| Triglycerides (mg/dl) (adjusted for duration) (geometric means ± SE) | 52.5 ± 1.1 | 58.3 ± 1.0 | 71.4 ± 1.1 | 0.0003 | 81.4 ± 1.1 | 103.4 ± 1.1 | 0.0227 |
| Triglycerides | |||||||
| Percent with high triglycerides‖‖ | 3 | 6.7 | 21.4 | <0.0001 | 24.1 | 40.6 | 0.0357 |
| Percent with high triglycerides (adjusted for duration) | 3.5 | 6.8 | 18.6 | 0.0058 | 22.1 | 41.6 | 0.0199 |
| HDL cholesterol (mg/dl) (means ± SD) | 61 ± 14 | 57.9 ± 12 | 54.2 ± 13.2 | 0.0008 | 44.7 ± 10.2 | 45.2 ± 11.4 | 0.7693 |
| HDL cholesterol (mg/dl) (adjusted for duration) (means ± SE) | 61.7 ± 1.3 | 58.1 ± 1.1 | 53.7 ± 1.4 | 0.0002 | 46.2 ± 1.4 | 44.6 ± 1.1 | 0.3615 |
| HDL cholesterol | |||||||
| Percent with low HDL cholesterol¶¶ | 5.8 | 6.1 | 13.5 | 0.0665 | 36.4 | 35.9 | 0.9455 |
| Percent with low HDL cholesterol (adjusted for duration) | 5.7 | 6.1 | 13.7 | 0.0938 | 27.9 | 37.2 | 0.2342 |
| Apolipoprotein B (geometric means ± SD) | 67.2 ± 1.3 | 70.8 ± 1.3 | 82.7 ± 1.5 | 0.0003 | 78.5 ± 1.4 | 92.2 ± 1.3 | 0.0077 |
| Apolipoprtein B (adjusted for duration) (geometric means ± SE) | 69.7 ± 1 | 71.4 ± 1 | 80.7 ± 1 | 0.0202 | 78.9 ± 1.1 | 91.9 ± 1 | 0.0210 |
| Apolipoprotein B | |||||||
| Percent with apolipoprotein B ≥90 mg/dl§§ | 11.5 | 16.1 | 36.1 | 0.0008 | 34.3 | 51.3 | 0.0937 |
| Percent with apolipoprotein B ≥90 mg/dl (adjusted for duration) | 13.7 | 16.4 | 32.5 | 0.0273 | 33.6 | 51.7 | 0.1011 |
| Percent with apolipoprotein B ≥80 mg/dl§§ | 21.3 | 28.7 | 48.6 | 0.0020 | 45.7 | 69.2 | 0.0173 |
| Percent with apolipoprotein B ≥80 mg/dl (adjusted for duration) | 25.3 | 29.5 | 44.9 | 0.0642 | 51.4 | 67.2 | 0.1417 |
| Current lipid-lowering medication (% yes) | 0 | 0.6 | 0.9 | 0.5208 | 0 | 3.3 | 0.1033 |
| Current lipid-lowering medication (adjusted for duration) | 0 | 0.1 | 0.01 | 0.5430 | 0 | 3 | 0.9999 |
P value for categorical variables using χ2 test for the association between variable levels and age-groups.
P value for continuous variables using ANOVA for the overall effect of age-group.
P value for adjusted variables using logistic regression (categorical variables) or linear regression (continuous variables) for the overall effect of age-group.
For non–laboratory-based variables, numbers in cells may vary slightly due to occasional missing data. For laboratory-based variables that required fasting (C-peptide, triglycerides, and LDL cholesterol), ∼30% of type 1 participants and 25% of type 2 participants had missing values. For the remaining laboratory-based variables, ∼20% of participants had missing values. Percents reflect total percent of type 1 diabetes and total percent of type 2 diabetes.
Reported for incident cases only; defined as blood bicarbonate <15 mmol/l or pH <7.25 (venous) or <7.30 (arterial or capillary) or DKA ICD-9 code (250.1) documented in the medical records or DKA diagnosis mentioned in the medical records with or without biochemical confirmation or ICD-9 code.
Family history includes parents, grandparents, and biological siblings.
Emergency room or hospitalization in the last 6 months.
High blood pressure defined as measured blood pressure (systolic and diastolic) ≥ age-, sex-, and height-specific 95th percentile.
Albumin-to-creatinine ratio ≥30 μg/mg.
American Diabetes Association/American College of Cardiology consensus statement on lipoprotein management (ref. 20).
≥110 mg/dl.
