Abstract
DNA damage has been associated with prostate cancer risk. Men who were referred for initial prostate biopsy for elevated prostate-specific antigen or abnormal digital rectal examination are often found with no cancer but have a higher risk of developing prostate cancer than the general population of men in their lifetime. In this study, we investigated whether DNA damage is one of the factors that predispose these men referred for prostate biopsies to a higher risk of prostate cancer. We found significantly elevated levels of 8-oxo-2-deoxyguanosine immunoreactivity in the prostates of the referred men (n = 50) in comparison to the control prostates of men (n = 32) with no indication for referral for prostate biopsy. Twelve of these control men were healthy middle-aged men and 20 of them were older men whose conditions were diagnosed with bladder cancer but with normal serum prostate-specific antigen and digital rectal examination and no evidence of prostate disease. In all the 8-oxo-2-deoxyguanosine-positive prostates, we detected phosphorylation of the ataxia telangiectasia mutated kinase and expression of the immune-stimulatory molecule MIC in the prostate epithelium. These data suggest that: 1) oxidative DNA damage has occurred in the “referred” but pathologically normal prostates, indicating that these prostates may be subjected to genomic instability and eventually neoplastic transformation; 2) in response to DNA damage, two surveillance pathways, represented by ataxia telangiectasia mutated phosphorylation and induction of the NKG2D ligand MIC, were activated to prevent tumorigenesis.
Introduction
The tumor suppressor ATM (ataxia telangiectasia mutated) is constitutively expressed but generally inactive in normal human tissues except in specialized cell types, for example, germ cells and lymphocytes [1]. In response to genotoxic insults, human cells, if not ATM-deficient, immediately activate the ATM protein kinase by autophosphorylation to initiate DNA-damage responses [2,3]. Thus, activation of ATM signifies the occurrence of DNA damage in cells. Activated ATM can interact with multiple pathways in response to DNA damage and serves as a “sensor” of oxidative stress to maintain DNA integrity [4,5]. It is well understood that ATM respond to severe DNA damage such as double-strand breaks (DSBs) by immediate phosphorylation of downstream target phospho-histones [6,7]. ATM can also respond to more subtle forms of DNA damage, such as single-strand base modification, among which, the oxidation of guanine, 8-oxo-2-deoxyguanosine (8-oxo-dG), is the major lesion [5,8,9]. Although complicated pathways are involved in ATM-mediated DNA-damage responses [10], phosphorylation of ATM results in either activation of cell cycle checkpoints to facilitate DNA repair or induction of p53-mediated apoptosis of severely damaged cells [1,2]. Activation of ATM has been shown in preneoplastic lesions of human breast, bladder, and colon and thus suggested to be a very early DNA damage surveillance mechanism to protect against transformation [11–14].
Recent studies indicate that exposure of nontransformed cell lines to genotoxic stresses can induce expression of the ligands for the immune receptor NKG2D through the same DNA damage response pathway as that activates ATM [15]. NKG2D is an activating receptor expressed by human natural killer (NK) cells, γδ T cells, CD8 T cells, and some activated CD4 T cells [16,17]. This finding revealed a possible new dimension to the mechanisms of early surveillance against tumorigenesis. In humans, the stress-induced MHC I chain-related molecules A and B (MICA/B, generally termed MIC) are the most frequently studied human NKG2D ligands [17]. MIC is often found expressed by human epithelial tumors but generally absent in normal tissues [18]. Engagement MIC to NKG2D can activate innate NK cell antitumor immunity as cytotoxicity and cytokine production [19]. Induced expression of MIC has been proposed to be one of the innate protective mechanisms for the host in response to epithelial transformation [16,17]. Conversely, tumor cells can shed MIC and the resulting soluble form of MIC (sMIC) can impair host immunity, which is suggested to be one of the mechanisms of tumor immune evasion and progression [20].
