Abstract
Bcl2 phosphorylation at Ser-70 may be required for the full and potent suppression of apoptosis in IL-3-dependent myeloid cells and can result from agonist activation of mitochondrial protein kinase C (PKC). Paradoxically, expression of exogenous Bcl2 can protect parental cells from apoptosis induced by the potent PKC inhibitor, staurosporine (stauro). High concentrations of stauro of up to 1 μM only partially inhibit IL-3-stimulated Bcl2 phosphorylation but completely block PKC-mediated Bcl2 phosphorylation in vitro. These data indicate a role for a stauro-resistant Bcl2 kinase (SRK). We show that aurintricarboxylic acid (ATA), a nonpeptide activator of cellular MEK/mitogen-activated protein kinase (MAPK) kinase, can induce Ser-70 phosphorylation of Bcl2 and support survival of cells expressing wild-type but not the phosphorylation-incompetent S70A mutant Bcl2. A role for a MEK/MAPK as a responsible SRK was implicated because the highly specific MEK/MAPK inhibitor, PD98059, also can only partially inhibit IL-3-induced Bcl2 phosphorylation, whereas the combination of PD98059 and stauro completely blocks phosphorylation and synergistically enhances apoptosis. p44MAPK/extracellular signal-regulated kinase 1 (ERK1) and p42 MAPK/ERK2 are activated by IL-3, colocalize with mitochondrial Bcl2, and can directly phosphorylate Bcl2 on Ser-70 in a stauro-resistant manner both in vitro and in vivo. These findings suggest a role for the ERK1/2 kinases as SRKs. Thus, the SRKs can serve to functionally link the IL-3-stimulated proliferative and survival signaling pathways and, in a novel capacity, may explain how Bcl2 can suppress stauro-induced apoptosis. In addition, although the mechanism of regulation of Bcl2 by phosphorylation is not yet clear, our results indicate that phosphorylation may functionally stabilize the Bcl2-Bax heterodimerization.
Bcl2 and other related proteins of the Bcl2 family regulate the effector stage of apoptosis (1, 2). Bcl2 can suppress cell death induced by a variety of stress applications including growth factor withdrawal (3), chemotherapy (4), irradiation (4), and viral infection (5). Although the mechanism of Bcl2 antiapoptotic function is not clear, it is postulated that Bcl2 may help maintain mitochondrial integrity (6) and/or block the activation of the downstream caspase cascade that leads to apoptosis (7).
We recently discovered that phosphorylation of Bcl2 at the evolutionarily conserved Ser-70 site may be required for its full and potent antiapoptotic function (8, 9). The hematopoietic growth factors IL-3 and erythropoietin as well as the potent protein kinase-C (PKC) activator, Bryostatin-1 (Bryo), were discovered to stimulate a PKC-dependent survival mechanism that featured functional phosphorylation of Bcl2 (8, 9). Similar results now have been reported for nerve growth factor-induced phosphorylation of Bcl2, which is also associated with the suppression of apoptosis in rat PC12W pheochromocytoma cells (10). The serine/threonine protein kinase Raf-1 can be activated directly via a Ras-Raf-1 or PKC-Raf-1 mechanism (11, 12), and the Raf-MEK-mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase 1 (ERK) signaling pathway is required for growth factor-induced proliferation (11, 13, 14). Recently, we discovered that mitochondrial PKCα is a physiologic Bcl2 kinase (15). However, PKC is not likely to be the sole physiologic Bcl2 kinase because forced overexpression of Bcl2 can prolong cell survival even when high concentrations of the potent PKC inhibitor staurosporine (stauro) are used, which can fully inhibit PKC (15, 16). Thus, we postulate that one or perhaps more stauro-resistant Bcl2 kinases (SRK) may exist to explain this apparent paradox.
As stated, MAPK family members have been implicated in fundamental cell processes such as proliferation and differentiation (17). Recent findings suggest that MAPK/ERK may also play a role in cell survival (10, 17). However, any physiological downstream survival substrates targeted by the MAPK/ERKs are not known. Results here identify the p44 MAPK/ERK1 and p42 MAPK/ERK2 as SRKs that can function in concert with PKC to functionally phosphorylate Bcl2. In addition, we find that Bcl2 phosphorylation at Ser-70 may affect the stability of interaction with its proapoptotic heterodimeric partner, Bax, an interaction which is thought to regulate cell survival (18).
Materials and Methods
Materials.
Anti-Bcl2, ERK1, ERK2, and CPP32 antibodies were purchased from Santa Cruz Biotechnology. Antihemagglutinin (anti-HA) antibody (HA-11) was obtained from Babco. Antiprohibitin antibody was purchased from Research Diagnostics (Flanders, NJ). Bax antisera was produced, characterized, and used as described previously (8, 9). Synthetic murine IL-3 was kindly provided by Ian Clark-Lewis (University of British Columbia, Canada). Bryo was the kind gift of G. R. Pettit (Arizona State University, Tempe). Staurosporine, aurintricarboxylic acid (ATA), PD98059, and purified active ERK1 and ERK2 were obtained from Calbiochem. Myelin basic protein (MBP) and Lipofectamine were purchased from Life Technologies (Gaithersburg, MD). All other reagents were from commercial sources, unless stated otherwise.
Cell Lines, Plasmids, and Transfections.
