Abstract
In 1991, a group of 21 scientists gathered at the Wingspread Conference Center to discuss evidence of developmental alterations observed in wildlife populations after chemical exposures. There, the term “endocrine disruptor” was agreed upon to describe a class of chemicals including those that act as agonists and antagonists of the estrogen receptors (ERs), androgen receptor, thyroid hormone receptor, and others. This definition has since evolved, and the field has grown to encompass hundreds of chemicals. Despite significant advances in the study of endocrine disruptors, several controversies have sprung up and continue, including the debate over the existence of nonmonotonic dose response curves, the mechanisms of low-dose effects, and the importance of considering critical periods of exposure in experimental design. One chemical found ubiquitously in our environment, bisphenol-A (BPA), has received a tremendous amount of attention from research scientists, government panels, and the popular press. In this review, we have covered the above-mentioned controversies plus six additional issues that have divided scientists in the field of BPA research, namely: 1) mechanisms of BPA action; 2) levels of human exposure; 3) routes of human exposure; 4) pharmacokinetic models of BPA metabolism; 5) effects of BPA on exposed animals; and 6) links between BPA and cancer. Understanding these topics is essential for educating the public and medical professionals about potential risks associated with developmental exposure to BPA and other endocrine disruptors, the design of rigorously researched programs using both epidemiological and animal studies, and ultimately the development of a sound public health policy.
I. Introduction
- II. The Synthetic Estrogen (Xenoestrogen), Bisphenol-A
- A. Biochemical properties of BPA
- B. Estrogenic activities of BPA
- C. Other activities of BPA
III. The Controversy about Nonmonotonic Dose Response Curves
- IV. Physiological Conditions Support Low-Dose Effects
- A. Intrauterine positional effects in rodents
- B. Uterine environments in other animal models and humans
V. Critical Periods of Exposure during Development
- VI. Controversies Specific to BPA
- A. Controversy 1: What is the mechanism for low-dose BPA action?
- B. Controversy 2: Are humans exposed to truly significant levels of BPA?
- C. Controversy 3: Does human exposure occur exclusively through the oral route?
- D. Controversy 4: Is BPA inactivated by conjugation in the digestive system? Are animal studies using other modes of exposure relevant?
- E. Controversy 5: Are there any definitive patterns to the effects seen in BPA-exposed animals?
- F. Controversy 6: Could low doses of BPA affect cancer incidence?
VII. Expert Opinions and Government Decisions
VIII. Conclusions
I. Introduction
IMAGINE A WORLD WHERE both livestock and wild animals become weak, sicken, and then die; where insects do not roam, pollination cannot occur effectively, and so there are no fruits; where vegetation withers and browns along the roadsides; where silence falls across the land because there are no birds left to sing. This is the world that Rachel Carson asked readers to picture in her 1962 book, “Silent Spring” (1), which detailed countless examples of poisonings by pesticides, insecticides, and herbicides. This analysis was the first of its kind, and it brought attention to the danger inherent in the ubiquitous release of man-made chemicals into the environment. The observations made by Carson are still valid today. Over 80,000 chemicals are in use in the United States, and approximately 1000–2000 new chemicals are introduced into commerce each year, but the U.S. Environmental Protection Agency (EPA) does not routinely assess the safety and risks associated with all existing or new chemicals (2). Carson outlined several important points in “Silent Spring” that are especially relevant to the current situation, namely: 1) very low doses of chemicals can have profound effects on exposed animals; 2) mixtures of chemicals can lead to compounded effects; and 3) timing of exposures is critical.
After the publication of “Silent Spring”, researchers continued to make connections between chemical exposures and adverse outcomes in wildlife and humans. In the 1980s, Theo Colborn, then at the World Wildlife Fund, was researching the health of vertebrates living in the Great Lakes (3). Her analysis of the body of the literature revealed that adverse health outcomes had been measured repeatedly in birds and fish. Colborn created a spreadsheet to tally the effects she and others were observing across dozens of species. She concluded that animals were being affected in a variety of ways: diminished reproduction, thyroid problems, altered behavior, and metabolism changes including wasting. Each of these outcomes suggested that the endocrine system was perturbed. Perhaps the most important observation made by Colborn was that these problems were observed in the offspring of exposed animals, and not the adult animals themselves.