DNA damage or failure of DNA damage surveillance has been associated with increased prostate cancer risk [21–23]. Several clinical studies have indicated that men who were referred for prostate biopsy because of elevated prostate-specific antigen (PSA > 4.0 ng/ml) or abnormal digital rectal examination (DRE) results but no evidence of cancer on biopsy have a higher incidence of developing prostate cancer than men in general population [24–27]. It has been suggested that there are preneoplastic changes in the “referred” prostate, which may be associated with increased risk of prostate cancer [28]. Here, we formulated the hypotheses that biological changes, such as DNA damage, have occurred in the “referred” prostates to predispose these men to increased risk of prostate cancer and that early tumor surveillance against these changes may form a barrier to tumorigenesis. To address these assertions, we examined oxidative DNA damage and responses to DNA damage, the ATM phosphorylation and the induction of MIC expression, in these “referred” prostates.
Materials and Methods
Patient and Sample Demographics
The collection and the use of tissue specimens and of relevant clinical information were approved by the University of Washington Institutional Review Board. Formalin-fixed and paraffin-embedded “referred” prostate needle core biopsies (n = 50) were obtained from the outpatient Urology Clinics at the Veterans Affair Puget Sound Health Care System. These biopsies were from men who had either an elevated serum PSA (>4.0 ng/ml) or an abnormal DRE results and had not undergone a previous prostate biopsy. All men underwent a 12-core biopsy protocol of the peripheral zone of the prostate. Pathological examination of the biopsies showed no histologic evidence of prostate cancer or prostatic intraepithelial neoplasia based on independent review by two pathologists experienced in prostate cancer. Two biopsy cores were chosen for this study.
Two groups of “healthy” prostate biopsies were used as normal or negative controls. The first group consisted of prostate biopsies from healthy middle-aged men (n = 12; median age, 42 years) with a serum PSA ≤2.5 ng/ml and a normal DRE result who had biopsies as part of a separate investigation [29,30]. The other group consisted of prostate specimens from aged men whose conditions were diagnosed with bladder cancer but with a serum PSA <4.0 ng/ml (n = 20; median age, 62 years), a normal DRE result, and no evidence of prostate cancer in microscopic examination of the prostate.
Lastly, we constructed tissue microarrays composed of prostate adenocarcinoma (n = 19; median age, 64 years) from men undergoing radical prostatectomy for clinically localized prostate cancer (Gleason grades 5–7) for comparison.
Immunohistochemistry
To evaluate DNA damage responses, we performed immunohistochemistry (IHC) staining of the prostate specimens using the following IHC-specific antibodies: anti-8-oxo-dG (Clone N45.1; Japan Institute for Control of Aging [31,32]), anti-ATM(Ab-8; NeoMarkers, Fremont, CA), monoclonal anti-Ser 1981-phosphorylated-ATM (pS-ATM) antibody (Rockland Immunochemicals, Gilbertsville, PA [12,13]), and MHC I chain-related molecule (MIC)-specific rabbit polyclonal antiserum H-300 (Santa Cruz Biotechnology Inc., Santa Cruz, CA [24]). Rabbit anti-phospho-H2AX antibody was from cell signaling (Danvers, MA).
Indirect IHC on formalin-fixed, paraffin-embedded tissues sections or microarrays was performed as previously described [33], using Vectastin Elite Kit (Vector Laboratories, Burlingame, CA). Briefly, slides containing sections of prostate lesions were dewaxed and rehydrated. After antigen retrieval procedure, endogenous peroxidase activity was quenched. After being blocked with 1.5%normal goat serum, slides were incubated with specific antibody for 1 hour followed by sequential incubation with biotinylated secondary antibody, peroxidase-labeled avidin, and diaminobenzidine/hydrogen peroxide chromogen substrate. Some slides were counterstained with hematoxylin before mounting. For the negative control, species-matched immunoglobin controls (Vector Laboratories) were used instead of the primary antibody.
Evaluation of Immunohistochemistry Staining
The intensity of specific staining was double-blind graded on a scale of 1 to 3 (1 = weak staining, 2 = moderate staining, and 3 = strong staining). A semiquantitative score on a 10% increment scale ranging from 0 to 100% was used to assess the percentage of stained cells of the intensity. Approximately 500 cells were analyzed for each case. The final composite staining score for immunostaining was based on the intensity of staining (1, 2, or 3) multiplied by the percentage of immunopositive cells (0–100). The maximum composite score is 300.