≤40 mg/dl.
After adjustment for diabetes duration, both systolic and diastolic blood pressure were higher among older compared with younger youth with type 1 diabetes (Table 1). However, the prevalence of high blood pressure (based on measurement of blood pressure) did not differ significantly by age category and was <10%. Similarly, <10% of all youth had been told by a health care provider they had high blood pressure; however, this proportion increased significantly with age and was 12% among those aged >15 years.
The prevalence of having high LDL cholesterol (defined as LDL cholesterol ≥100 mg/dl) was high in all age-groups (57, 39.2, 65.5 for ages 0–9, 10–14, and ≥15 years, respectively), and >85% of all age-groups exceeded the recent diabetes-specific guidelines for adults of LDL cholesterol <70 mg/dl (17). These guidelines also suggest apolipoprotein B of <80 mg/dl for adults with diabetes; the prevalence of high apolipoprotein B was significantly higher among older compared with younger age-groups and was 48.6% for the age-group ≥15 years. The prevalence of having either high triglyceride or low HDL cholesterol concentrations was lower (<25% for either lipid abnormality across all age-groups). Less than 1% of participants with type 1 diabetes were taking lipid-lowering medication.
Table 2 presents behavioral and psychosocial characteristics for youth with type 1 diabetes aged ≥10 years. Slightly >10% had high CES-D scores suggestive of a high degree of depression-related symptoms. Of the dietary variables, the recommendation most commonly met was for consumption of dairy products; however, nearly 70% of youth aged 10–14 years and 80% of those aged ≥15 years consumed less than the recommended two servings per day. A majority of youth reported participation in moderate or vigorous physical activity; however, physical inactivity was also common (84% of youth aged ≥15 years reported watching television ≥2 h/day).
Table 2.
Behavioral and psychosocial characteristics of African American youth with diabetes, according to current age*: the SEARCH study, cases from 2001 (prevalent) and 2002–2005 (incident)
| Type 1 diabetes |
Type 2 diabetes |
|||||
|---|---|---|---|---|---|---|
| 10–14 years | ≥15 years | P†‡ | 10–14 years | ≥15 years | P†‡ | |
| n (%)§ | 162 (59) | 113 (41) | 0.0031 | 81 (38) | 131 (62) | 0.0006 |
| CES-D score (means ± SD) | 12.9 ± 8.8 | 11.0 ± 8.3 | 0.1003 | 12.9 ± 8.2 | 15.3 ± 10 | 0.1085 |
| High CES-D score (% ≥24) | 11.6 | 10.4 | 0.7639 | 9.9 | 17.4 | 0.1578 |
| Smoking (% current) | 0 | 12.4 | <0.0001 | 4.2 | 13 | 0.0481 |
| Diet | ||||||
| Percent total kcal from total fat (means ± SD) | 39.3 ± 6.0 | 39.6 ± 5.9 | 0.7385 | 39.4 ± 6.3 | 37.6 ± 6.6 | 0.0736 |
| Percent total kcal from saturated fat (means ± SD) | 13.8 ± 2.3 | 13.9 ± 2.4 | 0.6902 | 13.8 ± 2.5 | 13.3 ± 2.5 | 0.1756 |
| Percent with ≥10% of kcal from saturated fat | 96.7 | 96.7 | 0.9855 | 96.8 | 87.9 | 0.0482 |
| Percent with ≥7% of kcal from saturated fat | 100 | 100 | NA | 100 | 100 | NA |
| Percent servings fruit and vegetables <5 per day | 86 | 84.8 | 0.8101 | 84.1 | 80.8 | 0.5911 |
| Percent with <2 servings dairy per day | 69.4 | 80.4 | 0.0689 | 77.8 | 83.8 | 0.3330 |
| Physical activity (% 3–7 days/week moderate or vigorous activity) | 64.8 | 52.8 | 0.0575 | 62 | 43.5 | 0.0143 |
| Physical inactivity (% watching television ≥2 h/day) | 68.3 | 84.0 | 0.0049 | 81.7 | 75.7 | 0.3345 |
These data were collected only among youth aged ≥10 years.
P value for categorical variables using χ2 test for the association between variable levels and age-groups.
P value for continuous variables using ANOVA for the overall effect of age-group.
Percents reflect total percent of type 1 diabetes and total percent of type 2 diabetes for age categories shown.