Murine wild-type or S70A mutant Bcl2 cDNA was cloned and stably transfected in murine IL-3 dependent NSF/N1.H7 cells as described previously (8). Factor-dependent murine FDC-P1 cells expressing high levels of endogenous Bcl2 were maintained as described (8). The murine wild-type Bcl2 cDNA was modified by addition of the HA tag sequence at its NH2 terminus and inserted into the mammalian expression plasmid pCI-neo (Promega), resulting in plasmid pCI-neo/HA-Bcl2. Cos7 cells were grown in 100-mm dishes to 80% confluence and transfected by using Lipofectamine according to the manufacturer's instructions. Triple transfection of cells was performed by using 20 μg of pLXSN/HA-MEK1 (ΔN3-S218E-S222D, a constitutively active MEK-1; ref. 19), 20 μg of pCMV5/WT-ERK1 or pMV7/HA-Xenopus WT-ERK2 (a kind gift of Lynn Heasley, University of Colorado Health Science Center, Denver; ref. 19), and 20 μg of pCI-neo/HA-Bcl2 expression plasmids in combination. After 5 h of incubation with Lipofectamine and plasmid DNA, Cos7 cells were washed and grown for 48 h in medium before metabolic labeling.
Metabolic Labeling, Immunoprecipitation, and Western Blot Analysis.
Cells were washed with phosphate-free RPMI medium and incubated with 32P-labeled orthophosphoric acid for 60 min as described previously (9). After agonist or inhibitor addition as indicated, cells were washed with ice-cold PBS and lysed in detergent buffer and Bcl2 was immunoprecipitated as described previously (9). The samples were subjected to 10–20% gradient SDS/PAGE, transferred to a nitrocellulose membrane, and exposed to Kodak X-Omat film for the times indicated at −80°C. The filters then were probed by Western blot analysis with a Bcl2 antibody and developed by using an ECL kit (Amersham) as described previously (8).
Assay of ERK1/ERK2 Activity and Bcl2 Phosphorylation in Vitro.
ERK1 or ERK2 was immunoprecipitated from cell lysates with polyclonal ERK1 or ERK2 antibodies after agonist treatment. MBP was used as substrate and incubated in assay buffer for 30 min at 30°C. Assay buffer contains 10 mM Hepes (pH 8.0), 100 μM ATP, 10 mM MgCL2, 1 mM DTT, 0.5 mM benzamidine, and 2 μCi [γ-32P]ATP (20). To observe Bcl2 phosphorylation, Bcl2 immune complexes obtained from cell lysates as described earlier were resuspended in the assay buffer as above. Purified, activated ERK1 or ERK2 enzyme (0.5 μg) was added and incubated for 30 min at 30°C. The reaction was stopped by the addition of 2× SDS sample buffer and boiling the sample for 5 min. The samples were analyzed by SDS/PAGE as described above. Bcl2 phosphorylation using purified PKCα (Panvera, Madison, WI) was performed as described previously (15).
Subcellular Fractionation.
Subcellular fractionation was performed to isolate heavy membrane (HM), light membrane, cytosol, and nuclear membrane as described previously (15, 21–23). Protein (50 μg) from each fraction was subjected to SDS/PAGE and analyzed by Western blotting by using antibodies to Bcl2, ERK1, and ERK2. The purity of fractions was confirmed by assessing localization of fraction-specific proteins including prohibitin (HM) and CPP32 (cytosolic) (24, 25).
Cell Viability Assay.
Cell viability was measured by the trypan blue dye exclusion method as described previously (8).
Results
Stauro or PD98059 Alone Only Partially Inhibit IL-3-Induced Bcl2 Phosphorylation Whereas the Combination Can Completely Block Bcl2 Phosphorylation and Synergistically Enhance Apoptosis.
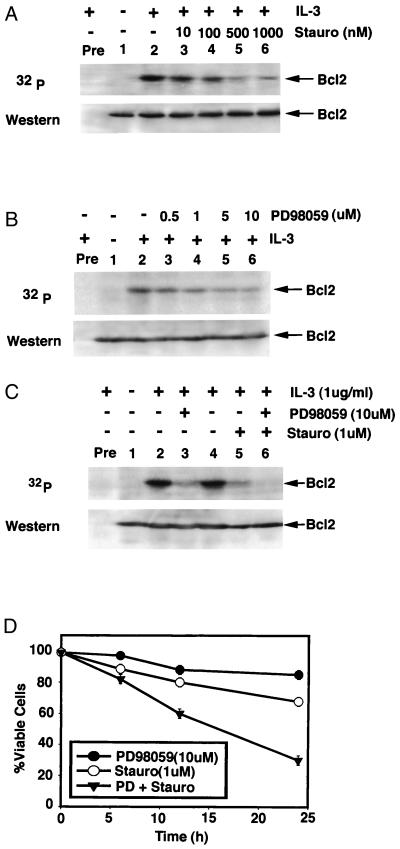
Phosphorylation of Bcl2 may be required for its full and potent function in IL-3-dependent myeloid cells (8), and expression of exogenous Bcl2 can protect cells from stauro-induced apoptosis (8, 15, 16). The effect of high concentrations of stauro on Bcl2 phosphorylation was tested. Factor-dependent NSF/N1 H7 (H7) cells expressing exogenous wild-type (wt) Bcl2 were metabolically labeled with 32P-labeled orthophosphoric acid and treated with varying concentrations of stauro, and Bcl2 was immunoprecipitated from cell lysates. Although the IC50 for stauro inhibition of intracellular PKC is reported to be 7 nM (26), the addition of even extremely high concentrations (e.g., 1 μM) to cells fails to completely inhibit IL-3-induced Bcl2 phosphorylation greater than 80% (as determined by densitometry; Fig. 1A). Similar results were reported previously in another IL-3-dependent cell line, FDC-P1 (9), indicating that the effect is not cell line-limited or an artifact of expression of recombinant Bcl2. These findings indicate that a SRK also exists in addition to staurosporine-sensitive PKCα.
Figure 1.