Statistical Analysis
Statistical analyses were performed using STATA (College Station, TX). The composite staining scores for 8-oxo-dG, pS-ATM, and MIC between subject groups were compared using analysis of variance with a Scheffe correction for multiple comparisons. Paired t-tests with a Bonferroni correction were performed for significance. For all comparisons, a P value < .05 was considered statistically significant. All P values are 2-tailed.
Results
The Referred and Control Subjects
The basic clinical characteristics of the referred and control subjects are given in Table 1. There was no significant difference in age or prostate size between the referred subjects and the aged control subjects. However, the referred subjects have significantly higher levels of serum PSA than the control aged subjects (P < .05). One third of the middle-aged control subjects were under androgen-suppression treatment for 4 weeks because of participating in an androgen-suppression investigation [29,30]. These subjects had reduced serum and intraprostatic tissue levels of testosterone (T) and dihydrotestosterone (DHT) at the time of biopsy [30]. However, the short-term androgen suppression did not affect the current study results.
Table 1.
Clinical Characteristics of the Referred and Control Subjects.
| Subject Group | Referred (n = 50) | Control Middle-aged (n = 12) | Control Aged (n = 20) |
| Age (years) | 61.4 ± 5.9 | 42.7 ± 3.4 | 62.4 ± 6.7 |
| PSA (ng/ml) | 7.6 ± 7.1 | <2.0 | <4.0 |
| Prostate volume (cm3) | 40.4 ± 24.6 | 22.7 ± 4.2 | 35.6 ± 29.4 |
Oxidative DNA Damage in the “Referred” Prostates
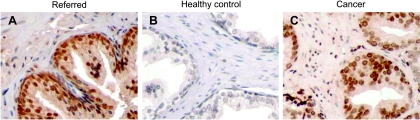
Extensive observations suggest that reactive oxygen species may cause accumulated DNA damage during aging, which contributes to increased incidence of cancer [34]. We thus examined the levels of oxidative DNA damage in the referred prostates. Fifty paraffin-embedded “referred” prostate biopsy specimens were IHC-stained with a monoclonal antibody specific to the oxidative DNA damage marker, 8-oxo-dG [31,32]. Sections of prostate biopsies from 12 middle-aged healthy men and 20 age-matched older men who have a PSA <4.0 ng/ml, a normal DRE result, and no evidence of prostate cancer were used as negative controls. Of 50 “referred” prostates, 36 (74%) showed positive 8-oxo-dG immuoreactivity. In contrast, 8-oxo-dG immunoreactivity was not found in the prostates from the 12 healthy middle-aged men and only found in 1 (5%) of 20 prostates from the control age-matched older men. The immunoreactivity of 8-oxo-dG was predominantly localized to the luminal epithelium of the prostates (Figure 1, A and B). We also assessed 8-oxo-dG immunoreactivity in the prostate carcinomas as a comparison. Nearly all the carcinomas from the 19 subjects showed strong 8-oxo-dG immunoreactivity (Figure 1C).
Figure 1.
8-oxo-dG immunostaining showing oxidative DNA damage in the referred prostates: (A) referred prostate, (B) age-matched control prostate, (C) prostate carcinoma. The IHC-specific anti-8-oxo-dG antibody (Clone N45.1) was used. Original magnification, x250.
We evaluated the level of 8-oxo-dG immunoreactivity in the epithelium of these specimens using a semiquantitative method by multiplying the intensity of immunostaining with the percentage of immunopositive cells (see details in Materials and Methods). 8-oxo-dG immunoreactivity was significantly elevated in the “referred” prostate epithelium compared with the two control groups (Table 2, P < .001) and was significantly elevated in the carcinomas compared with the referred prostates (Table 2, P < .01).
Table 2.