Type 2 diabetes
Figure 1 shows prevalence and incidence of type 2 diabetes among African American boys and girls, with numeric details provided in the online appendix. Type 2 diabetes was exceedingly rare among youth aged <10 years. During the 4 incident years, only 16 individuals were <10 years old at diagnosis (15 girls, 1 boy) out of a total of 298 case subjects (∼5%). Girls had substantially greater prevalence and incidence of type 2 diabetes than boys; for example, annual incidence among youth aged 10–14 years was 29.8/100,000 (95% CI 24.8–35.8) among girls compared with 12.2/100,000 (9.2–16.1) among boys.
Nearly 60% of youth with type 2 diabetes lived in households with an annual income <$25,000, and 62.6% lived in single-parent households (Fig. 2). Displayed in Fig. 3, the vast majority of both boys and girls with type 2 diabetes were obese; <10% of boys and girls were normal or underweight.
Clinical characteristics are presented in Table 1. Type 2 diabetes was diagnosed during a routine check-up in 41.3% of youth aged 10–14 years and among 34.4% of those aged ≥15 years, and slightly <70% reported at least one symptom of diabetes. Over 90% of youth with type 2 diabetes reported a family history of diabetes. Insulin, either alone or in combination with metformin, was used by 43.6% of youth aged 10–14 years and by 50% of older youth. A majority of youth with type 2 diabetes had A1C <8.0%, although 13.6% of those aged 10–14 years and 27.5% of older youth had A1C ≥9.5%. A majority of participants with type 2 diabetes had acanthosis nigricans.
About one-quarter of youth had high blood pressure, and about the same proportion had been told by a health care provider that they had high blood pressure. High albumin-to-creatinine ratio was found among 15.2% of youth with type 2 diabetes aged 10–14 years and among 13.4% of older youth.
A majority of youth with type 2 diabetes exceeded consensus guidelines for LDL cholesterol, including 95.1% of older youth who had LDL cholesterol ≥70 mg/dl. After adjustment for duration of diabetes, the prevalence of high triglycerides was higher among older compared with younger youth with type 2 diabetes (41.6 vs. 22.1%, respectively; P < 0.05). About one-third of youth with type 2 diabetes had low HDL cholesterol with no difference between age-groups.
High CES-D score was found among 9.9% of younger and among 17.4% of older youth with type 2 diabetes (Table 2). Thirteen percent of youth aged ≥15 years smoked. Over 80% of youth reported high intake of saturated fat and low intake of fruits and vegetables and dairy products. Participation in moderate or vigorous physical activity three or more times per week was lower in older youth with type 2 diabetes (43.5%) compared with younger youth (62%) (P < 0.05). Physical inactivity was observed among >75% of youth with type 2 diabetes.
CONCLUSIONS
African American youth aged <10 years have a relatively low prevalence of type 1 diabetes, but by the age of 10–19 years, an estimated 2.17/1,000 African American girls have type 1 diabetes, as do 1.91/1,000 African American boys (Fig. 1). The burden of type 2 diabetes in African American youth aged >10 years is more than twice as high among African American girls (prevalence of 1.47/1,000 [95% CI 1.24–1.73]) than among African American boys (0.67/1,000 [0.53–0.86]). African American youth with type 2 diabetes commonly live in low socioeconomic conditions. In adults, low socioeconomic status has been associated with increased prevalence of type 2 diabetes (24,25). Consistent with this observation, nearly 60% of African American youth with type 2 diabetes who participated in the SEARCH study visit were from households with low income. Metabolic control was generally poor in youth with either type 1 or type 2 diabetes. Particularly across the full age range for type 1 diabetes (0–9 to ≥15 years), several metabolic parameters were worse among older compared with younger youth even after adjustment for duration of diabetes.
From the worldwide DIAMOND Study Group (26), incidence rates of type 1 diabetes ranged from 0.1 to 40.9/100,000 per year. Incidence rates from countries in Africa were much lower (0.8 to 1.3/100,000 per year) than those reported for African Americans in the SEARCH study or other U.S. studies (27). Registries of childhood diabetes in the 1980s consistently reported lower incidence rates for African American youth compared with white youth. For 1990–1994 in Allegheny County, Pennsylvania, Lipman et al. (28) reported lower incidence of insulin-treated diabetes among nonwhite (primarily African American) youth compared with white youth aged <10 years, nearly identical rates for nonwhite and white youth aged 10–14 years, and for youth age 15–19 years, incidence rates were higher among nonwhite youth (30.4/100,000 per year [95% CI 18.3–47.4]) than among white youth (11.2/100,000 per year [7.6–15.9]). A similar pattern was reported from Philadelphia, Pennsylvania. For ages 0–17 years in the Chicago, Illinois, registry (29), for youth with presumed type 1 diabetes, a smaller difference in incidence rates between African American and NHW girls was observed than between African American and NHW boys. From the Allegheny County cohort, Libman et al. (27) described lower prevalence of diabetes autoantibodies among African American youth with insulin-treated diabetes and higher prevalence of obesity and other characteristics commonly associated with type 2 diabetes among African American compared with white youth (30). The Allegheny County, Philadelphia, or Chicago registries did not published data by race/ethnicity or according to diabetes type and sex within age-group, presumably due to sample size limitations.