Stauro or PD98059 partially inhibits IL-3-induced Bcl2 phosphorylation whereas the combination of stauro with PD98059 completely blocks Bcl2 phosphorylation and synergistically inhibits cell survival. (A–C) IL-3-dependent NSF.N1/H7 cells expressing wt Bcl2 were metabolically labeled with 32P-labeled orthophosphoric acid and pretreated with increasing concentrations of stauro (A) or PD98059 (B) or 10 μM PD98059 plus 1 μM stauro (C) for 15 min before adding synthetic murine IL-3 for 15 min. Bcl2 was immunoprecipitated by using a Bcl2 antibody (lanes 1–6) or preimmune serum (Pre). Phosphorylation was determined by autoradiography (Upper). Western blot analysis was performed to confirm and quantitate Bcl2 (Lower). (D) Wt Bcl2 H7 cells were treated with 10 μM PD98059, 1 μM stauro, or the combination for various times. Cell viability was determined by trypan blue dye exclusion. Data represent the mean ± SEM of three separate determinations.
In vitro, PD98059 has been shown to specifically inhibit MEK-1-mediated activation of MAPK (27) or a MEK-1 mutant that has low levels of constitutive activity (28) but does not directly inhibit ERK1 or ERK2 (28). The IC50 for PD98059 inhibition of intracellular MEK-1 is reported to be 2 μM (29). However, increasing the concentration of this selective MEK-1 inhibitor to 10 μM still only partially inhibits (≈70%) IL-3-induced Bcl2 phosphorylation (Fig. 1B). However, when 1 μM stauro is added with 10 μM PD98059, IL-3-induced Bcl2 phosphorylation is virtually blocked (Fig. 1C, lane 6). More importantly, cell survival in the presence of IL-3 at 24 h is reduced dramatically from 80% for each inhibitor alone to 30% for the combination (Fig. 1D). This is apparently specific for the ERKs because the specific MAPK p38 inhibitor, SB202190, fails to affect either IL-3- or Bryo-induced Bcl2 phosphorylation (data not shown). Collectively, these pharmacological data suggest a functional role for both a stauro-sensitive (i.e., PKC) and an SRK involving Bcl2 phosphorylation and point to MEK1 and/or a MAPK/ERK as potential candidate SRKs.
ATA Induces Bcl2 Phosphorylation at Ser-70 in a PD98059-Sensitive, Stauro-Resistant Manner and Enhances Cell Survival.
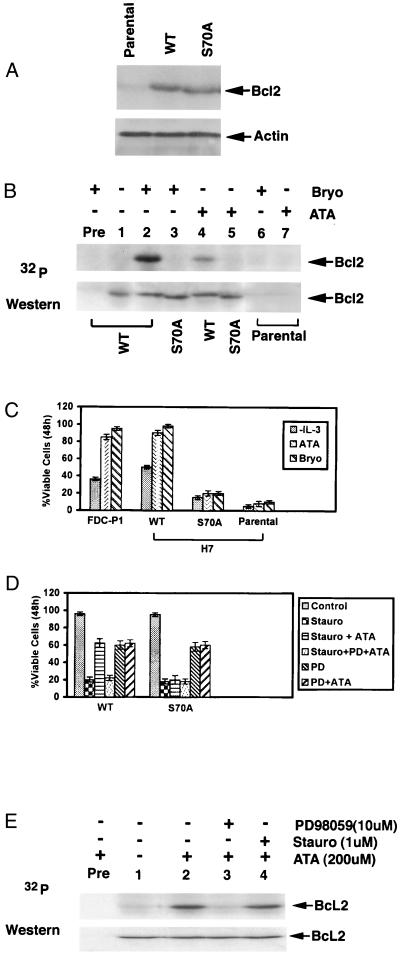
Recently, ATA, a nonpeptide activator of MEK-MAPK, was reported to replace nerve growth factor or serum and facilitate survival of PC12 neuroblastoma or Nb2 T lymphoma cells, respectively (30, 31). To pharmacologically test a potential role for involvement of the MEK/MAPK pathway in regulation of Bcl-2 phosphorylation, metabolic labeling studies were performed in H7 cells stably expressing wt or S70A Bcl2 as described previously (8). Importantly, Bcl2 protein levels were similar in both wt Bcl2 and S70A Bcl2 transfectants (Fig. 2A). Results demonstrate that ATA can induce Bcl2 phosphorylation exclusively at the Ser-70 site (Fig. 2B) and can protect wt but not S70A-expressing or parental cells, consistent with a role for the Ser-70 phosphorylation site in survival (Fig. 2C). Furthermore, ATA was found to protect another murine IL-3-dependent cell line, FDC-P1, which expresses high levels of endogenous Bcl2 as reported previously (Fig. 2C; ref. 9). These results also reveal that ATA or Bryo apparently only protect cells from apoptosis if wt but not the loss of function, nonphosphorylatable S70A mutant Bcl2 is exogenously expressed. As in previous studies (8), three independently derived clones expressing mutant S70A and wt Bcl2 were tested with similar results (data not shown), eliminating clonal variation as an experimental outcome. Thus, in the absence of an intact Ser-70 phosphorylation site (S70A) or significant levels of expression of Bcl2 (parental), neither ATA nor Bryo can significantly prolong survival in the absence of IL-3.
Figure 2.
