Abstract
Cyclic diguanylate (c-di-GMP) is a unique bacterial intracellular signaling molecule capable of stimulating enhanced protective innate immunity against various bacterial infections. The effects of intranasal pretreatment with c-di-GMP, or intraperitoneal coadministration of c-di-GMP with the pneumolysin toxoid (PdB) or PspA before pneumococcal challenge, was investigated in mice. We found that c-di-GMP had no significant direct short-term effect on the growth rate of S. pneumoniae either in vitro or in vivo. However, intranasal pretreatment of mice with c-di-GMP resulted in significant decrease in bacterial load in lungs and blood after serotypes 2 and 3 challenge, and significant decrease in lung titers after serotype 4 challenge. Potential cellular mediators of these enhanced protective responses were identified in lungs and draining lymph nodes. Intraperitoneal coadministration of c-di-GMP with PdB or PspA before challenge resulted in significantly higher antigen-specific antibody titers and increased survival of mice, compared to that obtained with alum adjuvant. These findings demonstrate that local or systemic c-di-GMP administration stimulates innate and adaptive immunity against invasive pneumococcal disease. We propose that c-di-GMP can be used as an effective broad spectrum immunomodulator and vaccine adjuvant to prevent infectious diseases.
Keywords: Streptococcus pneumoniae, c-di-GMP, immunomodulator, adjuvant, vaccine
1. Introduction
Streptococcus pneumoniae is the leading cause of bacterial pneumonia, meningitis, and otitis media in the United States [1]. In spite of the availability of antimicrobials, the capsular polysaccharide (PS) vaccine, and the 7-valent protein-PS conjugate vaccine, pneumococcal disease continues to be responsible for high morbidity and mortality worldwide, especially in groups at high risk [2]. Consequently, global efforts are focused on exploring alternative pneumococcal vaccine strategies to address the shortcomings of existing formulations, without compromising efficacy. One of these approaches involves the development of vaccines based on pneumococcal proteins that contribute to pathogenesis and are common to all serotypes. To date, the most promising vaccine candidates are the pneumolysin toxoid (PdB), pneumococcal surface protein A (PspA), pneumococcal surface protein C (PspC, also referred to as choline binding protein A) and the 37-kDa metal-binding lipoprotein PsaA (reviewed by [2]).
We have shown that c-di-GMP (3′,5′-cyclic diguanylic acid or cyclic diguanylate or cGpGp) initially identified in the bacterium Acetobacter xylinum, is an intracellular signaling molecule [3–5]. It is present in multiple bacterial species but not in eukaryotes [6–11], and is now recognized to control many key functions in bacteria, including survival, adherence, colonization, and biofilm formation [8, 9, 12–15]. Recent studies indicate that it might also modulate host cellular responses [14], resulting in an enhanced control of infection [16]. Consistent with this notion, we recently demonstrated that c-di-GMP increases MIG/CXCL9 (a chemoattractant for activated T cells) suggesting possible antitumor activity [17], and inhibits basal and growth factor-induced proliferation of human colon carcinoma cells [18].
Recently, c-di-GMP was shown to modulate the immune system to prevent and fight various lethal bacterial infections [17]. Intranasal (i.n.) or subcutaneous administration of c-di-GMP before intratracheal challenge with Klebsiella pneumoniae also resulted in significantly increased survival and reduction in bacterial counts in lung and blood [19]. The response was characterized by enhanced accumulation of neutrophils, αβ T cells, and activated NK and αβ T lymphocytes, associated with earlier and more vigorous expression of chemokines and type-1 cytokines [19]. Moreover, lung macrophages recovered from Klebsiella-infected mice pretreated with c-di-GMP expressed greater quantities of iNOS (inducible nitric oxide synthase) and nitric oxide ex vivo than those isolated from mice pretreated with control cGMP [19]. These findings demonstrate that c-di-GMP delivered locally or systemically stimulates protective innate immunity in the lung, decrease bacterial burden and enhances protective responses against infection.
In this study, we investigated the ability of c-di-GMP to enhance resistance against systemic pneumococcal infection, using established mouse models. We provide additional direct evidence that c-di-GMP is immunostimulatory, can protect against infection, and acts as an effective vaccine adjuvant against systemic disease.
2. Materials and Methods
2.1. Bacterial strains
The pneumococcal strains used in this study were D39, a virulent type 2 strain [20], strain T4, a type 4 encapsulated strain [21], and Xen10, a bioluminescent derivative of type 3 strain A66.1 [22] that has been engineered to express luciferase so infections can be followed using bioluminescent imaging (Xenogen Corp.,Hopkinton, MA).
2.2. Mice
I.n. challenge studies with D39 and T4 were carried out using 6–8-week old female Balb/cByJ mice (Jackson Laboratory, Bar Harbor, ME) at St. Jude Children’s Research Hospital. For bioluminescence studies, 6–8-week old female C57BL/6 mice (Charles River, Margate, U.K.), were used at the University of York. For intraperitoneal (i.p.) active immunization/challenge studies, 5-week old male outbred CD1 (Swiss) mice were used at the University of Adelaide. All animal experiments were approved by the Animal Care and Use and Ethics committees of the respective institutions.
2.3. c-di-GMP
The c-di-GMP used in these studies was chemically synthesized and prepared as described previously [23]. Control cGMP was purchased from Sigma (St. Louis, MO). c-di-GMP and control cGMP were reconstituted at the appropriate concentration in sterile 0.9% NaCl (saline). Control groups received either saline alone or control cGMP (Sigma). All c-di-GMP preparations were free of endotoxin contamination [17], and did not have any direct antimicrobial effects on S. pneumoniae (not shown).
2.4. In vitro effects of c-di-GMP on S. pnenumonie
We grew S. pneumoniae Xen10 to log phase (as per intranasal challenge protocol) and then added either a volume of saline (control) or 50, 200 or 500 uM of c-di-GMP. Cultures were incubated for a further 30 min at 37°C prior to serial dilution and plating for CFU.
2.5. Pre-treatment of S. pneumoniae with c-di-GMP
To test the effect of pre-incubating S. pneumoniae with c-di-GMP, we prepared doses of Xen10 (as per intranasal challenge protocol) and immediately prior to intranasal challenge added saline or c-di-GMP to give a final concentration of 200 uM per 50 ul dose. Groups of 5 mice in each case were then imaged at 30 min, 1, 2, 4 and 18 h post-challenge.