In July of 1991, Colborn summoned a group of 21 scientists to Racine, Wisconsin, at the Wingspread Conference Center (3). These scientists came from diverse backgrounds including ecology, endocrinology, medicine, law, reproductive physiology, toxicology, wildlife management, and cancer biology and presented their work relevant to the topic “Chemically-Induced Alterations in Sexual Development: The Wildlife/Human Connection.” Reports covered the effects of endocrine disruptors on gene imprinting, sexual differentiation, and reproductive function in mammals and fish, neurobehavioral development, and autoimmune diseases.
About this meeting, Colborn later said: “The reason these people were brought together was because we had seen such very blatant, open evidence among various wildlife species and populations concerning this problem of transgenerational exposure … by the third morning, these people were so moved by what they heard that they decided they wanted to produce what was called a consensus statement. They wanted the rest of the world to know what they had discovered that weekend” (4).
The following consensus statement was composed by the conference attendees: “We are certain of the following: a large number of man-made chemicals that have been released into the environment, as well as a few natural ones, have the potential to disrupt the endocrine system of animals, including humans” (5).
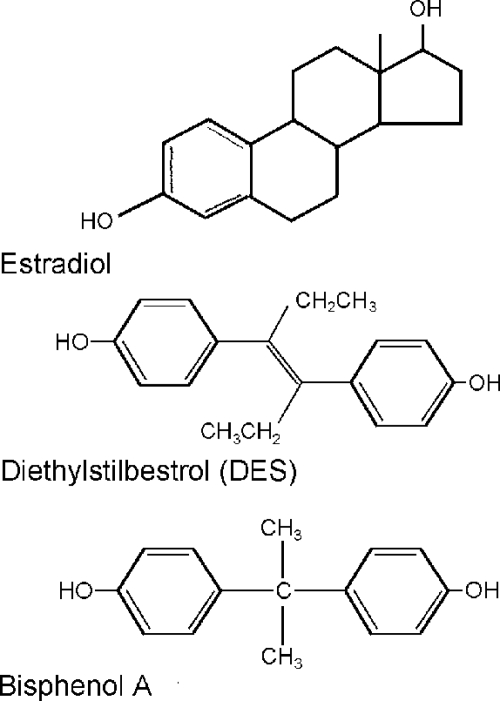
From this 1991 Wingspread meeting, the term “endocrine disruptor” became widely accepted in the scientific community. Additionally, the conference attendees noted similarities between exposure to endocrine disruptors and the potent estrogen diethylstilbestrol (DES), a pharmacological agent administered to pregnant women from 1948–71 (Fig. 1). DES produced striking effects in exposed offspring but much less serious effects in exposed mothers (6). Hence, there was particularly strong concern about exposure during critical periods of development.
Figure 1.
Chemical structures of BPA, DES, and estradiol. The structures of BPA and DES are more similar to one another than they are to the endogenous estradiol, indicating that chemicals with variable structures are capable of binding to the ER.
In 1995, the EPA sponsored a workshop to assess research needs for the risk assessment of the effects of endocrine disruptors on wildlife and human populations. At that time, an endocrine disruptor was defined as “an exogenous agent that interferes with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development and/or behavior” (7). In several other meetings held since 1995, scientists have examined evidence of the effects of endocrine disruptors on wildlife and humans. In particular, the National Toxicology Program’s report of the Endocrine Disruptors Low-Dose Peer Review in 2000 confirmed that there was sufficient evidence to support the claim that many of these chemicals had effects at low, environmentally relevant doses (8). Pointedly, “Low-dose effects … were demonstrated in laboratory animals exposed to certain endocrine active agents. The effects are dependent on the compound studied and the endpoint measured … The toxicological significance of many of these effects has not been determined.”