ATA induces Bcl2 phosphorylation at Ser-70 in a PD98059-sensitive, stauro-resistant manner and enhances cell survival. (A) Bcl2 protein expression was determined in H7 cells transfected with wt or S70A Bcl2 by Western analysis using Bcl2 antibodies. (B) H7 cells expressing exogenous wt or S70A mutant Bcl2 were metabolically labeled with 32P-labeled orthophosphoric acid and treated with Bryo (100 nM) or ATA (200 μM) as indicated. Cells were lysed and analyzed as described in Fig. 1. (C) FDC-P1 cells expressing endogenous Bcl2, parental H7 cells, and H7 cells expressing exogenous wt or S70A Bcl2 were deprived of IL-3 and treated with or without ATA (200 μM) or Bryo (100 nM) for 48 h, and cell viability was determined by trypan blue dye exclusion. Data represent the mean of three separate determinations. (D) H7 cells expressing wt or S70A Bcl2 in the presence of IL-3 were treated with 1 μM stauro or 10 μM PD98059 in the presence or absence of 200 μM ATA for 48 h, and cell viability was determined as described above. (E) H7 cells expressing wt Bcl2 were metabolically labeled with 32P-labeled orthophosphoric acid and treated with 200 μM ATA with or without PD98059 (10 μM) or Stauro (1 μM) as indicated. Immunoprecipitated Bcl2 was analyzed as described in Fig. 1.
Significantly, Bcl2 phosphorylation and cell survival data for ATA are similar for Bryo, a potent natural product and potent activator of PKC. Both agents can replace IL-3 as a survival agonist (refs. 8 and 9; Fig. 2C). Because Bryo does not directly activate MEK-1/MAPK but can do so indirectly by activating PKC, which can directly activate Raf-1, the upstream MEKK (11, 13), this indicates that PKC may be involved in survival signaling in both MEK/MAPK-dependent and MEK/MAPK-independent pathways. Importantly, ATA also was found to protect H7 cells expressing wt but not S70A mutant Bcl2 from stauro-induced apoptosis (Fig. 2D). However, treatment with the highly specific MEK-1/MAPK inhibitor PD98059 could block ATA's ability to protect these cells, further supporting a role for MEK/MAPK in survival signaling. These results suggest that ATA may function in survival signaling by potentially activating the MAPK/ERK pathway, which can couple to downstream signaling involving Bcl2. However, although MAPK/ERK can be activated by IL-3, either in a Raf-1-MEK-MAPK cascade (13, 32) or after the direct phosphorylation and activation of RAF-1 by PKC (11), ATA apparently activates MAPK/ERK independently of PKC. To test this, H7 cells expressing wt Bcl2 were metabolically labeled with 32P-labeled orthophosphate and treated with ATA to stimulate Bcl2 phosphorylation. Cells also were treated with high concentrations of stauro or PD98059 as indicated. Results reveal that although ATA-stimulated Bcl2 phosphorylation is completely blocked by PD98059, even high concentrations of stauro that can completely inhibit PKC have no effect (Fig. 2E). Thus, ATA-induced Bcl2 phosphorylation may be mediated by a PD98059-sensitive, stauro-resistant Bcl2 kinase.
ERK1 and ERK2 Are Activated by IL-3 and Colocalize with Bcl2 in Mitochondria.
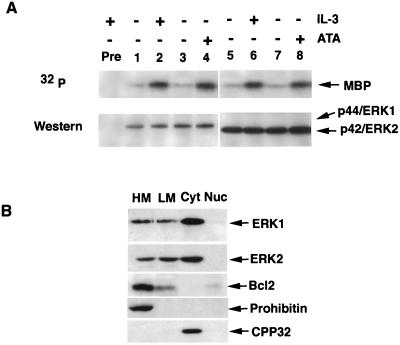
We tested whether MAPK/ERK1 or ERK2, the immediate downstream substrates for MEK-1, might be SRKs. First, IL-3 and ATA were assessed for their ability to activate ERK1 and ERK2 in H7 cells expressing wt Bcl2. Cells were washed in IL-3-free medium and incubated in the presence of IL-3 or ATA for 15 min. The ERKs were immunoprecipitated and used in an in vitro kinase assay along with [γ-32P]ATP and MBP as the substrate for phosphotransfer. Results indicate that both IL-3 and ATA can rapidly activate ERK1 and ERK2 to phosphorylate MBP (Fig. 3A). Again, FDC-P1 cells also were tested with similar results (data not shown). Second, to assess a potential direct role for an MAPK/ERK as an SRK, subcellular fractionation of H7 cells expressing Bcl2 was performed as described (15, 23). Bcl2 is thought to function in survival signaling by protecting mitochondrial integrity (6, 33). Although the majority of ERK1/2 is cytoplasmic, as previously reported (34), there is a distinct population of ERK1 and ERK2 colocalized with Bcl2 in the HM fraction, which contains mitochondrial membranes (Fig. 3B). To determine the purity of the subcellular fractions obtained, fraction-specific proteins were assessed by probing the same filters. Prohibitin, an exclusively mitochondrial protein (24), was detected only in the HM fraction whereas CPP32 (caspase 3), which is a cytosolic protease in growing cells (25), was detected exclusively in the cytosol (Fig. 3B). These results indicate that the mitochondria-containing HM and cytosolic fractions are >98% pure and that artifactual cross-contamination is unlikely to account for these findings.
Figure 3.
ERK1 and ERK2 are activated by IL-3 and colocalize with Bcl2 in mitochondria. (A) H7 cells expressing wt Bcl2 were treated with 1 μg/ml IL-3 or 200 μM ATA as indicated. ERK1 or ERK2 was immunoprecipitated from cell lysates and incubated with purified MBP in an in vitro kinase assay as described in Materials and Methods. Reaction mixtures were analyzed by SDS/PAGE, followed by autoradiography or Western blot by using ERK1 or ERK2 antibodies. (B) Subcellular fractionation, as described previously (14, 23), was performed to isolate HM, light membrane (LM), cytosol (Cyt), and nuclear membrane (Nuc). Prohibitin and CPP32 were used as positive and negative control to verify further the HM without contamination. Western blot analysis of subcellular fractions was performed to detect Bcl2 and ERK1/2.