2.6. Preparation of antigens
A 43 kDa N-terminal His6-tagged PspA fragment was cloned, expressed, and purified as described previously [24–26]. Pneumolysin toxoid (PdB) was cloned as a His6-tagged fusion protein from plasmid pJCP202 [27] by amplification with primers AD20 5′-GGAACTTATTAGGATCCAGAAGATGGC-3′ and AD21 5′-TTGTCGCGAGCTCTCTCCTCTCCTA-3′ (BamHI and SacI restriction sites underlined). The PCR fragment was cloned into the corresponding restriction sites in the polylinker of pQE32 (Qiagen Inc.) and then used to transform a lipid A (lpxM) mutant of Escherichia coli BL21 (DE3) [28]. Recombinant His6-PdB was expressed, purified and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie brilliant blue staining, as described previously [29]. The protein migrated at approx. 53 kDa, consistent with its predicted size from the DNA sequence. His6-PspA fragment was analyzed in parallel and also migrated at the expected size (approx. 43 kDa). Both antigens were judged to be >95% pure (not shown).
2.7. I.n. challenge
For bioluminescence studies, mice were directly challenged i.n. with 2 × 106 CFU of Xen10 in a 50 μl volume under light anesthesia with 2.5% isoflurane (Baxter Healthcare Corporation, Deerfield, IL). Before challenge, groups of mice were treated i.n. with either c-di-GMP (200 nmol ), cGMP (200 nmol; Sigma) or PBS at −48 h and −24 h. Infection was then followed in individual mice using the IVIS Imaging 100 system (Xenogen). Luminescence (photons/sec/cm2) was determined at various time points and quantified using LivingImage software (Xenogen).
For studies using c-di-GMP pretreatment to protect against i.n. challenge and to enumerate bacterial titers in the lungs and blood, mice were anesthetized 48 and 24 h before infection and given 200 nmol per mouse of either c-di-GMP, or control cGMP (Sigma). Both were diluted in saline and delivered in a final volume of 50 μl (25 μl per nostril). Before infection, mice were anesthetized and then challenged directly with 1 × 104, 1 × 105, or 1 × 106 CFU of D39 or 1 × 105 CFU of T4 in 100 μl sterile PBS (50 μl per nostril). Twenty-four hours after infection, mice were lightly anesthetized with isoflurane and approx. 100 μl of blood was obtained via retro-orbital puncture. Mice were then euthanized by CO2 inhalation followed by cervical dislocation. Lungs were removed aseptically, washed three times in sterile PBS, and homogenized in 500 μl sterile PBS. Pneumococci were enumerated by plating serial 10-fold dilutions of lung homogenates or blood on tryptic soy agar (Difco) plates supplemented with 3% v/v sheep erythrocytes.
2.8. Immunization of mice
Six groups of 5-to 6-week-old male CD1 mice (15 per group) were immunized i.p. with PdB or PspA, in either alum adjuvant (Imject Alum no. 77161; Pierce, Rockford, Ill.) or c-di-GMP. Each group received either PdB+alum, PspA+alum, PdB+ c-di-GMP, PspA+c-di-GMP, or a placebo. Each mouse received three doses of 10 μg of each antigen in either 100 μg of alum or 200 nmol of c-di-GMP at 14-day intervals. Mice given the placebo received an identical course of saline plus alum or c-di-GMP. Sera were collected from individual mice by retro-orbital bleeding 1 week after the third immunization.
2.9. ELISA and Western blotting
Aliquots of sera from individual mice in each group were pooled and assayed for protein-specific antibodies by enzyme-linked immunosorbent assay (ELISA), using 96-well polystyrene microtiter trays (Nunc) coated with purified antigens. Pooled sera were analyzed for total IgG and IgG subclass (IgG1, IgG2a) antibodies, while sera from individual mice were assayed for total IgG, as described previously [26, 29]. Pooled sera were also subjected to Western immunoblotting using whole-cell lysates of D39 or purified proteins as the antigen, as described previously [29].
2.10. I.p. challenge
Mice were challenged 2 weeks after the third immunization using opaque-phase variant of D39, selected on THY-catalase plates [30]. Bacteria were grown as described previously [26] and the dose adjusted to 3 × 102 CFU (approx. 30 LD50 dose of D39 for CD1 mice). Mice were closely monitored for 21 days, and survival times recorded.
2.11. Flow cytometry
Preparation of single cell suspensions of lung and draining lymph nodes (dLN), and subsequent flow cytometric staining were carried out as previously described [31, 32]. Total viable cell counts were determined by trypan blue exclusion. The following antibodies were used (all BD Pharmingen, Oxford, UK, unless otherwise stated): HL3 (CD11c), RB6–8C5 (Ly6G/Ly6C; Gr1), 2G9 (MHC-II), M1/70 (CD11b), 7/4 (7/4 antigen; Serotec, Oxford, U.K.), 145.2C11 (CD3), H57–597 (TCR b), GK1.5 (CD4), 53–6.7 (CD8a), RA3.6B2 (B220), 1D3 (CD19), 3/23 (CD40) and GL1 (CD86). All samples were treated with anti-FcR (2.4G2) prior to specific staining. Samples were acquired on a Cyan ADP flow cytometer.
2.12. Statistics
Lung and blood titers were compared by Tukey’s analysis of variance, using SigmaStat for Windows (SysStat Software, Inc., V3.11). Differences in luminescent bacterial titers were determined by Student’s t test. Median survival times of groups in the i.p challenge experiment were compared by the Mann-Whitney U test (two-tailed). Differences in the overall survival rate between groups were analyzed by the Fisher exact test. Antibody titers from individual mice were compared by Student’s t test (two-tailed). Data from flow cytometry were analyzed with Summit 4.3 software (Dako, Ely, U.K.). A P value of <0.05 was considered statistically significant.
3. Results
3.1. c-di-GMP has no direct effect on growth of c-di-GMP
In two independent experimental, we found no effect in either promoting or inhibiting growth of S. pneumoniae Xen10 was observed as a result of incubation with c-di-GMP (data not shown).