Evaluation of 8-oxo-dG, ATM, pATM, and MIC Immunostaining in Prostate Epithelium.
| Type of Prostates | Composite Staining Scores* | |||||||
| 8-oxo-dG | pATM | ATM | MIC | |||||
| Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | |
| Healthy (middle-aged) | 0 | 0 | 0 | 0 | 300 | 300.0 ± 0.0 | 0 | 4.8 ± 19.2 |
| Healthy (aged) | 0 | 5.2 ± 20.4 | 0 | 4.2 ± 18.4 | 300 | 300.0 ± 0.0 | 0 | 4.8 ± 19.2 |
| referred | 120 | 153.0 ± 103.2† | 200 | 208.2 ± 58.7† | 300 | 300.0 ± 0.0 | 185 | 197.5 ± 59.1† |
| Cancer | 300 | 284 ± 48.4‡ | 120 | 123 ± 42.1 | 170 | 172.6 ± 55.3 | 80 | 88.4 ± 37.5 |
Composite score = staining intensity (1, 2, or 3) x percentage of positive cells (0–100). Maximum score is 300.
Composite score is significantly higher in the referred than in the healthy (P < .001).
Composite score is significantly higher in carcinoma than in the referred (P < .01).
Reactive oxygen species can also cause DNA DSBs. We thus also examined the level of DSBs in the referred prostates by IHC staining with a specific antibody to phospho-H2AX [7]. Thirty-nine of the referred prostates showed weak immunoreactivity of phospho-H2AX. Neither the frequency nor intensity of phospho-H2AX immunoreactivity has correlation with the positivity of 8-oxo-dG in the referred prostates (data not shown). These data suggest that single-strand DNA base oxidative modification is the major form of oxidative DNA damage in the referred prostates.
Cellular DNA Damage Surveillance in the “Referred” Prostate Through Activation of ATM Kinase in the Epithelia
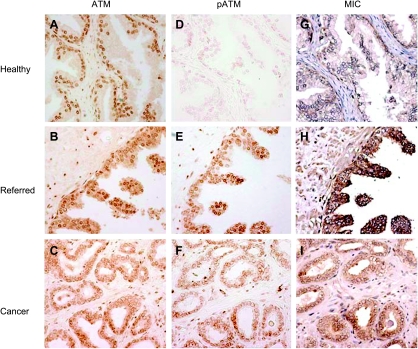
ATM kinase is a key regulator of biological response to DNA damage [2], and activation of ATM by phosphorylation is one of the first indications of cellular response to DNA damage [3]. To evaluate ATM phosphorylation status in the “referred” prostates in response to oxidative DNA damage, we first examined ATM expression in these biopsies. ATM was constitutively expressed in all the prostate tissues (Figure 2, A–C). We next evaluated ATM phosphorylation in these prostate biopsies using IHC with the monoclonal antibody pS-ATM, which is specific to serine1981-phosphorylated-ATM [12,13]. In the 50 “referred” prostate specimens, phosphorylation of ATM was evident in the epithelium of 42 specimens (Figure 2E), including the 36 specimens that are 8-oxo-dG-positive. Phosphorylation of ATM was not seen in the middle-aged and only weakly seen in 1 of 20 of the older “control” prostate epithelium (Table 2 and Figure 2D).
Figure 2.
Immunohistochemistry staining showing activation of ATM and induction of MIC in the luminal epithelium of the representative “referred” prostate. ATM is constitutively expressed in the prostate (A–C). Phosphorylation of ATM is rare in the healthy prostate (D) but evident in the “referred” prostate (E) and heterogenic in carcinomas (F). MIC expression is detected only where ATM was phosphorylated (G–I). Original magnification, x200.
In carcinomas, ATM immunostaining showed a great degree of heterogeneity, which may be due to the heterogeneity of mutations in the ATM gene [35]. Phosphorylation of ATM was seen in the epithelium of all the specimens but with a high degree of heterogeneity in intensity and reduced levels in most cells (Figure 2F). This attenuated or defective ATM activation has been suggested to be a cause of cancer progression [36].