ERK1 and ERK2 Can Directly Phosphorylate Bcl2 at Ser-70 in a Stauro-Resistant Manner.
The consensus phosphorylation site for the MAPK/ERK substrates is characterized by a target serine/threonine residue located immediately amino-terminal to a proline residue in a putative, proline-directed phosphorylation site (35). Interestingly, both murine and human Ser-70 Bcl2 phosphorylation sites are conserved and contain this potential consensus sequence (… VRTS*PL… ; ref. 36). An in vitro kinase assay was developed to test whether activated ERK1 and ERK2 can phosphorylate Bcl2. Results reveal that both ERK1 and ERK2 directly phosphorylate Bcl2 exclusively at Ser-70 (Fig. 4A) in a stauro-resistant manner (Fig. 4B). Purified PKCα was included as a positive control as shown previously (15). PKC-mediated Bcl2 phosphorylation is completely inhibited by stauro (Fig. 4B, compare lanes 7 and 8). These findings suggest that both ERK1 and ERK2 are candidate SRKs. To determine whether ERK1 and ERK2 may be SRKs in vivo, triple transfection studies were carried out in Cos7 cells. HA-Bcl2, a constitutively active MEK-1, and wild-type, activatable ERK1 or ERK2 cDNA expression plasmids were transiently transfected as described in Materials and Methods. After transfection for 48 h, either 10 μM PD98059, to inhibit ERK activation by constitutively active MEK-1 as described previously (27, 28), or 1 μM stauro was added to cells for 1 h as indicated. Cells then were metabolically labeled with 32P-labeled orthophosphate and treated with the same inhibitors for an additional 4 h before detergent lysis and isolation of HA-Bcl2 by isolation of Bcl2 by immunoprecipitation using an anti-HA antibody as described in Materials and Methods. The results clearly demonstrate that both ERK1 and ERK2 can be activated by MEK-1 to phosphorylate HA-Bcl2 and that this reaction is apparently specific, being PD98059-sensitive and stauro-resistant (Fig. 4C). The selectivity of the ERKs as potential direct Bcl2 kinases in vivo is demonstrated in these studies because cotransfection with the constitutively active MEK-1 and HA-Bcl2, in the absence of ERK, fails to lead to phosphorylation of Bcl2 (Fig. 4C, lane 4). Because even high concentrations of stauro are unable to inhibit ERK-mediated Bcl2 phosphorylation (Fig. 4C, lanes 7 and 11), these findings also verify the selectivity of PD98059 for inhibiting MEK-1 activation of ERK and confirm that ERK1 and ERK2 are potential physiologic SRKs.
Figure 4.
ERK1 and ERK2 can directly phosphorylate Bcl2 at Ser-70 in a stauro-resistant manner. (A) Wt or the phosphorylation-defective S70A mutant Bcl2 was immunoprecipitated from cell lysates and incubated with purified, activated ERK1 or ERK2 in an in vitro kinase assay as described in Materials and Methods. (B) As in A, immunoprecipitated wt Bcl2 was used as substrate for purified, active ERK1 or ERK2 or PKCα with or without 1 μM stauro as indicated. (C) Transfection of Cos7 cells using constitutively active MEK1, wt, activatable ERK1 or ERK2, and HA-Bcl2 expression plasmids was performed as described in Materials and Methods. At 48 h after transfection, the cells were pretreated with either 10 μM PD98059 or 1 μM stauro for 1 h as indicated and then metabolically labeled with 32P-labeled orthophosphate and treated with the same inhibitor for an additional 4 h. HA-Bcl2 was immunoprecipitated with monoclonal HA-11 antibody and analyzed as in Fig. 1.
Phosphorylation of Bcl2 at Ser-70 Is Necessary for Maximal Association with Its Heterodimeric Partner, Bax.
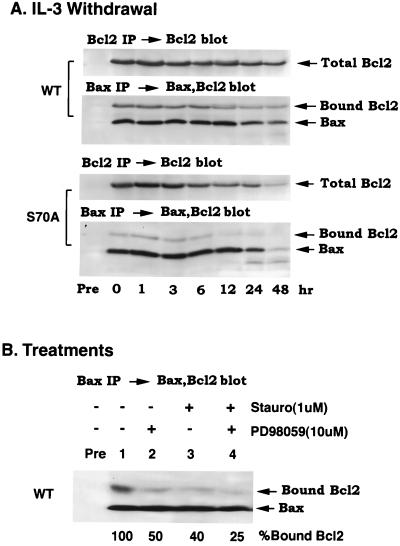
The discovery of p44 MAPK/ERK1 and p42 MAPK/ERK2 in addition to PKC as agonist-activated Bcl2 kinases underscores the potential importance of this posttranslational modification in Bcl2 survival function. Although the mechanism(s) by which Bcl2 actually functions to protect the integrity of mitochondria and suppress apoptosis is still unclear, one popular model holds that heterodimerization with Bax may block Bax's proapoptotic properties (18). Although we have found previously that phosphorylation of Bcl2 is not required for interaction with Bax under nonstress, growth conditions (8), we did not measure the stability of such an interaction after a stress application that leads to death. Now, we find that during IL-3 withdrawal at various times up to 48 h, the phosphorylation-negative, S70A Bcl2 mutant associates poorly with Bax as compared with wt Bcl2 when assessed by coimmunoprecipitation from detergent-soluble cell lysates (Fig. 5A, compare WT and S70A). Furthermore, the stability of the Bcl2:Bax association under these conditions is reduced significantly when cells are treated with either PD98059 or stauro (i.e., a reduction of 50% and 60%, respectively) or the combination (i.e., 75% reduced) as determined by densitometry (Fig. 5B, compare lanes 2, 3, and 4 with 1). These findings are consistent with the notion that Bcl2 phosphorylation may affect its antiapoptotic function by potentially enhancing or stabilizing a Bcl2:Bax interaction.