3.2. Pre-treatment of S. pneumoniuae with c-di-GMP has no direct effect on growth
In experiments to test the effect of pre-incubating S. pneumoniae with c-di-GMP, we found that in contrast to the reduced early control at 0–4 h seen in pre-treated mice, mice given c-di-GMP at the time of challenge showed responses matching those of the control group. No significant differences were observed in the early responses between the two groups (data not shown). However, at 18 h mice given c-di-GMP showed lower luminesence than did control mice, suggesting c-di-GMP can have a beneficial effect at these later time points (data not shown). In summary, these experiments show that c-di-GMP has no significant direct short-term effect on the growth of S. pneumoniae either in vitro or in vivo.
3.3. c-di-GMP enhances innate protection against lethal pneumococcal infection
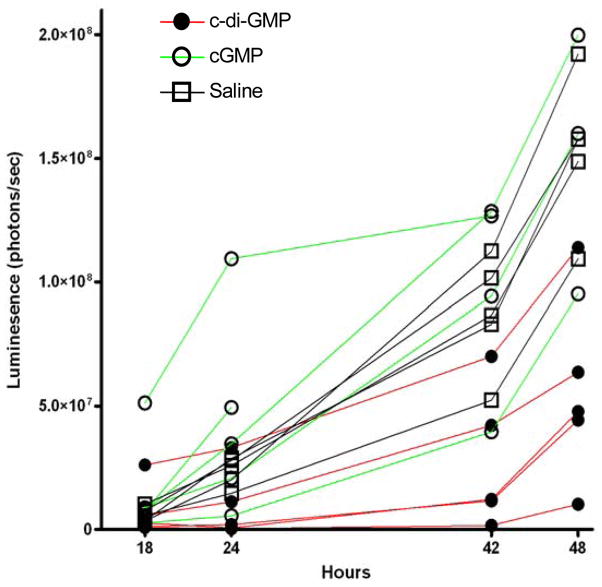
Previous Xenogen mouse model of pneumococcal infection indicated that i.n. infection with 1–2 × 106 CFU of S. pneumoniae Xen10 typically establishes a lung-restricted lethal infection [22, 33] while allowing disease progression to be followed from as early as 5 min post challenge [31]. The intensity of the bioluminescent signal has been shown to correlate with bacterial load [22]. To examine the effects of c-di-GMP on host survival and bacterial growth in this model, mice were pretreated at 48 h and 24 h with c-di-GMP, cGMP or PBS (10 mice per group) before i.n. challenge with a lethal dose of Xen10 and imaged at various time points thereafter.
At early time points (1–4 h post-challenge), mice pretreated with PBS or cGMP showed a general tendency towards control of bacterial numbers, reflected by relatively small increases in luminescence (Fig. 1a, b). However, c-di-GMP-pretreated mice generally showed rapidly increasing luminescence, suggesting an inability to control bacterial growth at this stage. Surprisingly, from 8 h post-infection, c-di-GMP-pretreated mice exerted a rapid and effective control over bacterial growth. Instead of continued multiplication, luminescence in lungs of the mice tended to plateau, followed by a much slower increase in bacterial titers over the following 24–48 h (Fig. 1b, c). Mice in both control groups, despite their lower bacterial loads at early time points, appeared to succumb to infection before 18 h post-challenge. In these groups, luminescence increased significantly in the lungs (Fig. 1b, c–f) compared to c-di-GMP-treated mice at 42 and 48 h. Together, these data suggest that, despite a short initial lag, c-di-GMP pretreatment is beneficial to the host and aids in controlling fulminant pneumococcal infection.
Fig. 1.
The effect of c-di-GMP pretreatment on S. pneumoniae Xen10 infection. Luminescence was quantified in the thorax of individual mice given c-di-GMP, cGMP or PBS before S. pneumoniae Xen10 infection at: (a) 1, 2.5 and 4 h, (b) 18, 24, 40 and 48 h and (c) 1, 4, 18, 24, 40 and 48 h post challenge. Mean (±SEM) luminescences were calculated for each group at each time point. *P<0.01 vs. PBS control group by Student’s t-test. (d–f) Luminescence images taken at 48 h post Xen10 challenge. Two mice within the cGMP group died as a result of infection at 24–42 h. In all cases n=5 mice per group. Results are representative of 2 separate experiments.
3.4. Pneumococcal counts in lung and blood of pretreated mice
To test the effect of c-di-GMP on invasive disease by virulent strains, groups of 6 mice were infected with 1 × 106 CFU of S. pneumoniae D39 or 1 × 105 CFU of T4. At 24 h post-infection, bacteria were enumerated from blood cultures and lung homogenates. Mice treated twice with c-di-GMP 48 and 24 h before D39 challenge had greater than 100-fold reduction in mean lung titers compared to mice treated with cGMP (Fig. 2a). Mice challenged with T4 also showed significant reductions in mean lung titers. Blood titers were also lower in both groups, but the difference was only statistically significant for D39-challenged mice (Fig. 2b).
Fig. 2.
Pneumococcal counts in lung and blood of c-di-GMP and cGMP pretreated mice. Groups of mice (n=6) were pretreated with either c-di-GMP or control GMP then infected with 1 × 106 CFU of S. pneumoniae D39 or 1 × 105 CFU of T4 intranasally and (a) lung and (b) blood cultures were assayed 24 h later. (c) In a second experiment lung titer data were collected following challenge with either 104 or 105 CFU of D39. The 25th – 75th percentiles are represented by the shaded box-plots with the horizontal bar indicating the mean value. Error bars indicate the standard deviation of the measurements. An asterisk (*) indicates a significant difference by ANOVA compared to the group treated with c-di-GMP (P<0.05).
To determine whether this effect could be seen across a broader range of infectious doses, groups of mice were treated as above and infected with 1 × 104 or 1 × 105 CFU of D39 (Fig. 2c). Mice treated with c-di-GMP had approx. 100-fold reductions in lung titers at both additional doses tested, although this was only statistically significant in the group challenged with the lower dose.