From the SEARCH study, the incidence rates (2002–2003) among African American youth aged 0–4 and 5–9 years were significantly lower than NHW youth (31), and this was true for both boys and girls. Similar to younger age-groups, incidence was significantly lower among African American boys aged 10–14 years than among NHW boys of the same age. Taking advantage of the greater sample size from 4 years of incidence data (2002–2005), for girls aged 10–14 years, incidence of type 1 diabetes was significantly higher among the African American girls (26.1/100,000 per year [95% CI 21.5–31.8]) than among African American boys (16.7/100,000 per year [13.1–21.2]) and was not different from incidence among NHW girls (29.1/100,000 per year [26.6–32.0]) (32). Patterns of incidence were similar for ages 15–19 years compared with patterns for ages 10–14 years.
Thus, the large sample size of the SEARCH study, inclusive of a total of 450 African American validated cases of provider-diagnosed type 1 diabetes, allowed observation of patterns of incidence that raise at least two critical questions for future research. First, what are the genetic, behavioral, or environmental factors that protect African American youth aged <10 years and African American boys aged 10–19 years against the higher incidence of type 1 diabetes observed in their NHW counterparts? Second, what are the factors that cause African American girls aged 10–19 years to lose this protective advantage? Beginning with the observation that studies consistently show a marked race/ethnic difference in obesity among women and much smaller race/ethnic differences among men (33), we propose one avenue for consideration as a possible explanation for the latter.
Previously, the SEARCH study reported limited evidence for the accelerator hypothesis (34), which postulates that obesity-mediated insulin resistance contributes to the development of type 1 diabetes by accelerating destruction of pancreatic β-cells (35). We observed that current BMI was associated with younger age at diagnosis among individuals with β-cell function below the median, as measured by fasting C-peptide. From the National Heart, Lung and Blood Institute Growth and Health Study (36), adolescent African American girls were more commonly overweight than NHW girls, as expected. Interestingly, the rate of new-onset overweight was similar between African American and NHW girls for ages 13–19 years; however, for ages 9–12 years, new-onset overweight was notably higher among African American girls (9.4%) than among NHW girls (5.8%). Thus, it is possible that earlier (prepubertal) development of obesity in African American girls is acting during pubertal development to markedly increase risk at this vulnerable time for onset of type 1 diabetes.
Banerji (37) has described a high proportion (40%) of African American youth with type 2 diabetes with DKA at the time of diagnosis, which may reflect the phenomenon of Flatbush diabetes as observed among African American adults (38), in which DKA is common despite absence of common diabetes autoantibodies and low prevalence of HLA genotypes associated with high risk for type 1 diabetes (39). In the SEARCH study, the percent of youth with provider-diagnosed type 2 diabetes who presented with DKA was ∼12%, much lower than that reported by Banerji and similar to previously reported prevalence of DKA at diagnosis among other race/ethnic groups in SEARCH (20). Future analyses of SEARCH data will further explore this issue and will incorporate diabetes autoantibody data as well as HLA genotype.
Glycemic control among African American youth in the present study was quite poor, evidenced by ∼50% of youth aged ≥15 years with type 1 diabetes with A1C ≥9.5% and 27.5% of youth aged ≥15 years with type 2 diabetes with similarly high A1C. Other studies have documented worse glycemic control among African American youth with diabetes compared with NHW youth (40–42), as well as more rapid decline in metabolic control postdiagnosis among African American youth compared with white youth with type 1 diabetes (43). Living in a one-parent household may partly explain this phenomenon (42,43), and, in fact, youth from single-parent households experienced decline in metabolic status nearly three times as fast as those from two-parent households (43). Over 50% of African American SEARCH study participants with type 1 diabetes reported living in a single-parent household, as did 63% of African American youth with type 2 diabetes. In an adult population, medication adherence did not fully explain elevated A1C observed in African American compared with white patients (44); thus, it is possible that biological as well as social and behavioral factors contribute to suboptimal glycemic control among African American individuals.