To further confirm that ATM was specifically activated in the “referred” prostates, we evaluated the level of ATM phosphorylation in the epithelium of these specimens using a semiquantitative method as described above. Phosphorylation of ATM in the “referred” prostate epithelium was significantly elevated compared with the two control groups (Table 2, P < .001) and carcinomas (Table 2, P = .03). Whereas the differences in the levels of ATM phosphorylation between the “referred” biopsy and the carcinoma may be in part due to ATM mutation and thus reduced ATM expression in the carcinomas [12] (Table 2), the significantly elevated ATM phosphorylation in the “referred” specimens compared with the normal controls is not a result of difference in ATM expression (Table 2). The normal and the “referred” prostates expressed comparable levels of ATM protein (Table 2 and Figure 2, A and B).
Systemic DNA Damage Surveillance by Induction of MIC Expression in the Secretory Epithelial Cells
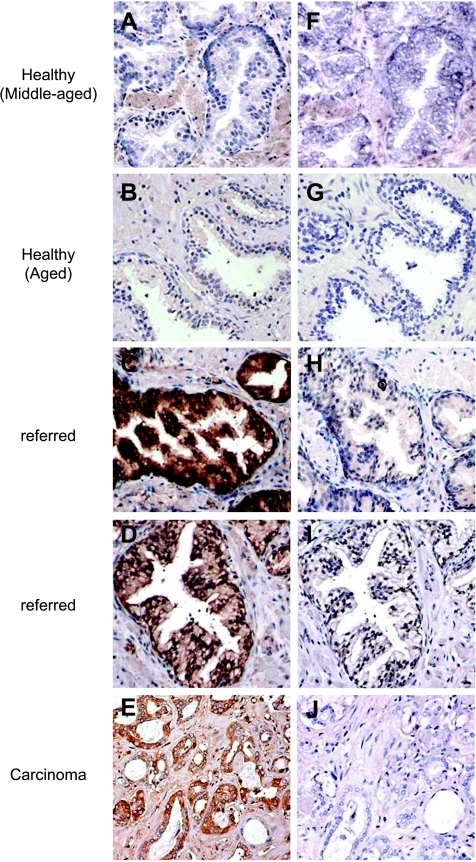
Gasser et al. [15] have shown that induced expression of MIC was associated with DNA damage in cells. We thus examined MIC expression in these prostate biopsies. With a specific antibody to MIC, we showed positive MIC immunoreactivity in the luminal secretory epithelium of the “referred” prostate biopsies, which showed positive to pATM (Figure 2H). In comparison, all the middle-aged and 19 of the 20 older healthy prostate glands were negative for MIC (Table 2 and Figure 2G). In the carcinomas, the luminal epithelia showed heterogeneity in MIC immuoreactivity (Figure 2I). Overall, MIC expression was detected where phosphorylation of ATM was apparent. To confirm the specificity of the anti-MIC antibody, we preabsorbed the anti-MIC antibody with purified recombinant soluble MIC. No MIC immune reactivity was yielded in any of the specimens (Figure 3), thus confirming the specificity of the anti-MIC antibody.
Figure 3.
Peptide blocking demonstrating specificity of anti-MIC antiserum. (A–E) MIC immunoreactivity with the rabbit anti-MIC anti-serum H-300 in various prostate specimens. (A and B) No positive MIC immunoreactivity was seen in the healthy prostate epithelium. (C and D) Different intensity of MIC immunoreactivity in the “referred” prostate epithelium. (F–J) No MIC immunoreactivity was seen in any of the specimens when the anti-MIC antibody was preabsorbed with 5 ng/µl purified sMICA. A and F are frozen sections. The rest are paraffin-embedded sections. Original magnification, x200.
We also assessed MIC expression in the epithelium of these specimens using the semiquantitative method as described above. As shown in Table 1, MIC expression was significantly elevated (P < .001) in the “referred” prostate epithelium compared with either the middle-aged or the age-matched normal controls. Although MIC was prevalently expressed in the carcinomas, the level was significantly lower when compared with the “referred” epithelium (P < .05). The reduced level of MIC expression shown in the carcinomas is due to tumor shedding of MIC as we have previously reported [33].