3.5. Analysis of sera
ELISA analysis of pooled sera from groups of mice immunized with PdB and PspA indicated strong, antigen-specific antibody responses, and IgG1 response was predominant (table 1). Interestingly, antibody titers elicited to PdB+c-di-GMP and PspA+c-di-GMP were significantly higher than PdB+alum and PspA+alum (P=0.003 and P=0.0005, respectively). When antibody titers from 3 mice that survived the challenge and from 3 mice that died soon after challenge in each group were compared, the titers for surviving mice immunized with PdB+alum and PspA+c-di-GMP were significantly higher (P=0.01 in both cases). However, antibody titers for surviving mice immunized with PspA+alum and PdB+c-di-GMP were not significantly different from those obtained from their counterparts that died early.
Table 1.
Antibody titers obtained from immunized mice
| ELISA titersa |
||||||
|---|---|---|---|---|---|---|
| Immunogen | Alum-immunized group | c-di-GMP-immunized group | ||||
| Total IgG | IgG1 | IgG2a | Total IgG | IgG1 | IgG2a | |
| PdB | 30,000 | 40,000 | 8,000 | 60,000 | 40,000 | 25,000 |
| (28,200 ± 3,250) | (54,200 ± 5,800) | |||||
| PspA | 30,000 | 35,000 | 8,000 | 140,000 | 160,000 | 50,000 |
| (24,700 ± 1,600) | (112,000 ± 17,200) | |||||
| Alum | <500 | ND | ND | ND | ND | ND |
| c-di-GMP | ND | ND | ND | <500 | ND | ND |
ELISA titers were determined as the reciprocal of the dilution of sera giving 50% of the highest absorbance reading above the background at 405nm. Values in parenthesis represent mean antibody titers ± SEM from six individual mice.
ND= Not Determined.
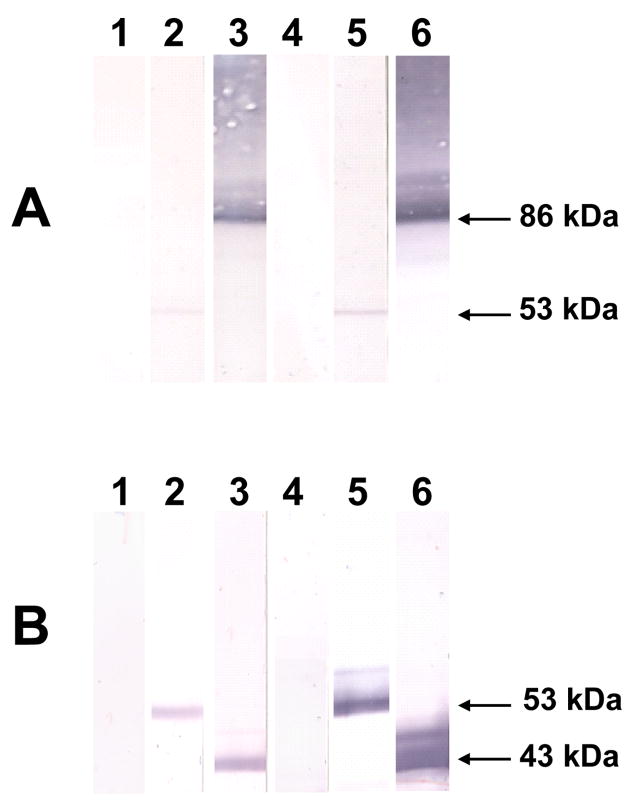
Western immunoblot analysis of whole cell lysates of D39 also demonstrated protein-specific antibody responses (Fig. 3A). Anti-PdB sera specifically labeled pneumolysin, but a characteristic labeled smear was observed with anti-PspA sera [28, 34]. Western blot analysis of the purified proteins also demonstrated specific antibody responses to purified PdB [53 kDa] and the purified 43 kDa N-terminal fragment of PspA (Fig. 3B).
Fig. 3.
Western blot of (A), whole-cell lysates of S. pneumoniae D39, and (B), purified PdB and the 43 kDa N-terminal fragment of PspA with anti-PdB and anti-PspA sera. The blots were reacted with specific antisera generated from mice immunized with the indicated antigens. Results for nitrocellulose membranes reacted with sera from mice immunized with alum (lane 1), PdB+alum (lane 2), PspA+alum (lane 3), c-di-GMP (lane 4), PdB+c-di-GMP (lane 5), and PspA+c-di-GMP (lane 6) are shown. The corresponding molecular mass of each protein is indicated by an arrow: pneumolysin or purified PdB (53 kDa), full length PspA (86 kDa) and the N-terminal fragment of PspA (43 kDa).
3.6. Protection studies
In the i.p. challenge experiment, mice immunized with PdB+alum and PspA+alum survived significantly longer than the placebo group [P<0.02 in both cases] (Fig. 4). Furthermore, PdB+c-di-GMP-immunized mice survived significantly longer than those immunized with c-di-GMP alone (P<0.05). Surprisingly, the median survival time for mice immunized with PspA+c-di-GMP, although longer than for those immunized with c-di-GMP alone (>504 h vs 60 h), did not reach statistical significance. Interestingly, mice immunized with PdB+c-di-GMP survived significantly longer than those that received PdB+alum (P<0.05).
Fig. 4.
Survival times for mice after intraperitoneal challenge. Groups of 15 CD1 mice were immunized with the indicated antigens and challenged 2 weeks after the third immunization with approximately 3 × 102 CFU of the capsular type 2 strain D39. The broken lines denote the median survival time for each group.
The overall survival rate for mice immunized with Alum+PdB or Alum+PspA (4 survivors vs no survivors in the Alum only group) was statistically significant (P=0.05 in both cases). However, there were no significant differences in the overall survival rates between mice immunized with c-di-GMP alone and those that were immunized with either c-di-GMP+PdB (4 survivors vs 9 survivors) or c-di-GMP+PspA (4 survivors vs 8 survivors). This is likely due to the fact that c-di-GMP by itself is capable of stimulating protective innate immunity against infection.