Among U.S. adults, the prevalence of hypertension is higher among African Americans (33.5%) than among Mexican Americans (20.7%) or NHWs (28.9%) (P < 0.01) (45), and this pattern is present among children and adolescents as well (46). In SEARCH study participants with type 2 diabetes, nearly a quarter had high blood pressure and a similar proportion had been told by a provider that they had high blood pressure. This observation is of critical public health importance because systolic blood pressure in childhood has been prospectively associated with increased carotid artery intima-media thickness in adulthood (47). In addition, among African Americans, but not NHWs, elevated blood pressure in childhood predicted microalbuminuria in adulthood (48).
The prevalence of elevated LDL cholesterol was surprisingly high among African American SEARCH study youth with either type 1 or type 2 diabetes. Over 90% of youth aged ≥15 years had LDL cholesterol >70 mg/dl, and >60% had LDL cholesterol >100 mg/dl. In addition, nearly half of older youth with type 1 diabetes and ∼70% of youth with type 2 diabetes had high apolipoprotein B (>80 mg/dl). These values are established cut points for identification of individuals at high risk for cardiovascular disease based on predictive models in adult populations (17) and thus highlight the potential for substantial future cardiovascular disease in youth with either type 1 or type 2 diabetes. It is possible that the poor glycemic control among the African American youth contributes substantially to this finding of high apolipoprotein B (49).
Similar to results of national survey data for adults (50) and youth (51), in the SEARCH study we previously reported that African American youth had lower prevalence of low HDL cholesterol compared with NHW youth (16). Low HDL cholesterol, however, remains an important consideration both for youth with type 1 and type 2 diabetes. For youth with type 1 diabetes aged ≥15 years, 13.5% had low HDL cholesterol. Of youth with type 2 diabetes, approximately one-third had low HDL cholesterol. After adjustment for diabetes duration, the prevalence of elevated triglyceride concentration increased with age in both type 1 and type 2 diabetes; among youth aged ≥15 years, 18.6% of youth with type 1 diabetes and 41.6% of those with type 2 diabetes had elevated triglycerides, again highlighting the adverse cardiovascular disease risk profile in these youth.
Identification of lifestyle factors that may contribute to the adverse metabolic profile described herein for youth with either type 1 or type 2 diabetes may offer opportunities for intervention to improve long-term prognosis. Smoking was reported by 12.4% of youth aged ≥15 years with type 1 diabetes and by 13% of youth with type 2 diabetes. Less than 10% of youth with either type 1 or type 2 diabetes consumed <10% of total calories from saturated fat, and 100% of youth consumed >7% of total calories from saturated fat as recommended for people with diabetes (52). The vast majority of youth reported low intake of fruits and vegetables and dairy products. Although close to half the youth reported participation in moderate or vigorous physical activity on ≥3 days per week, the prevalence of physical inactivity was high.
Clinical trials of diabetes self-management interventions among youth with type 1 diabetes that included behavioral family systems therapy (53) or motivational interviewing (54) have demonstrated improvement in glycemic control; however, studies to date generally have not focused on metabolic status beyond glycemic control and have not focused on the African American population. A large clinical trial now underway among youth with type 2 diabetes, including a diverse study population, will compare three approaches, one of which includes lifestyle combined with metformin, with the primary outcome of time to loss of glycemic control and an array of secondary outcomes to include metabolic, clinical, and behavioral outcomes (55).
In conclusion, African American youth experience a substantial health burden due to both type 1 and type 2 diabetes, and African American youth with diabetes have generally poor metabolic status. Lifestyle behaviors likely to be detrimental to good health are common. The high proportion of youth living in single-parent households and with adverse socioeconomic status may contribute to less than optimal health behaviors and metabolic status. There are opportunities for future research to better understand the occurrence of diabetes in youth particularly related to whether and why overweight and obesity may contribute not only to type 2 diabetes but also to type 1 diabetes, particularly among African American girls. Research on behavioral and pharmacologic approaches to sustainable improvements in lifestyle habits, weight status, and metabolic status including glycemia, blood pressure, and lipid profile among African American youth with either type 1 or type 2 diabetes is urgently needed.