II. The Synthetic Estrogen (Xenoestrogen), Bisphenol-A
Bisphenol-A (BPA) is one of the highest volume chemicals produced worldwide, with over 6 billion pounds produced each year and over 100 tons released into the atmosphere by yearly production. BPA is the building block of polycarbonate plastic. Numerous studies found that BPA leaches from polycarbonate baby bottles (see Ref. 9) and reusable water bottles (10). Other polycarbonate containers intended to be used as reusable food containers, food-contact items such as polyvinyl chloride stretch films, and some paper and cardboard used as food containers have been examined for their BPA content (reviewed in Ref. 9). Metallic food cans are protected from rusting and corrosion by the application of epoxy resins as inner coatings. Many of these resins are synthesized by the condensation of BPA with epichlorhydrin to create BPA diglycidyl ether. When incomplete polymerization occurs, residual BPA leaches from the epoxy resin and has the potential to contaminate stored foods. Several studies have documented conditions that support or enhance BPA migration from the coating of cans (9). Others have also examined BPA levels leaching from epoxy resins lining cans to specific foods including canned pet foods, vegetables, and fish, whereas still others have found BPA contamination in canned infant formula (9).
Toxicology studies have determined that the maximum tolerated dose for BPA is 1000 mg/kg body weight (BW) · d (11). The EPA calculated a reference dose of 50 μg/kg · d using a safety factor of 1000. [Three safety factors of 10-fold were applied to account for the following: human risk estimated from animal studies; variability within the human population; and extrapolation for subchronic to chronic exposures (12).] A reference dose is typically calculated using the NOAEL (no-observed-adverse-effect level), but the reference dose for BPA was calculated using the LOAEL (lowest-observable-adverse-effect level) because a NOAEL had not been determined and adverse responses were detected even at the lowest dose administered (12).
A. Biochemical properties of BPA
BPA contains two phenol functional groups (Fig. 1), and it is prepared by the combination of two equivalents of phenol with one equivalent of acetone. BPA was first synthesized by A. P. Dianin in 1891 and was later investigated in the 1930s during the search for synthetic estrogens. It was tested for its estrogenic properties at that time but abandoned for pharmaceutical use when DES was determined to be much more potent (13).
BPA, with its two benzene rings and two (4, 4′)-OH substituents, fits in the ER binding pocket. Biochemical assays have examined the kinetics of BPA binding to ER and have determined that BPA binds both ERα and ERβ, with approximately 10-fold higher affinity to ERβ (14,15).
A study of BPA and 19 related compounds determined the minimum structural requirements for estrogenic activity: a 4-OH group on the A-phenyl ring and a hydrophobic moiety at the 2-position of the propane (16). However, the affinity of BPA for the ERs is approximately 10,000-fold weaker than that of estradiol (15). Extensive biochemical studies of ERα have identified two distinct gene transactivating regions, termed AF1 (found in the amino terminus) and AF2 (located in the carboxyl terminus) (14). ER agonists are subdivided based on their ability to activate these regions. Binding of xenoestrogens to the ERs alters their ability to recruit coactivators that may be important for differences in tissue- dependent responses. For instance, biochemical analyses indicate that BPA is able to induce greater changes in downstream gene expression in cells containing ERβ where TIF2 is the main coactivator, but may be equally effective in cells expressing either ERα or ERβ if the steroid receptor coactivator-1a is present (17). Based on these biochemical studies, it has been proposed that differences in the ability of ERα or ERβ to recruit coactivators when BPA is bound may contribute to the complex tissue-specific responses to BPA exposure (18).
B. Estrogenic activities of BPA
Several in vitro assays are available for measuring the estrogenic activity of possible endocrine disruptors including BPA (reviewed in Ref. 19). The E-SCREEN assay uses the estrogen sensitive MCF7 breast epithelial cell line to measure cell proliferation after treatment with a range of concentrations of the chemical to be tested, as well as estradiol for a positive control (20). The E-SCREEN is the most sensitive assay for estrogenicity; it can discriminate between partial and full agonists, can accurately identify antagonists, and has verified the estrogenic properties of BPA. Until recently, BPA was considered a weak environmental estrogen because of its relatively low affinity for the nuclear ERs compared with estradiol [EC50 = 2–7 × 10−7 m compared with 1–6 × 10−13 m for estradiol (21,22)]. However, results from recent studies have revealed a variety of pathways through which BPA can stimulate cellular responses at very low concentrations, below the levels where BPA is expected to bind to the classical nuclear ERs (reviewed in Ref. 23).