3.7. c-di-GMP treatment affects both lung and draining LN populations
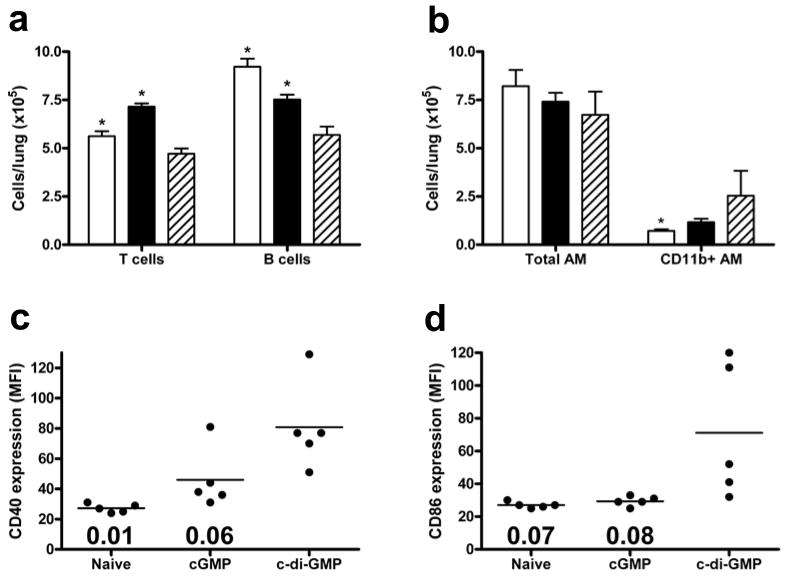
To examine potential mechanisms underlying the protective effect of c-di-GMP, immune cell populations from lungs and lung draining lymph nodes (dLN) were quantified in naïve, cGMP and c-di-GMP treated mice. Draining LN and lungs were analyzed at day 0, following doses of c-di-C-DI-GMP (or control) at −48 h and −24 h. Compared with naïve lungs, no significant effect of cGMP (P=0.17) or c-di-GMP (P=0.19) on overall cell number was observed. Treatment with c-di-GMP reduced both T and B lymphocyte counts in lungs compared with control groups (Fig. 5a), although the proportion of CD44low naïve T cells was slightly increased in c-di-GMP-treated mice compared with naïve (P=0.01) and cGMP groups (P=0.001). c-di-GMP treatment had no significant effect on total CD11chiMHCIIlow alveolar macrophage (AM) numbers (Fig. 5b), but increased the proportion of these cells expressing CD11b (Fig. 5b), suggesting an increase in newly influxed AM [32]. Similarly, while numbers of lung CD11chiMHCIIhi dendritic cells (DC) were not significantly influenced by c-di-GMP treatment (data not shown), DC from these mice expressed higher levels of CD40 and CD86 than did DC from either control group (Fig. 5c, d). Finally, neither monocyte nor neutrophil populations in the lungs were significantly altered by c-di-GMP treatment compared with control groups.
Fig. 5.
Effect of c-di-GMP treatment on lung cells. Lung cell populations were quantified (a, b) in naïve mice (open bars), or following cGMP (closed bars) or c-di-GMP treatment (hatched bars). Graphs show mean numbers of CD3+TCRb+CD19− T cells, CD3−TCRb−CD19+ B cells (a), and CD11c+MHCIIlowCD11b+/− AM (b). *P<0.05 compared with c-di-GMP group (Student’s t-test). Expression of CD40 (c) and CD86 (d) on CD11c+MHCIIhigh lung dendritic cells is shown for individual mice in each group as mean fluorescence intensity (MFI). Bars represent mean values and numbers indicate P value compared with c-di-GMP group.
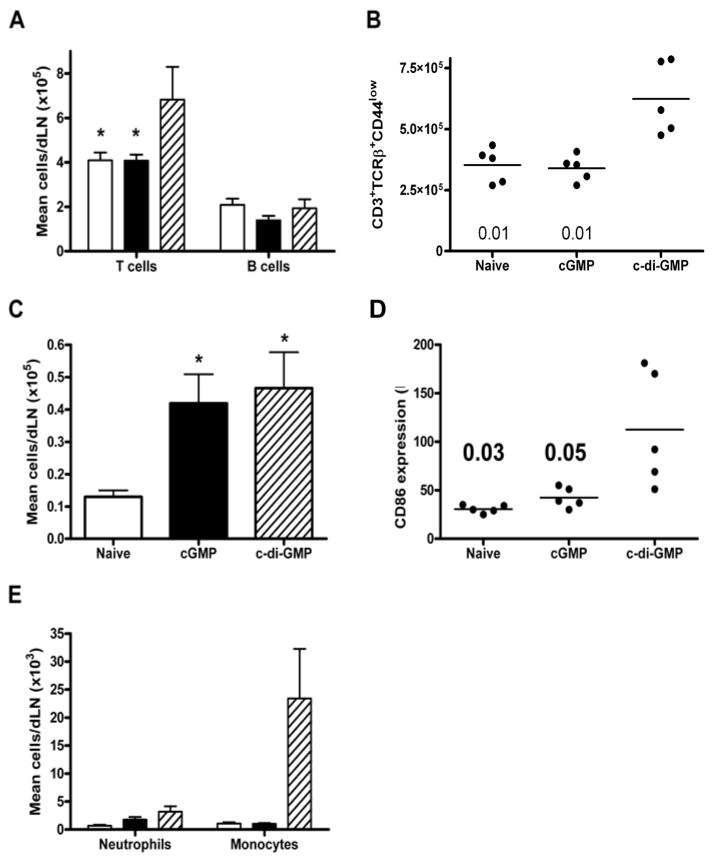
In contrast to the lungs, c-di-GMP treatment significantly increased the total cell number of lung dLN compared to cGMP (P=0.01) or naïve groups (P=0.01), reflected in significant increases in T lymphocyte, but not B lymphocyte, numbers in the dLN (Fig. 6a). Furthermore, there was an increase in the proportion of CD44low naïve cells c-di-GMP-treated mice (Fig. 6b). DC population significantly increased in both cGMP and c-di-GMP treated groups compared with controls (Fig. 6c). However, expression of CD86 was significantly increased only in the c-di-GMP group (Fig. 6d), but not CD40 (not shown). Finally, while c-di-GMP treatment had no significant effect on dLN neutrophil counts (Fig. 6e), mean counts of monocytes within the lung dLN of the c-di-GMP group were >20-fold higher than in either control group (Fig. 6e). Taken together, these data demonstrate cellular consequences of c-di-GMP treatment which may facilitate more potent immune responses.
Fig. 6.