Acknowledgments
The SEARCH for Diabetes in Youth Study is funded by the Centers for Disease Control and Prevention (PA no. 00097 and DP-05-069) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site contract numbers are as follows: Kaiser Permanente Southern California (U01 DP000246), the University of Colorado Health Sciences Center (U01 DP000247), the Pacific Health Research Institute (U01 DP000245), the Children's Hospital Medical Center (Cincinnati) (U01 DP000248), the University of North Carolina (U01 DP000254), the University of Washington School of Medicine (U01 DP000244), and the Wake Forest University School of Medicine (U01 DP000250). The authors acknowledge the involvement of general clinical research centers at the following institutions in the SEARCH for Diabetes in Youth Study: the Medical University of South Carolina (grant no. M01 RR01070), Cincinnati Children's Hospital (grant no. M01 RR08084), Children's Hospital and Regional Medical Center and the University of Washington School of Medicine (grant nos. M01RR00037 and M01RR001271), and the Colorado Pediatric General Clinical Research Center (grant no. M01 RR00069).
No potential conflicts of interest relevant to this article were reported.
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Pinhas-Hamiel O, Zeitler P: The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 146 :693 –700,2005 [DOI] [PubMed] [Google Scholar]
- 2.Liese AD, D'Agostino RB Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, Loots B, Linder B, Marcovina S, Rodriguez B, Standiford D, Williams DE: The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 118 :1510 –1518,2006 [DOI] [PubMed] [Google Scholar]
- 3.Egede LE, Gogo-Jack S: Epidemiology of type 2 diabetes: focus on ethnic minorities. Med Clin North Am 89 :949 –975, viii, 2005 [DOI] [PubMed]
- 4.Crook ED, Patel SR: Diabetic nephropathy in African-American patients. Curr Diab Rep 4 :455 –461, 2004 [DOI] [PubMed]
- 5.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD: Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: the Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21 :518 –524,1998 [DOI] [PubMed] [Google Scholar]
- 6.Kirk JK, D'Agostino RB Jr, Bell RA, Passmore LV, Bonds DE, Karter AJ, Narayan KM: Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care 29 :2130 –2136,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SEARCH Study Group: SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 25 :458 –471,2004 [DOI] [PubMed] [Google Scholar]
- 8.Ingram DD, Parker JD, Schenker N, Weed JA, Hamilton B, Arias E, Madans JH: United States Census 2000 population with bridged race categories. Vital Health Stat 21 –55,2003 [PubMed]
- 9.Mayer-Davis EJ, Nichols M, Liese AD, Bell RA, Dabelea DM, Johansen JM, Pihoker C, Rodriguez BL, Thomas J, Williams D: Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Am Diet Assoc 106 :689 –697,2006 [DOI] [PubMed] [Google Scholar]
- 10.Brener ND, Kann L, Kinchen SA, Grunbaum JA, Whalen L, Eaton D, Hawkins J, Ross JG: Methodology of the youth risk behavior surveillance system. MMWR Recomm Rep 53 :1 –13,2004 [PubMed] [Google Scholar]
- 11.Radloff L: The CES-D scale: a self report depression scale for research in the general population. Applied Psychological Measurement 1 :385 –401,1977 [Google Scholar]
- 12.Lawrence JM, Standiford DA, Loots B, Klingensmith GJ, Williams DE, Ruggiero A, Liese AD, Bell RA, Waitzfelder BE, McKeown RE: Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics 117 :1348 –1358,2006 [DOI] [PubMed] [Google Scholar]
- 13.Kershnar AK, Daniels SR, Imperatore G, Palla SL, Petitti DB, Pettitt DJ, Marcovina S, Dolan LM, Hamman RF, Liese AD, Pihoker C, Rodriguez BL: Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr 149 :314 –319,2006 [DOI] [PubMed] [Google Scholar]
- 14.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Deeb L, Grey M, Anderson B, Holzmeister LA, Clark N: Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 28 :186 –212,2005 [DOI] [PubMed] [Google Scholar]
- 15.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH: Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 157 :821 –827,2003 [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez BL, Fujimoto W, Mayer-Davis E, Imperatore G, Williams DE, Bell RA, Wadwa RP, Palla SL, Liu L, Kershnar A, Daniels S, Linder B: Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for Diabetes in Youth Study. Diabetes Care 29 :1891 –1896,2006 [DOI] [PubMed] [Google Scholar]