Correlation of MIC Expression with ATM Phosphorylation
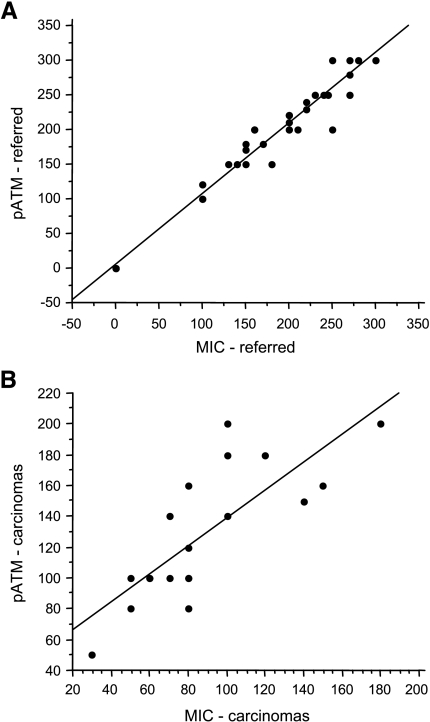
Using simple regression analysis, we show that there is a significant correlation of MIC expression and ATM phosphorylation in the “referred” prostate epithelium (R = 0.99, P < .001; Figure 4A). There was also a significant correlation between MIC expression and ATM phosphorylation in the carcinomas (R = 0.78, P < .001; Figure 4B). Together, these results demonstrate that, in the “referred” prostate, induction of MIC expression is correlated with ATM phosphorylation, presumably a manifestation of the cellular response to DNA damage.
Figure 4.
Correlation of MIC expression with ATM phosphorylation. (A) Significant correlation of ATM phosphorylation and MIC expression in the prostate epithelium of the “referred” men, R = 0.99, P < .001. (B) A lesser degree but still significant correlation of ATM phosphorylation and MIC expression in the prostate carcinomas, R = 0.78, P < .001. ATM phosphorylation and MIC immunostaining were evaluated using a semiquantitative composite score method as described in the Materials and Methods section. Data were analyzed using Pearson's correlation test.
Discussion
We have shown that two DNA damage response pathways, as indicated by phosphorylation of ATM kinase and expression of the stress-induced MIC, are activated in most (84%) of the “referred” prostate epithelium. Among these prostates, a high percentage (36/42) expressed an oxidative DNA damage phenotype as indicated by oxidative modification of guanine. These manifestations are absent or rare in healthy prostates. Our data indicate that genomic damage, predominantly single-strand DNA base oxidative modification, has occurred in the epithelium of these histologically normal but “referred” prostates.
DNA damage, caused by oxidative stress and/or free radical, has been associated with an increased cancer risk [21–23]. It has been reported that 42% of men aged 55 to 80 years exhibited a DNA-damaged phenotype in the prostate [23].Our data suggest that DNA damage, largely due to oxidative stress as indicated by 8-oxo-dG, has occurred in the prostate of a majority of men referred for prostate biopsy in our institution. We have no evidence that our referral pattern for prostate biopsy is different from that of other academic institution. However, studies from other institutions suggest that 25% to 40% of these “referred” men will be expected to have their conditions diagnosed with prostate cancer in future biopsies [24–27]. The lower incidence of prostate cancer development in contrast to the high incidence of DNA damage of the “referred” men may be due to activation of two surveillance pathways as our study has indicated, which prevents or delays the development of cancer (Figure 5). The primary pathway is phosphorylation of ATM, which initiates the DNA repair or apoptosis. The secondary pathway alerts the immune system by inducing the expression of the immune stimulatory molecule MIC and potentially activating NK cell immunity to eliminate cells with genomic damage and prevent tumorigenesis. We speculate that, in some men, mutations accumulate in the DNA damage response pathways during aging, such as pTEN, p53, CHEK, and so on [37], thus unscheduled cell replication would likely occur, which will ultimately result in genomic instability and tumorigenesis. In fact, this speculation is supported by our current data showing the elevated level of DNA damage and impaired activation of ATM in the carcinomas. In addition, tumor cells may shed MIC, which can lead to the failure of MIC-induced innate immune surveillance [33].