Effect of c-di-GMP treatment on lung draining LN cells. Draining LN populations were quantified (a, c, e) in naïve mice (open bars), or following cGMP (closed bars) or c-di-GMP treatment (hatched bars). Graphs show mean numbers of T and B lymphocyte (a, as defined in Fig. 5), CD11c+ DC (c) and non-overlapping Gr1+CD11b+7/4+ neutrophil and monocyte populations, conclusively differentiated based on size and granularity characteristics (e). *P<0.05 compared with c-di-GMP group (a) or naïve group (c) (Student’s t-test). The percentage of CD44low T cells was in individual mice is shown (b), as is the MFI level of CD86 expression on DCs (d). Bars represent mean values and numbers indicate p value compared with c-di-GMP group.
4. Discussion
In this study, we report that pretreatment of mice either i.n. or i.p. with c-di-GMP, but not control nucleotide (cGMP) or saline, can induce a significant protective immune response against i.n. or i.p. challenge with virulent pneumococci in various murine infection models. Our current findings are consistent with previous studies with Staphylococcus aureus and K. pneumoniae, as well as recent in vivo and in vitro studies showing that c-di-GMP is immunomodulatory and immunostimulatory [16, 17, 19].
In bacterial pneumonia, clearance of pathogens is primarily dependent upon a vigorous innate immune response [35–38]. The present study clearly demonstrates that c-di-GMP administration enhances several key aspects of the cytokine-mediated innate immunity in the lung. Although the cellular components of c-di-GMP-stimulated immunity have not been clearly defined, several candidate cell populations, such as neutrophils, MIP-2, NK, NKT and αβ T cells, are likely to be involved, as shown previously [19]. Neutrophils are important for bacterial clearance from the lung [36, 38], and c-di-GMP-induced upregulation of MIP-2 may contribute to increased neutrophil trafficking. Moreover, NK cells are considered to be the primary source of IFN-γ in the lung early during bacterial infection [39, 40], while NKT cells can be primed to secrete prodigious quantities of IFN-γ during infection [41–43], as well as contributing to innate immunity against pulmonary pneumococcal challenge [44]. The promotion of enhanced type 1 immunity in response to c-di-GMP administration supports the possibility that c-di-GMP directly stimulates DC mediated responses in the lung via activation of p38 MAPK (mitogen activated protein kinase) and enhanced IL-12 p40 expression, as demonstrated recently [17]. However, other cells, including lung macrophages, may also contribute.
We also demonstrate that c-di-GMP can prime or directly stimulate multiple beneficial aspects of innate and adaptive immunity. Therefore, its use avoids current concerns with drug resistance associated with traditional antibiotics. Since c-di-GMP has fast-acting broad spectrum activity and flexibility in route of administration but does not directly affect the bacterial cell, rather the host, its use may also be complementary to antibiotics (prophylactically or therapeutically), as previously proposed [17].
In the bioluminescence studies, c-di-GMP pretreatment resulted in reduced early control of bacterial numbers at 0–4 h, but a significant reduction in bacterial titers at 42–48 h. Although the reasons for these observations are unclear, mice pretreated with c-di-GMP, but not with cGMP or saline, rapidly exhibited physical symptoms of mild toxic shock (piloerection, severe sweating) following challenge (unpublished observations). This suggests a rapid pro-inflammatory cytokine response initiated by pneumococcal challenge in c-di-GMP ‘primed’ mice which did not occur in control groups. While it was previously shown that TNF, a major pro-inflammatory cytokine involved in toxic shock responses, is neither significantly induced nor required for a complete cellular response to S. pneumoniae [31], it remains to be seen whether such a locally initiated shock response may be detrimental to early cellular responses in the lungs. It was striking that after approximately 8 h, c-di-GMP-pretreated mice exerted control over bacterial numbers. Since early anti-pneumococcal responses in previously naïve mice are dominated by neutrophils and other phagocytes [31], we propose that c-di-GMP pretreatment results in the ability to mount a more effective and prolonged phagocytic response. This may occur either through increased phagocyte recruitment or more efficient phagocytic killing [19]. These possibilities and the mechanism involved are under investigation.
Although the data using the serotype 3 strain Xen10 were compelling, different pneumococcal strains often behave quite differently in mice [33]. We therefore sought to determine whether amelioration of invasive disease was possible in strains with pathogenicity profiles distinct from Xen10. While Xen10 stays confined to the lung, the type 2 strain D39 causes both lung infection and a high grade sepsis with bacteremia, while the type 4 strain T4 causes primary bacteremia which progresses to meningitis in untreated mice; with seeding of the lungs occurring secondary to bacteremia [33]. With both these virulent strains, pretreatment with c-di-GMP decreased lung and blood titers compared to mock treated controls, indicating the effects are not specific to the lung and can be generalized to several pneumococcal strains and serotypes. Instead of “cherry-picking” a single observation with a single strain to suggest the compound works, we have demonstrated an effect with 3 very different strains of pneumococcus. The strain utilized in Fig 1 is limited to the lung, while the other 2 strains also get into the bloodstream and cause systemic infection. It is likely that these strain-specific differences account for differences in the effectiveness of the compound. This is exactly what we would expect to see in the real world and suggests broad applicability of the compound, which is balanced by variability of response. This provides additional support for the proposed mechanism suggesting these results with S. pneumoniae (and our previous results with S. aureus and K. pneumoniae) are based on changes to the host’s innate immunity, rather than a direct effect on any particular bacterial strain.
In the i.p. immunization/challenge studies, the enhanced protection elicited by PdB and PspA is in agreement with previous findings [26–28]. Passive-immunization-i.p.-challenge experiments demonstrated that the enhanced protection elicited by PdB and PspA was, at least, partly antibody mediated [26]. In this work, mice immunized with PdB and PspA in c-di-GMP elicited significantly higher antibody titers than mice that received the antigens in alum. Indeed, mice immunized with PdB+c-di-GMP were significantly better protected than those immunized with PdB+alum. While high antibody titers correlated with protection, this may not necessarily represent the true or only mechanism of protection. Recent studies in mice have indicated T-cell mediated, antibody-independent immunity against pneumococcal colonization [45, 46] or systemic disease [47, 48]. Indeed, low CD4 T-cell immunity to pneumolysin was found to be associated with pneumococcal carriage in children [49], while PspA was effective at eliciting T cell-mediated responses during invasive disease in adults [50]. Therefore, enhanced protection afforded by either PdB+c-di-GMP or PspA+c-di-GMP may be T-cell mediated, although this remains to be demonstrated directly.