Several membrane steroid receptors have been described, including a membrane-bound form of ERα (mER) that is similar but not identical to the nuclear ERα (24,25) and a transmembrane ER called G protein-coupled receptor 30 (GPR30) (the first membrane ER identified that is structurally dissimilar to the nuclear ERs) (26). BPA has been shown to bind to both mER and GPR30, and studies have determined that these membrane-bound receptors are capable of nongenomic steroid actions (25,26,27) (also reviewed in Ref. 18). GH3/B6 pituitary cells, which naturally express mER, respond to low level BPA exposure (in the picomolar to nanomolar range) by producing a calcium flux which leads to prolactin release (25). However, examination of other nongenomic signaling pathways in these same cells (i.e., ERK activation) revealed no effect of BPA exposure. This suggests that BPA, like other xenoestrogens, differentially utilizes signaling pathways downstream of mER activation. Pancreatic α-cells treated with BPA also demonstrate nongenomic signals occurring via mER, suggesting that the affected nongenomic signaling pathways are not specific to a single cell type (28,29). A third example indicates that BPA can signal through a nongenomic pathway in cultured mouse endothelial cells to increase nitric oxide production, although it was not specifically demonstrated that these effects were mediated via mER (30). In fact, most studies of nongenomic actions of BPA and other xenoestrogens do not specify whether they are due to actions via ER in the plasma membrane, the cytosol, or elsewhere (31). However, based on the results of these studies, it is now widely accepted that BPA not only has the efficacy of estradiol but is also equally potent regarding several of its effects (12,18,27).
In vivo assays have also been used to determine the estrogenicity of BPA. When prepubescent CD-1 mice were treated with doses of BPA ranging from 0.1 to 100 mg/kg BW, estrogenic responses including increased uterine wet weight, luminal epithelial height, and increased expression of the estrogen-inducible protein lactoferrin were observed (32). Additionally, single, high doses of BPA (up to 150 mg/kg BW) induced proliferation of the uterine and vaginal epithelial cells of ovariectomized rats (33). Other organs including the mammary and pituitary glands displayed estrogenic responses to BPA exposure as well, but at lower doses than those needed to generate a significant response in the uterotrophic assay (34). Thus, the characterization of BPA as a weak estrogen is likely to underestimate the impact of BPA exposure on different target organs.
C. Other activities of BPA
In addition to its estrogenic activity, there is some evidence that BPA binds to thyroid hormone receptor, acting as a thyroid hormone antagonist by preventing the binding of T3. One study found the affinity of BPA for this receptor severalfold lower than its affinity for the ERs (35). However, other studies have been unable to duplicate these results, finding that BPA does not competitively inhibit the binding of labeled T3 to the thyroid hormone receptor or induce thyroid hormone-dependent production of GH in GH3 cells (16,36). Halogenated BPA (tetrachlorobisphenol A and tetrabromobisphenol A) used as flame retardants were also shown to inhibit the binding of T3 to the thyroid hormone receptor (36). In vivo studies examining the effects of BPA on thyroid hormone signaling have been conducted in rats exposed to 1, 10, or 50 mg/kg BW starting on embryonic d 6 (37). Perinatally exposed rats had elevated T4 levels on postnatal day (PND) 15 and up-regulation of a thyroid hormone-responsive gene in the brain. It was also observed in medaka fish that the acceleration in embryonic development and time to hatch induced by BPA were blocked by a thyroid hormone receptor antagonist, suggesting that BPA is acting through a thyroid hormone pathway (38).
The antiandrogenic properties of BPA are still somewhat in dispute. Using a competitive binding assay with labeled dihydroxytestosterone and a yeast reporter assay, antiandrogenic activity of BPA was detected in the 10−5 to 10−7 m range (39). However, other studies have shown a half- maximal response of approximately 50 nm BPA, suggesting a dose response curve shifted to the left (40). There have also been mixed results with mammalian cell reporter assays, i.e., some groups measured antiandrogenic activity of BPA with a half-maximal response at 2.14–3.2 μm (41,42), whereas others were unable to demonstrate any antagonist activity (43). Addressing these discrepancies in an animal model may be difficult because distinguishing estrogenic effects and antiandrogenic effects in vivo is not easy (39). For instance, evidence that BPA inhibits testicular steroidogenesis at low exposure levels has been suggested to occur via the ER and is likely due to its estrogenicity (44).