Figure 5.
Bcl2 phosphorylation at the Ser-70 site is necessary for maximal association with its heterodimeric, proapoptotic partner Bax. (A) H7 cells expressing wt or S70A Bcl2 were grown in IL-3-containing medium, and IL-3 was deprived for various times up to 48 h. The cells were harvested, washed, and lysed in detergent buffer at the indicated times, and the lysates were immunoprecipitated by using Bcl2 antibodies or a Bax-specific antisera as described (8). Total cell Bcl2 was determined by quantitative immunoprecipitation by using a Bcl2 antibody (Upper). Bax and bound Bcl2 were analyzed by Western blotting with a mixture of Bcl2 and Bax antibodies (Lower). (B) H7 cells expressing wt Bcl2 in the presence of IL-3 were treated with 1 μM stauro, 10 μM PD98059, or the combination for 30 min before harvesting, washing, lysis in detergent buffer, and immunoprecipitation by using a Bax-specific antiserum. The immunoprecipitates were analyzed as described in A. The percentage of bound Bcl2 after various treatments was determined by densitometry.
Discussion
Recently we discovered that phosphorylation of Bcl2 at Ser-70 may be required but is not sufficient for Bcl2's full and potent antiapoptotic function (8) and that the stauro-sensitive PKCα can functionally phosphorylate Bcl2 (15). However, a paradox arose because it was reported that forced expression of exogenous Bcl2 can prolong survival of cells treated with stauro (15, 16). Thus, the existence of a physiologic SRK was predicted. Evidence reported here suggests that a MEK/MAPK may be an SRK because the specific MEK/MAPK inhibitor PD98059 can only partially inhibit IL-3-induced Bcl2 phosphorylation whereas the combination of PD98059 and stauro can completely block phosphorylation. Furthermore, ERK1 and ERK2 can colocalize with Bcl2 in mitochondria and can directly phosphorylate Bcl2 in vitro at the functional Ser-70 site in a stauro-resistant, PD90859-sensitive manner, indicating their potential direct role as SRKs. Confirmation of the ERKs as physiologic SRKs was obtained in vivo from results of transfection studies that demonstrated that either activatable ERK1 or ERK2, when cotransfected with constitutively active MEK1, resulted in the stauro-resistant, PD98059-sensitive phosphorylation of Bcl2. The mechanism is apparently specific because activated MEK-1 alone cannot induce Bcl2 phosphorylation under these conditions. Recent reports supporting our findings indicate that activation of MAPK/ERK1/2 can be associated with cell survival; however, the mechanism(s) was not identified (17, 30). Our findings extend those studies by demonstrating that agonist-activated MAPK/ERKs may play a role in suppressing apoptosis, at least in part, by directly phosphorylating Bcl2. This novel finding may help to explain why ATA, a pharmacological activator of MAPK/ERKs but not PKC, can substitute for IL-3 in cell survival. Thus, ATA not only induces Bcl2 phosphorylation but also protects H7 cells expressing wt, but not the loss of function and nonphosphorylatable S70A mutant Bcl2, from apoptosis after IL-3 withdrawal (Fig. 2). Importantly, because ATA stimulation of Bcl2 phosphorylation is stauro-resistant, this supports a mechanism for MEK/MAPK in regulating this survival substrate.
Our findings also demonstrate that neither protein kinase A inhibitor HA1004 (9) nor MAPK/p38-specific inhibitor SB202190 can block IL-3- or Bryo-induced Bcl2 phosphorylation (data not shown), suggesting that PKA and p38 are not likely to be involved. However, reports recently have suggested that another MAPK, JNK/SAPK, may be a Bcl2 kinase (37, 38). In one report, JNK, but neither ERK1 nor p38, was found to phosphorylate Bcl2 in Cos7 cells when coexpressed with constitutively active Rac-1 to activate JNK (37). However, no specific upstream activator of ERK1 was used, and so a negative result may not be surprising. By contrast, in our transfection studies we used a constitutively active MEK-1 mutant (19) to ensure activation of ERK1/2 in Cos7 cells. Because the resulting HA-Bcl2 phosphorylation was inhibited by the MEK inhibitor PD98059 but not stauro and because activated MEK-1 alone did not result in phosphorylation of HA-Bcl2, these data are consistent with the notion that the ERKs1/2 are specific, direct SRKs. Thus, although it is formally possible that other SRKs also may exist, the ERKs1/2 are physiologic SRKs. Furthermore, because we can account for virtually all of the agonist-induced Bcl2 phosphorylation observed after IL-3 addition to cells through the concerted action of both PKC (a stauro-sensitive Bcl2 kinase) and the SRKs, ERK1/2, we would not need to postulate the existence of additional SRKs. This may explain, at least in part, how Bcl2 can prolong cell survival in cells treated with high concentrations of the potent PKC inhibitor, stauro.