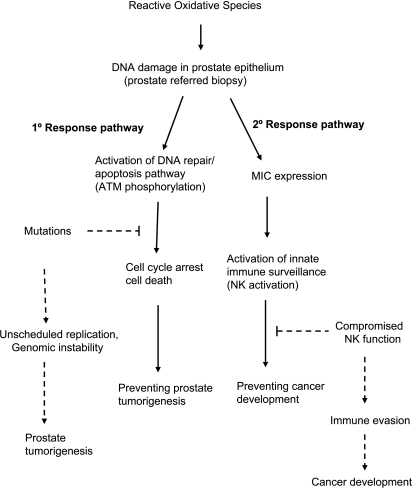
Figure 5.
Proposed etiology of DNA damage in the “referred” prostate biopsies and prostate cancer risk. In response to DNA damage in the prostate, cellular and systemic tumor surveillance pathways could be initiated to prevent tumorigenesis or cancer development. Protection at cellular level is through activation of ATM to initiate DNA repair or apoptosis of damaged cells. Protection at systemic level is through the induction of MIC expression to alert of NK cell-mediated innate immunity. In the incidence of accumulation of mutations in DNA repair or apoptosis pathways, genomic instability will occur and lead to prostate tumorigenesis. Compromised NK cell function will enable tumor immune evasion and facilitate cancer development.
Before this report, MIC was only shown to be expressed by transformed cells, either in some viral-infected cells or in tumor cells. Our data demonstrate that MIC can be detected in preneoplastic human tissues where DNA damage has occurred. This suggests that MIC is unlikely to be a tumor-specific biomarker. In fact, we show that the level of MIC expression correlates with ATM phosphorylation in tissues, suggesting that induction of MIC expression may serve as a general DNA damage marker and signify a preneoplastic biological change in tissues.
Our study has several limitations. This is a clinical observation study designed to generate associations with plausible biologic mechanisms related to carcinogenesis. Because prostate cancer is a slowly developing disease, we do not have lifetime clinical follow-up data of the study subjects. Thus, our findings can only be used as preneoplastic markers to suggest potential cancer risk and cannot be powered to predict future cancer diagnosis.
In summary, our data indicate that, although the biopsy-based histological features may not identify a malignancy, genomic stress such as DNA damage has occurred, which may predispose the prostate to neoplastic transformation. In response to the DNA damage, activation of cellular DNA repair pathway and induction of the immune stimulatory MIC expression may form barriers to prevent cancer development.
Acknowledgments
The authors thank Catherine L. Atteridge and Michael Tao for technical assistance in IHC.
Footnotes
Supported by Department of Defense New Investigator's Award (W81XWH-04-1-0577), National Institutes of Health Temin Award (1K01CA116002), the Pacific Northwest Prostate Cancer SPORE Pilot Award (P50-CA97186) to J. Wu and S.R. Plymate; National Institutes of Health Award (K23DK65083) to D. Lin; and Veterans Affairs Research Service and National Institutes of Health awards (CA 085859) to S.R. Plymate.