- 17.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL: Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 31 :811 –822,2008 [DOI] [PubMed] [Google Scholar]
- 18.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW: Nephropathy in diabetes. Diabetes Care 27 (Suppl. 1):S79 –S83,2004 [DOI] [PubMed] [Google Scholar]
- 19.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents: The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114 :555 –576,2004 [PubMed] [Google Scholar]
- 20.Rewers A, Klingensmith G, Davis C, Petitti DB, Pihoker C, Rodriguez B, Schwartz ID, Imperatore G, Williams D, Dolan LM, Dabelea D: The presence of diabetic ketoacidosis at diagnosis mellitus in youth: the SEARCH for Diabetes in Youth Study. Pediatrics 121 :e1258 –e1266,2007 [DOI] [PubMed] [Google Scholar]
- 21.Rushton JL, Forcier M, Schectman RM: Epidemiology of depressive symptoms in the National Longitudinal Study of Adolescent Health. J Am Acad Child Adolesc Psychiatry 41 :199 –205,2002 [DOI] [PubMed] [Google Scholar]
- 22.Brown LD, Cai TT, DasGupta A: Interval estimation for a binomial proportion. Stat Sci 16 :101 –133,2001 [Google Scholar]
- 23.Misra A, Ganda OP: Migration and its impact on adiposity and type 2 diabetes. Nutrition 23 :696 –708,2007 [DOI] [PubMed] [Google Scholar]
- 24.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS: Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289 :76 –79,2003 [DOI] [PubMed] [Google Scholar]
- 25.Agardh EE, Ahlbom A, Andersson T, Efendic S, Grill V, Hallqvist J, Ostenson CG: Socio-economic position at three points in life in association with type 2 diabetes and impaired glucose tolerance in middle-aged Swedish men and women. Int J Epidemiol 36 :84 –92,2007 [DOI] [PubMed] [Google Scholar]
- 26.DIAMOND Project Group: Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med 23 :857 –866,2006 [DOI] [PubMed] [Google Scholar]
- 27.Libman IM, Pietropaolo M, Trucco M, Dorman JS, LaPorte RE, Becker D: Islet cell autoimmunity in white and black children and adolescents with IDDM. Diabetes Care 21 :1824 –1827,1998 [DOI] [PubMed] [Google Scholar]
- 28.Lipman TH, Jawad AF, Murphy KM, Tuttle A, Thompson RL, Ratcliffe SJ, Levitt Katz LE: Incidence of type 1 diabetes in Philadelphia is higher in black than white children from 1995 to 1999: epidemic or misclassification? Diabetes Care 29 :2391 –2395,2006 [DOI] [PubMed] [Google Scholar]
- 29.Smith TL, Drum ML, Lipton RB: Incidence of childhood type I and non-type 1 diabetes mellitus in a diverse population: the Chicago Childhood Diabetes Registry, 1994 to 2003. J Pediatr Endocrinol Metab 20 :1093 –1107,2007 [DOI] [PubMed] [Google Scholar]
- 30.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ: Evidence for heterogeneous pathogenesis of insulin-treated diabetes in black and white children. Diabetes Care 26 :2876 –2882,2003 [DOI] [PubMed] [Google Scholar]
- 31.Dabelea D, Bell RA, D'Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B: Incidence of diabetes in youth in the United States. JAMA 297 :2716 –2724,2007 [DOI] [PubMed] [Google Scholar]
- 32.Bell RA, Mayer-Davis EJ, Beyer JW, D'Agostino RB Jr, Lawrence JM, Linder B, Liu LL, Marcovina SM, Rodriguez BL, Williams D, Dabelea D: Diabetes in non-Hispanic white youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study.32 (Suppl. 2):102 –111,2009 [DOI] [PMC free article] [PubMed]
- 33.Wang MC, Kim S, Gonzalez AA, MacLeod KE, Winkleby MA: Socioeconomic and food-related physical characteristics of the neighbourhood environment are associated with body mass index. J Epidemiol Community Health 61 :491 –498,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabelea D, D'Agostino RB Jr, Mayer-Davis EJ, Pettitt DJ, Imperatore G, Dolan LM, Pihoker C, Hillier TA, Marcovina SM, Linder B, Ruggiero AM, Hamman RF: Testing the accelerator hypothesis: body size, β-cell function, and age at onset of type 1 (autoimmune) diabetes. Diabetes Care 29 :290 –294,2006 [DOI] [PubMed] [Google Scholar]
- 35.Wilkin TJ: The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia 44 :914 –922,2001 [DOI] [PubMed] [Google Scholar]