With respect to a functional role for IL-3-induced Bcl2 phosphorylation by either SRKs or PKC, our results also suggest a novel mechanism by which phosphorylation may, at least in part, regulate Bcl2's survival function. Thus, Bcl2 phosphorylation may somehow enhance and/or stabilize, in a mechanism that is not yet clear, the noncovalent interaction between Bcl2 and its proapoptotic partner, Bax, that recently has been documented to occur in cells (39). This interaction has been postulated to potentially mediate cell survival by quenching Bax's apoptotic function (18). Our findings indicate that an intact Ser-70 Bcl2 phosphorylation site may regulate the stable interaction with Bax. Thus, although the loss-of-function S70A Bcl2 mutant can associate with Bax under IL-3-replete growth conditions as demonstrated in immunoprecipitation studies (Fig. 5 at 0 time), the association is reduced significantly and rapidly after IL-3 withdrawal. Furthermore, in support of a role for Bcl2 phosphorylation in the stability of association, incubation of cells with either PD98059 or stauro can inhibit significantly any Bcl2 and Bax interaction. Thus, although Bcl2 phosphorylation is not required for heterodimerization with Bax as we reported previously (8), it may be required for the stable association of these two death regulators after stress application such as IL-3 withdrawal or treatment with certain protein kinase inhibitors (i.e., stauro). Additional studies, however, will be required to elucidate this mechanism. In addition, there also may be other functional roles for Bcl2 phosphorylation in suppressing apoptosis because Bcl2 also can bind to other death regulators and/or may maintain mitochondrial integrity via potential pore/channel properties (1, 6).
By contrast to our (8, 9, 15, 40) and other's findings (10, 41, 42), serine phosphorylation of Bcl2 also has been proposed to inactivate Bcl2 (38, 43, 44). The conclusions were drawn from studies using clinically useful antimitotic drugs such as paclitaxel, vincristine, and vinblastine that could induce apoptosis of cells in association with Bcl2 phosphorylation. However, the antimitotic drug-induced phosphorylation mechanism clearly differs from that of growth factor- or agonist-induced Bcl2 phosphorylation, which occurs exclusively at Ser-70 and which is rapid and reversible (8, 21). In addition, paclitaxel-induced Bcl2 phosphorylation occurs only after 2 h of incubation with the drug and appears to involve multiple sites, including Ser-70 and Ser-87 (43, 44). Furthermore, neither PKC nor the ERKs appear to be involved in paclitaxel-induced Bcl2 phosphorylation. Thus, although it is possible that multiple-site phosphorylation may differentially regulate Bcl2, perhaps by inducing an additional conformational change, it has not yet been demonstrated experimentally. Alternatively, antimitotic drug-induced Bcl2 phosphorylation could be a stress response intended to activate Bcl2 and prevent apoptosis. This explanation could account for why, for example, forced overexpression of Bcl2 can significantly prolong cell survival after exposure to paclitaxel (45).
The flexible loop domain (amino acids 32–80) of Bcl2 has been proposed as a negative regulatory domain (46). Although the function of this region is not yet understood, this domain contains the Ser-70 phosphorylation site (8) and a caspase-3 cleavage site (Asp-34; ref. 47). Previous reports have indicated that deletion of the loop region can enhance Bcl-XL or Bcl2's antiapoptotic function under certain conditions (46). Thus, although it is not clear how phosphorylation affects the function of the flexible loop, it is possible that phosphorylation may promote a conformational change(s) that somehow inhibits the negative function of the loop domain. Further studies will be required to demonstrate this hypothesis.
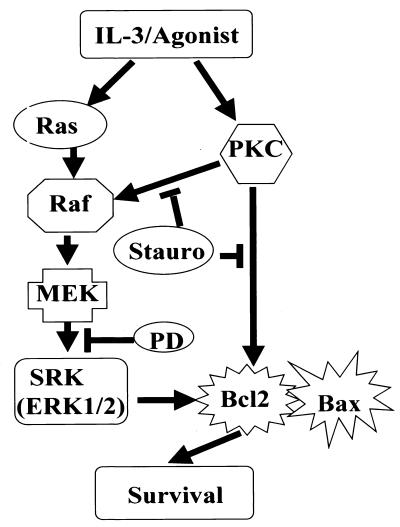
In summary, it now seems clear that in addition to a requisite role in growth factor-induced proliferative signaling, the MEK-1/MAPK (ERK1/2) pathway also may interface with survival signaling at the level of Bcl2 to directly link these two critical growth pathways (Fig. 6). By serving as an SRK, the ERKs may cooperate with survival-signaling pathways to ensure Bcl2's survival function. These results would explain the paradox with stauro and Bcl2 and suggest that novel apoptosis-inducing antineoplastic strategies aimed at functionally inactivating Bcl2 may require targeting of multiple upstream agonist-activated pathways.
Figure 6.
Proposed model of IL-3 survival signaling. IL-3 growth factor or survival agonists such as ATA or Bryo (9, 30) stimulate different upstream protein kinase cascades that retain selective sensitivity to either stauro or PD98059 with respect to Ser-70 Bcl2 phosphorylation and cell survival. IL-3 signal transduction includes PKC-dependent and PKC-independent pathways: PKC/Bcl2, PKC/Raf/MEK/SRK(ERK1/2)/Bcl2, and Ras/Raf/MEK/SRK(ERK1/2)/Bcl2.
Acknowledgments
We thank Dr. Lynn Heasley (University of Colorado Health Science Center) for kindly providing constitutively active MEK1, WT-ERK1, and WT-ERK2 plasmids. This work was supported by National Institutes of Health Grants CA44649 and CA47993.