References
- 1.Bassing CH, Alt FW. The cellular response to general and programmed DNA double strand breaks. DNA Repair (Amst) 2004;3:781–796. doi: 10.1016/j.dnarep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 3.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 4.Bhuller Y, Wells PG. A developmental role for ataxia-telangiectasia mutated in protecting the embryo from spontaneous and phenytoin-enhanced embryopathies in culture. Toxicol Sci. 2006;93:156–163. doi: 10.1093/toxsci/kfl045. [DOI] [PubMed] [Google Scholar]
- 5.Chen JH, Hales CN, Ozanne SE. DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res. 2007;35:7417–7428. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo LJ, Yang LX. Gamma-H2AX—a novel biomarker for DNA double-strand breaks. In Vivo. 2008;22:305–309. [PubMed] [Google Scholar]
- 8.Reliene R, Fischer E, Schiestl RH. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in atm-deficient mice. Cancer Res. 2004;64:5148–5153. doi: 10.1158/0008-5472.CAN-04-0442. [DOI] [PubMed] [Google Scholar]
- 9.Bhuller Y, Jeng W, Wells PG. Variable in vivo embryoprotective role for ataxia-telangiectasia-mutated against constitutive and phenytoin-enhanced oxidative stress in atm knockout mice. Toxicol Sci. 2006;93:146–155. doi: 10.1093/toxsci/kfl022. [DOI] [PubMed] [Google Scholar]
- 10.Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- 11.Bartek J, Lukas J, Bartkova J. DNA damage response as an anticancer barrier: damage threshold and the concept of “conditional haploinsufficiency”. Cell Cycle. 2007;6:2344–2347. doi: 10.4161/cc.6.19.4754. [DOI] [PubMed] [Google Scholar]
- 12.Bartkova J, Bakkenist CJ, Rajpert-De Meyts E, Skakkebaek NE, Sehested M, Lukas J, Kastan MB, Bartek J. ATM activation in normal human tissues and testicular cancer. Cell Cycle. 2005;4:838–845. doi: 10.4161/cc.4.6.1742. [DOI] [PubMed] [Google Scholar]
- 13.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 14.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr, Kastrinakis NG, Levy B, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 15.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess SJ, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan JE. The NKG2D receptor: immunobiology and clinical implications. Immunol Res. 2008;40:18–34. doi: 10.1007/s12026-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 17.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 18.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 20.Salih HR, Holdenrieder S, Steinle A. Soluble NKG2D ligands: prevalence, release, and functional impact. Front Biosci. 2008;13:3448–3456. doi: 10.2741/2939. [DOI] [PubMed] [Google Scholar]
- 21.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 22.Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001;61:6025–6028. [PubMed] [Google Scholar]
- 23.Lockett KL, Hall MC, Clark PE, Chuang SC, Robinson B, Lin HY, Su LJ, Hu JJ. DNA damage levels in prostate cancer cases and controls. Carcinogenesis. 2006;27:1187–1193. doi: 10.1093/carcin/bgi288. [DOI] [PubMed] [Google Scholar]
- 24.Smith DS, Humphrey PA, Catalona WJ. The early detection of prostate carcinoma with prostate specific antigen: the Washington University experience. Cancer. 1997;80:1852–1856. [PubMed] [Google Scholar]
- 25.Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167:2435–2439. [PubMed] [Google Scholar]
- 26.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 27.Welch HG, Fisher ES, Gottlieb DJ, Barry MJ. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99:1395–1400. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 28.De Marzo AM, Meeker AK, Zha S, Luo J, Nakayama M, Platz EA, Isaacs WB, Nelson WG. Human prostate cancer precursors and pathobiology. Urology. 2003;62:55–62. doi: 10.1016/j.urology.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 29.Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, Amory JK, Nelson PS, Wu JD. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290:E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- 30.Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, Nelson PS, Matsumoto AM, Bremner WJ. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 31.Toyokuni S, Tanaka T, Hattori Y, Nishiyama Y, Yoshida A, Uchida K, Hiai H, Ochi H, Osawa T. Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- 32.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 33.Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest. 2004;114:560–568. doi: 10.1172/JCI22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 35.Hall EJ, Schiff PB, Hanks GE, Brenner DJ, Russo J, Chen J, Sawant SG, Pandita TK. A preliminary report: frequency of A-T heterozygotes among prostate cancer patients with severe late responses to radiation therapy. Cancer J Sci Am. 1998;4:385–389. [PubMed] [Google Scholar]
- 36.Fan C, Quan R, Feng X, Gillis A, He L, Matsumoto ED, Salama S, Cutz JC, Kapoor A, Tang D. ATM activation is accompanied with earlier stages of prostate tumorigenesis. Biochim Biophys Acta. 2006;1763:1090–1097. doi: 10.1016/j.bbamcr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 37.Dong JT. Prevalent mutations in prostate cancer. J Cell Biochem. 2006;97:433–447. doi: 10.1002/jcb.20696. [DOI] [PubMed] [Google Scholar]