There remains an obvious and urgent need for improvements in our treatment regimens for pneumococcal infections. Strategies such as immunomodulation that go beyond simple antibiotic treatment are needed. We would argue that compounds such as c-di-GMP which may augment the innate immune response could be used alone as an immunostimulatory or immunoprophylactic agent for prevention of such infections in high risk patients, therapeutically in combination with antibiotics for treatment of infections once they develop, and as a vaccine adjuvant.
In conclusion, we demonstrate that innate immune stimulation with c-di-GMP ameliorates subsequent invasive pneumococcal disease and augments the immune response. Furthermore, the fact that the results are more generalizable and reproducible in different serotypes, and complementary in different infection models, is very encouraging. These findings, in addition to the possibility that formulation of vaccines with c-di-GMP is likely to be simpler, argues in favor of using c-di-GMP as an adjuvant of choice. This would make deployment of such vaccines to developing countries, where the need is greatest, more affordable. We propose that c-di-GMP treatment may play a potentially beneficial role in immunoprophylaxis by activating innate host defenses against respiratory and systemic infections.
Acknowledgments
The authors wish to acknowledge excellent technical support from Amy R. Iverson at St. Jude Children’s Research Hospital. D.K.R.K. is a recipient of a Burroughs Wellcome Career Award in the Biomedical Sciences. This research was supported by the Army Research Office Grant to DKRK, National Health and Medical Research Council of Australia Program Grant 284214, and Welcome Trust Grant #075846 to ACK and Paul Kaye and MRC Grant #G981148.
Footnotes
Potential conflicts of interest: D.K.R. Karaolis has three related patents: a method for attenuating virulence of microbial pathogens and for inhibiting microbial biofilm formation (PCT/US04/23498); a method for stimulating the immune, inflammatory, or neuroprotective response (U.S. 11/079, 886; PCT/US05/08447); and a method for inhibiting cancer cell proliferation or increasing cancer cell apoptosis (U.S. 11/079,779; PCT/US05/08448).
References
- 1.CDC. Assessment of Susceptibility Testing Practices for Streptococcus pneumoniae -United States, February 2000. MMWR. 2002;51(18):392–4. [PubMed] [Google Scholar]
- 2.Paton JC. New pneumococcal vaccines: basic science developments. In: Tuomanen EI, Mitchell TJ, Morrison DA, Spratt BG, editors. The pneumococcus. Washington, D.C: ASM Press; 2004. pp. 382–402. [Google Scholar]
- 3.Amikam D, Benziman M. Cyclic diguanylic acid and cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol. 1989;171(12):6649–55. doi: 10.1128/jb.171.12.6649-6655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross P, Mayer R, Weinhouse H, Amikam D, Huggirat Y, Benziman M, et al. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. J Biol Chem. 1990;265(31):18933–43. [PubMed] [Google Scholar]
- 5.Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinberger-Ohana P, Mayer R, et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature. 1987;325:279–81. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- 6.D’Argenio DA, Miller SI. Cyclic di-GMP as a bacterial second messenger. Microbiology. 2004;150(Pt 8):2497–502. doi: 10.1099/mic.0.27099-0. [DOI] [PubMed] [Google Scholar]
- 7.Galperin MY, Nikolskaya AN, Koonin EV. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett. 2001;203(1):11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones HA, Lillard JW, Jr, Perry RD. HmsT, a protein essential for expression of the haemin storage (Hms+) phenotype of Yersinia pestis. Microbiology. 1999;145(Pt 8):2117–28. doi: 10.1099/13500872-145-8-2117. [DOI] [PubMed] [Google Scholar]
- 9.Rashid MH, Rajanna C, Ali A, Karaolis DKR. Identification of genes involved in the switch between the smooth and rugose phenotypes of Vibrio cholerae. FEMS Microbiol Lett. 2003;227(1):113–9. doi: 10.1016/S0378-1097(03)00657-8. [DOI] [PubMed] [Google Scholar]
- 10.Römling U, Gomelsky M, Galperin MY. C-di-GMP: the dawning of a novel bacterial signalling system. Mol Microbiol. 2005;57(3):629–39. doi: 10.1111/j.1365-2958.2005.04697.x. [DOI] [PubMed] [Google Scholar]
- 11.Römling U, Amikam D. Cyclic di-GMP as a second messenger. Curr Opin Microbiol. 2006;9(2):218–28. doi: 10.1016/j.mib.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 12.D’Argenio DA, Calfee MW, Rainey PB, Pesci EC. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J Bacteriol. 2002;184(23):6481–9. doi: 10.1128/JB.184.23.6481-6489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht GB, Newton A. Identification of a novel response regulator required for the swarmer-to-stalked-cell transition in Caulobacter crescentus. J Bacteriol. 1995;177(21):6223–9. doi: 10.1128/jb.177.21.6223-6229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karaolis DKR, Rashid MH, Rajanna C, Luo W, Hyodo M, Hayakawa Y. c-di-GMP (3′–5′-cyclic diguanylic acid) inhibits Staphylococcus aureus cell-cell interactions and biofilm formation. Antimicrob Agents Chemother. 2005;49(3):1029–38. doi: 10.1128/AAC.49.3.1029-1038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Römling U, Rohde M, Olsen A, Normark S, Reinkoster J. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol Microbiol. 2000;36(1):10–23. doi: 10.1046/j.1365-2958.2000.01822.x. [DOI] [PubMed] [Google Scholar]
- 16.Brouillette E, Hyodo M, Hayakawa Y, Karaolis DKR, Malouin F. 3′,5′-cyclic diguanylic acid reduces the virulence of biofilm-forming Staphylococcus aureus strains in a mouse model of mastitis infection. Antimicrob Agents Chemother. 2005;49(8):3109–13. doi: 10.1128/AAC.49.8.3109-3113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karaolis DKR, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E, et al. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol. 2007;178(4):2171–81. doi: 10.4049/jimmunol.178.4.2171. [DOI] [PubMed] [Google Scholar]
- 18.Karaolis DKR, Cheng K, Lipsky M, Elnabawi A, Catalano J, Hyodo M, et al. 3′,5′-Cyclic diguanylic acid (c-di-GMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochemical and Biophys Res Comm. 2005;329(1):40–5. doi: 10.1016/j.bbrc.2005.01.093. [DOI] [PubMed] [Google Scholar]