Additional studies indicate that BPA also binds to an orphan nuclear receptor called estrogen-related receptor-γ (ERR-γ) (45). Although the endogenous ligand for ERR-γ remains unknown, the human receptor behaves as a constitutive activator of transcription and may play a role in differentiation and maturation of the fetal brain. When BPA is bound to ERR-γ, it preserves this receptor’s basal activity and can prevent its deactivation by antiestrogens (46).
BPA also binds the aryl hydrocarbon receptor (AhR) (42), a ligand-dependent transcription factor present in almost every tissue. AhR is thought to be activated by many chemicals with diverse structures and may mediate the toxic and/or biological effects of these chemicals. Although AhR has been implicated in several signal transduction pathways, the effects of BPA binding to AhR remain unknown at this time. Because AhR can cross-talk with other receptors including ERs and the androgen receptor, endocrine-related endpoints may be affected by its activation (reviewed in Ref. 47).
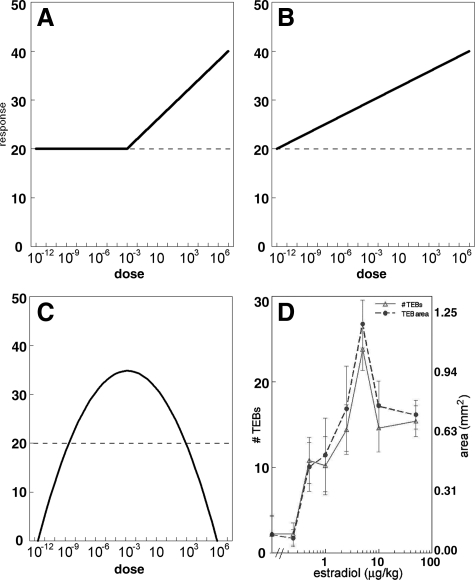
III. The Controversy about Nonmonotonic Dose Response Curves
For many years, when assessing the effects of possible endocrine disruptors, toxicologists have relied on the principle that “the dose makes the poison,” implying that higher doses were expected to cause greater harm. Thus, effects that are not seen at high doses are not expected at low doses. This threshold model is used most often for risk assessment of noncarcinogens (Fig. 2A) (12,48). This model identifies a “safe” dose by assessing different doses of a chemical until the NOEL (no observable effect level) is determined. Additionally, toxicologists depend on the linear nonthreshold model (Fig. 2B) to assess the danger of different doses of carcinogens and extrapolate these findings to very low doses of the chemical. In contrast to the above-mentioned dogma in toxicology, multiple studies have found that neither the threshold nor the linear nonthreshold models are applicable to the responses of hormones in which biphasic dose responses have been observed for many different endpoints at many levels of organization (reviewed in Refs. 12,48 and 49). These U-shaped and inverted U-shaped dose response curves are considered “nonmonotonic” (Fig. 2C) and are used as evidence that very low doses of natural and synthetic hormones can affect endpoints such as cell proliferation and organ size (12).
Figure 2.
Hypothetical curves to illustrate the threshold (A), linear nonthreshold (B), and nonmonotonic (C) model dose response curves. In the threshold model, treatment with increasing doses of a drug has no effect until the “threshold” dose is reached, at which point an increase in response is observed. In the linear nonthreshold model, a response occurs even at the lowest treatment dose, and therefore effects at high doses can be used to predict responses at low doses. With a NMDR curve, an increase in dose does not necessarily correspond to an increase in response, such that, in this example, doses from 10−12-10−3 m result in an increase in response, and doses from 10−3-107 m result in a decrease in response. These curves are common for endocrine endpoints. D, Examples of NMDR curves observed in mammary gland morphological parameters after administration of estradiol to ovariectomized females. The left y-axis is the number of terminal end buds (TEBs), and the right y-axis is total area of all TEBs; the TEB is an estrogen-dependent structure. [Panel D is reproduced from L. N. Vandenberg, et al.: J. Steroid Biochem Mol Biol 101:263–274 (49). Copyright 2006, with permission from Elsevier.]