Abbreviations
- ATA
aurintricarboxylic acid
- Bryo
bryostatin 1
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- stauro
staurosporine
- SRK
staurosporine-resistant Bcl2 kinase
- PKC
protein kinase C
- MBP
myelin basic protein
- HM
heavy membrane
- wt
wild type
References
- 1.Adams J M, Cory S. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Yang E, Korsmeyer S J. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 3.Vaux D L, Cory S, Adams J M. Nature (London) 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 4.Sentman D L, Shutter J R, Hockenbery D, Kanagawa O, Korsmeyer S J. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Nature (London) 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 6.Green D R, Reed J C. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 7.Reed J C. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito T, Deng X, Carr B, May W S. J Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 9.May W S, Tyler P G, Ito T, Armstrong D K, Qatsha K A, Davidson N E. J Biol Chem. 1994;269:26865–26870. [PubMed] [Google Scholar]
- 10.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau V J. J Biol Chem. 1997;272:19022–19026. doi: 10.1074/jbc.272.30.19022. [DOI] [PubMed] [Google Scholar]
- 11.Carroll M P, May W S. J Biol Chem. 1994;269:1249–1256. [PubMed] [Google Scholar]
- 12.Marais L, Light Y, Mason C, Paterson H, Olson M F, Marshall C J. Nature (London) 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 13.Howe L R, Leevers S J, Gomez N, Makielny S, Cohen P, Marshall C J. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 14.Kolch W, Heldecker G, Hochs G, Hummel R, Vahdid H, Mischak H, Finkenzeller G, Marme D, Rapp U R. Nature (London) 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 15.Ruvolo P P, Deng X, Carr B, May W S. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 16.Hunter J J, Bond B L, Parslow T G. Mol Cell Biol. 1996;16:877–883. doi: 10.1128/mcb.16.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadheim T A, Kucera G L. Biochem Biophys Res Commun. 1998;245:266–227. doi: 10.1006/bbrc.1998.8410. [DOI] [PubMed] [Google Scholar]
- 18.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 19.Mansour S J, Matten W T, Hermann A S, Canadia J M, Rong S, Fukassawa K, Woude G F, Ahn N G. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 20.Robbins D J, Zhen E, Owaki H, Vanderbilt C, Ebert D, Geppert T D, Cobb M H. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- 21.Deng X, Ito T, Carr B, Mumby M, May W S. J Biol Chem. 1998;273:34157–34163. doi: 10.1074/jbc.273.51.34157. [DOI] [PubMed] [Google Scholar]
- 22.Earl D C, Korner A. Biochem J. 1965;94:721–734. doi: 10.1042/bj0940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H G, Rapp U R, Reed J C. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 24.Ikonen E, Fielder K, Parton R G, Simons K. FEBS Lett. 1995;358:273–277. doi: 10.1016/0014-5793(94)01444-6. [DOI] [PubMed] [Google Scholar]
- 25.Krajewska M, Wang H G, Krajewski S, Zapata J M, Shabaik A, Gascoyne R, Reed J C. Cancer Res. 1997;57:1605–1613. [PubMed] [Google Scholar]
- 26.Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Biophys Res Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 27.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 29.Pang L, Sawada T, Deckers S J, Saltiel A. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 30.Okada N, Koizumi S. J Biol Chem. 1995;270:16464–16469. doi: 10.1074/jbc.270.27.16464. [DOI] [PubMed] [Google Scholar]
- 31.Rui H, Xu J, Mehta S, Fang H, Williams J, Dong F, Grimly P M. J Biol Chem. 1998;273:28–32. doi: 10.1074/jbc.273.1.28. [DOI] [PubMed] [Google Scholar]
- 32.Marshall C J. Cell Signalling. 1996;383:127–128. doi: 10.1038/383127a0. [DOI] [PubMed] [Google Scholar]
- 33.Zhu W, Cowie A, Wasfy G W, Penn L Z, Leber B, Adams D W. EMBO J. 1996;15:4130–4140. [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall C J. Curr Opin Cell. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez F A, Raden D L, Davis R J. J Biol Chem. 1991;266:22159–22163. [PubMed] [Google Scholar]
- 36.Takayama S, Cazals-Hatem D L, Kitada S, Tanaka S, Miyashita T, Hovey L R, Huen D, Rickinson A, Verapandian P, Krajewski S. DNA Cell Biol. 1994;13:679–692. doi: 10.1089/dna.1994.13.679. [DOI] [PubMed] [Google Scholar]
- 37.Maundrell K, Antonsson B, Magnenat E, Camps M, Muda M, Chabert C, Gilieron C, Boschert U, Via-Knecht E, Martinou J C, et al. J Biol Chem. 1997;272:25238–25242. doi: 10.1074/jbc.272.40.25238. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava R K, Mi Q S, Hardwick J M, Longo D L. Proc Natl Acad Sci USA. 1999;96:3775–3780. doi: 10.1073/pnas.96.7.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahajan N P, Linder K, Berry G, Gordon G W, Heim R, Herman B. Nat Biotech. 1999;16:547–552. doi: 10.1038/nbt0698-547. [DOI] [PubMed] [Google Scholar]
- 40.Ruvolo P P, Deng X, Ito T, Carr B K, May W S. J Biol Chem. 1999;274:20296–20300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 41.Chen C Y, Faller D V. J Biol Chem. 1996;271:2376–2379. doi: 10.1074/jbc.271.5.2376. [DOI] [PubMed] [Google Scholar]
- 42.Murata M, Nagai M, Fujita M, Ohmori M, Takahara J. Cell Mol Life Sci. 1997;53:737–743. doi: 10.1007/s000180050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haldar S, Jena N, Croce C M. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu A, Haldar S. Int J Oncol. 1998;13:659–664. doi: 10.3892/ijo.13.4.659. [DOI] [PubMed] [Google Scholar]
- 45.Gibson S, Widmann C, Johnson G L. J Biol Chem. 1999;274:10916–10922. doi: 10.1074/jbc.274.16.10916. [DOI] [PubMed] [Google Scholar]
- 46.Chang B S, Minn A J, Muchmore S W, Fesik S W, Thompson C B. EMBO J. 1997;16:968–977. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng E H, Kirsch D G, Clem R J, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]