- 19.Karaolis DKR, Newstead MW, Zeng X, Hyodo M, Hayakawa Y, Bhan U, et al. Cyclic-di-GMP stimulates protective innate immunity in bacterial pneumonia. Infect Immun. 2007;75(10):4942–50. doi: 10.1128/IAI.01762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avery OT, MacLeod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;79(2):137–58. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293(5529):498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 22.Francis KP, Yu J, Bellinger-Kawahara C, Joh D, Hawkinson MJ, Xiao G, et al. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun. 2001;69(5):3350–8. doi: 10.1128/IAI.69.5.3350-3358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyodo M, Hayakawa Y, Karaolis DKR. Organic Synthesis, Chemical Properties, and Biological Activities of Cyclic bis(3′–5′) Diguanylic Acid (c-di-GMP) and Its Analogs. J Synth Org Chem Jpn. 2006;64(4):359–70. [Google Scholar]
- 24.Briles DE, Hollingshead SK, King J, Swift A, Braun PA, Park MK, et al. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000;182(6):1694–701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 25.Nabors GS, Braun PA, Herrmann DJ, Heise ML, Pyle DJ, Gravenstein S, et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine. 2000;18(17):1743–54. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- 26.Ogunniyi AD, Folland RL, Briles DE, Hollingshead SK, Paton JC. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect Immun. 2000;68(5):3028–33. doi: 10.1128/iai.68.5.3028-3033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paton JC, Lock RA, Lee CJ, Li JP, Berry AM, Mitchell TJ, et al. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect Immun. 1991;59(7):2297–304. doi: 10.1128/iai.59.7.2297-2304.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cognet I, de Coignac AB, Magistrelli G, Jeannin P, Aubry JP, Maisnier-Patin K, et al. Expression of recombinant proteins in a lipid A mutant of Escherichia coli BL21 with a strongly reduced capacity to induce dendritic cell activation and maturation. J Immunol Methods. 2003;272(1–2):199–210. doi: 10.1016/s0022-1759(02)00506-9. [DOI] [PubMed] [Google Scholar]
- 29.Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect Immun. 2007;75(1):350–7. doi: 10.1128/IAI.01103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiser JN, Austrian R, Sreenivasan PK, Masure HR. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62(6):2582–9. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby AC, Raynes JG, Kaye PM. The role played by tumor necrosis factor during localized and systemic infection with Streptococcus pneumoniae. J Infect Dis. 2005;191(9):1538–47. doi: 10.1086/429296. [DOI] [PubMed] [Google Scholar]
- 32.Kirby AC, Raynes JG, Kaye PM. CD11b regulates recruitment of alveolar macrophages but not pulmonary dendritic cells after pneumococcal challenge. J Infect Dis. 2006;193(2):205–13. doi: 10.1086/498874. [DOI] [PubMed] [Google Scholar]
- 33.Orihuela CJ, Gao G, McGee M, Yu J, Francis KP, Tuomanen EI. Organ-specific models of Streptococcus pneumoniae disease. Scand J Infect Dis. 2003;35(9):647–52. doi: 10.1080/00365540310015854. [DOI] [PubMed] [Google Scholar]
- 34.McDaniel LS, Sheffield JS, Delucchi P, Briles DE. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect Immun. 1991;59(1):222–8. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R, 3rd, et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65(4):1139–46. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipscomb MF, Onofrio JM, Nash EJ. A morphological study of the role of phagocytes in the clearance of Staphylococcus aureus from the lung. J Reticuloend Soc. 1983;33(6):429–42. [PubMed] [Google Scholar]
- 37.Toews GB, Gross GN, Pierce AK. The relationship of inoculum size to lung bacterial clearance and phagocytic cell response in mice. Am Rev Respir Dis. 1980;120(3):559–66. doi: 10.1164/arrd.1979.120.3.559. [DOI] [PubMed] [Google Scholar]
- 38.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun. 2000;68(7):4289–96. doi: 10.1128/iai.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng JC, Tateda K, Zeng X, Standiford TJ. Transient transgenic expression of gamma interferon promotes Legionella pneumophila clearance in immunocompetent hosts. Infect Immun. 2001;69(10):6382–90. doi: 10.1128/IAI.69.10.6382-6390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferlazzo G, Morandi B, D’Agostino A, Meazza R, Melioli G, Moretta A, et al. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J of Immunol. 2003;33(2):306–13. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, et al. alpha -galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97(15):8461–6. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawakami K, Kinjo Y, Yara S, Koguchi Y, Uezu K, Nakayama T, et al. Activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun. 2001;69(1):213–20. doi: 10.1128/IAI.69.1.213-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 44.Kawakami K, Yamamoto N, Kinjo Y, Miyagi K, Nakasone C, Uezu K, et al. Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33(12):3322–30. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- 45.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci USA. 2005;102(13):4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCool TL, Weiser JN. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect Immun. 2004;72(10):5807–13. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadioglu A, Gingles NA, Grattan K, Kerr A, Mitchell TJ, Andrew PW. Host cellular immune response to pneumococcal lung infection in mice. Infect Immun. 2000;68(2):492–501. doi: 10.1128/iai.68.2.492-501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadioglu A, Coward W, Colston MJ, Hewitt CR, Andrew PW. CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect Immun. 2004;72(5):2689–97. doi: 10.1128/IAI.72.5.2689-2697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q, Bagrade L, Bernatoniene J, Clarke E, Paton JC, Mitchell TJ, et al. Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J Infect Dis. 2007;195(8):1194–202. doi: 10.1086/512617. [DOI] [PubMed] [Google Scholar]
- 50.Baril L, Dietemann J, Essevaz-Roulet M, Béniguel L, Coan P, Briles DE, et al. Pneumococcal surface protein A (PspA) is effective at eliciting T cell-mediated responses during invasive pneumococcal disease in adults. Clin Exp Immunol. 2006;145(2):277–86. doi: 10.1111/j.1365-2249.2006.03148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]