In some nonmonotonic dose responses (NMDRs), the dose response curve may be shaped like a U with high responses at low and at high levels of exposure, whereas others are shaped like an inverted U, with the greatest responses at intermediate doses (50). Calabrese and Baldwin (51) described the importance of experimental design to detect the presence of a NMDR curve. In particular, studies of dose response curves must use a wide range of doses, including doses below the established LOAEL. A meta-analysis of 20,285 toxicology studies conducted between 1962 and 1998 found that only 1% of the published studies met the criteria set a priori to determine whether a study was designed properly to detect a NMDR curve (51). Almost 40% of these studies satisfied the requirements for a NMDR, supporting the idea that the occurrence of NMDRs is nonrandom and may even be more common than monotonic dose response curves.
A curious criticism of NMDR curves has been the lack of a definitive mechanism to explain these nonmonotonic responses. Several studies have suggested that the shape of these curves can be explained by the down-regulation of receptors at higher hormone levels (52,53). There is also evidence that NMDR curves are generated by the integration of two or more monotonic dose response curves that occur through different pathways affecting a common end point with opposing effects (54). For instance, in vitro studies have shown that low doses of androgens can mediate a proliferative response in androgen-target cells, whereas at a higher dose they inhibit cell proliferation (55). When the end point is cell number, the resulting curve has the shape of an inverted U. The two arms of this curve are induced independently of each other; they can be segregated, generating two differently behaving cell types, i.e., one that shows a monotonic proliferative response (the cell number increases as the androgen dose increases), and another that shows a monotonic inhibitory response (the cell number decreases as the hormone concentration increases) (54). The biochemical events underlying these effects are distinct (56). Additional studies have indicated that NMDRs occur at various levels of organization (Fig. 2D and Ref. 49).
NMDR curves have been observed after exposure of cultured cells to BPA. For instance, the response of GH3/B6 pituitary cells to BPA followed a U-shaped NMDR curve, where doses of 10−12, 10−11, and 10−8 m elicited significant responses and doses of 10−10 and 10−9 m did not (57). LNCaP prostate cancer cells responded to BPA in a similar manner with maximal proliferation induced by 10−9 m (58). Fewer cells proliferated at lower (10−10 m) and higher (10−8 and 10−7 m) doses of BPA. Additionally, BPA inhibited adiponectin secretion from human adipose explants with a U-shaped NMDR curve; concentrations of 10−10 and 10−9 m inhibited release, whereas doses of 10−8 and 10−7 m were indistinguishable from unexposed controls (59). Finally, pancreatic islet cells exposed to BPA release insulin, displaying an inverted U-shaped NMDR curve where only doses of 10−9 and 10−10 m significantly increase insulin release (29).
Typically, only a few doses are tested in animal studies, so it is more difficult to determine whether the observed responses are truly nonmonotonic. However, many studies conducted with a low and a high dose of BPA show effects at the low dose that are not apparent after exposure to the high dose (60). For instance, one of the first in vivo studies of BPA demonstrated that female offspring born to pregnant dams exposed to 0.1 mg BPA/kg BW · d from d 5 gestation through the period of lactation were significantly heavier than controls when weighed as adults, but the body weight of females exposed to a higher dose (1.2 mg BPA/kg BW · d) were not different from controls (61). Low-dose perinatal BPA exposure (2 μg BPA/kg BW · d) increased anogenital distance in female offspring, but a higher dose (20 μg BPA/kg BW · d) had no effect (62). The low dose examined in this study also affected the number of days when cornified cells appeared in vaginal smears, whereas the high dose again had no effect. NMDRs are not observed in all endpoints examined after BPA exposure, but they have been observed in behaviors, protein expression, development of embryos, and organ size (60).