- 36.Thompson DR, Obarzanek E, Franko DL, Barton BA, Morrison J, Biro FM, Daniels SR, Striegel-Moore RH: Childhood overweight and cardiovascular disease risk factors: the National Heart, Lung and Blood Institute Growth and Health Study. J Pediatr 150 :18 –25,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerji MA: Diabetes in African Americans: unique pathophysiologic features. Curr Diab Rep 4 :219 –223, 2004 [DOI] [PubMed]
- 38.Banerji MA: Impaired beta-cell and alpha-cell function in African-American children with type 2 diabetes mellitus: “Flatbush diabetes.” J Pediatr Endocrinol Metab 15 (Suppl. 1):493 –501,2002 [PubMed] [Google Scholar]
- 39.Banerji MA, Chaiken RL, Huey H, Tuomi T, Norin AJ, Mackay IR, Rowley MJ, Zimmet PZ, Lebovitz HE: GAD antibody negative NIDDM in adult black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4: Flatbush Diabetes. Diabetes 43 :741 –745,1994 [DOI] [PubMed] [Google Scholar]
- 40.Delamater AM, Shaw KH, Applegate EB, Pratt IA, Eidson M, Lancelotta GX, Gonzalez-Mendoza L, Richton S: Risk for metabolic control problems in minority youth with diabetes. Diabetes Care 22 :700 –705,1999 [DOI] [PubMed] [Google Scholar]
- 41.Chalew SA, Gomez R, Butler A, Hempe J, Compton T, Mercante D, Rao J, Vargas A: Predictors of glycemic control in children with type 1 diabetes: the importance of race. J Diabetes Complications 14 :71 –77,2000 [DOI] [PubMed] [Google Scholar]
- 42.Auslander WF, Thompson S, Dreitzer D, White NH, Santiago JV: Disparity in glycemic control and adherence between African-American and Caucasian youths with diabetes. Family and community contexts. Diabetes Care 20 :1569 –1575,1997 [DOI] [PubMed] [Google Scholar]
- 43.Ellis DA, Templin T, Naar-King S, Frey MA, Cunningham PB, Podolski CL, Cakan N: Multisystemic therapy for adolescents with poorly controlled type I diabetes: stability of treatment effects in a randomized controlled trial. J Consult Clin Psychol 75 :168 –174,2007 [DOI] [PubMed] [Google Scholar]
- 44.Adams AS, Trinacty CM, Zhang F, Kleinman K, Grant RW, Meigs JB, Soumerai SB, Ross-Degnan D: Medication adherence and racial differences in A1C control. Diabetes Care 31 :916 –921,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hajjar I, Kotchen TA: Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 290 :199 –206,2003 [DOI] [PubMed] [Google Scholar]
- 46.Muntner P, He J, Cutler JA, Wildman RP, Whelton PK: Trends in blood pressure among children and adolescents. JAMA 291 :2107 –2113,2004 [DOI] [PubMed] [Google Scholar]
- 47.Li S, Chen W, Srinivasan SR, Tang R, Bond MG, Berenson GS: Race (black-white) and gender divergences in the relationship of childhood cardiovascular risk factors to carotid artery intima-media thickness in adulthood: the Bogalusa Heart Study. Atherosclerosis 194 :421 –425,2007 [DOI] [PubMed] [Google Scholar]
- 48.Hoq S, Chen W, Srinivasan SR, Berenson GS: Childhood blood pressure predicts adult microalbuminuria in African Americans, but not in whites: the Bogalusa Heart Study. Am J Hypertens 15 :1036 –1041,2002 [DOI] [PubMed] [Google Scholar]
- 49.Albers JJ, Marcovina SM, Imperatore G, Snively BM, Stafford J, Fujimoto WY, Mayer-Davis EJ, Petitti DB, Pihoker C, Dolan L, Dabelea DM: Prevalence and determinants of elevated apolipoprotein B and dense low-density lipoprotein in youths with type 1 and type 2 diabetes. J Clin Endocrinol Metab 93 :735 –742,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ford ES, Giles WH, Dietz WH: Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287 :356 –359,2002 [DOI] [PubMed] [Google Scholar]
- 51.Duncan GE, Li SM, Zhou XH: Prevalence and trends of a metabolic syndrome phenotype among U.S. adolescents, 1999–2000. Diabetes Care 27 :2438 –2443,2004 [DOI] [PubMed] [Google Scholar]
- 52.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML: Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 31 (Suppl. 1):S61 –S78,2008 [DOI] [PubMed] [Google Scholar]
- 53.Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Mauras N, White NH: Randomized trial of behavioral family systems therapy for diabetes: maintenance of effects on diabetes outcomes in adolescents. Diabetes Care 30 :555 –560,2007 [DOI] [PubMed] [Google Scholar]
- 54.Channon SJ, Huws-Thomas MV, Rollnick S, Hood K, Cannings-John RL, Rogers C, Gregory JW: A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care 30 :1390 –1395,2007 [DOI] [PubMed] [Google Scholar]
- 55.Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, Wilfley D: Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 8 :74 –87,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]