It has been suggested that the linear nonthreshold model and the threshold model routinely used for risk assessment purposes by government agencies, including the EPA, should be rejected and replaced entirely (12,48). Additionally, typical high-dose toxicology studies used for risk assessment purposes are designed to detect gross changes in morphology or development, with endpoints including mortality, body weight, and tumor incidence (48). Unfortunately, these studies are not designed to detect more subtle developmental effects that impact the health of the individual such as the presence and hormonal responsiveness of prostate lesions, tissue organization of the mammary gland, expression of sexually dimorphic behaviors, etc. Many of these studies examine only animals exposed during adulthood and thus lack information about offspring of animals treated during pregnancy. Making predictions about the safety of low doses by testing higher doses is not appropriate when very low doses of endocrine disruptors can alter biochemical and morphological endpoints in a manner that is not necessarily predicted by exposures at much higher doses (12).
IV. Physiological Conditions Support Low-Dose Effects
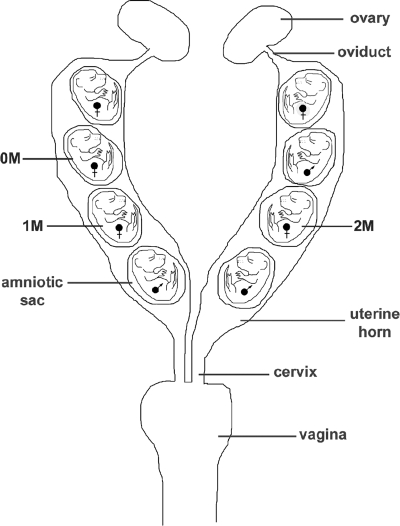
Evidence for the ability of low doses of hormone to induce and/or modify phenotypes comes from studies of the unique hormonal microenvironment of the rodent uterus. Each rodent fetus is fixed in position with respect to its neighbors in the bicornate uterus (Fig. 3); thus, delivery by cesarean section allows for the determination of the position of each fetus in the uterus. Researchers noted that at birth, statistically significant variation was found in anogenital distance in these otherwise identical mice (63). Differences in after-birth phenotype could be due to their intrauterine position and the resulting differential hormone exposure.
Figure 3.
Schematic diagram of the mouse reproductive tract showing the three possible intrauterine positions of female fetuses. Females located between two males are considered 2M, between a male and a female are considered 1M, and between only females are considered 0M. [Modified from F. S. vom Saal, et al.: J Reprod Fertil 62:33–37 (69). Copyright 1981, Society for Reproduction. Reproduced by permission.] Multiple studies have detected statistically significant differences in physiological, morphological, and behavioral parameters in mice dependent on position in the uterus during gestation.
A. Intrauterine positional effects in rodents
Male mice begin to produce testosterone at approximately 12–13 d gestation, and some of this testosterone is passively transferred to neighboring fetuses (reviewed in Ref. 64). A wide variety of physiological, morphological, and behavioral endpoints are affected by intrauterine position. These include several endocrine-related endpoints such as prostate development, mammary gland development, responsiveness to testosterone, steroid metabolism, age of pubertal onset, and estrous cycle length, among others (see Refs. 64 and 65 for review). Together, these data indicate that very small amounts of hormones during fetal development alter phenotypic endpoints that become apparent in adulthood. It has also been postulated that intrauterine position and endocrine disruption act via similar mechanisms, suggesting that intrauterine position may influence an individual’s sensitivity to an exogenous hormone (65).
The mechanism responsible for differences in development based on intrauterine position is still unknown. Work by vom Saal and Bronson (66) implicates testosterone in establishing position-specific phenotypes; females positioned in the uterus next to only males (2M females) have higher levels of testosterone in their serum and amniotic fluid compared with females positioned next to only females (0M females). The 2M females are more masculinized than their 0M counterparts, evidenced by longer anogenital distances at birth, a morphological measurement that is a sensitive signal of prenatal testosterone exposure (64).
Intrauterine position would explain the occurrence of offspring with a comparable genotype but variable phenotypes. This range of phenotypes would allow for a greater chance of survival under different environmental conditions. For instance, 0M females would be better suited to reproduce when resources are plentiful (67,68). However, under stressful conditions, the aggressive 2M females would be more successful breeders (69). There is evidence that a variable intrauterine environment is essential for normal development, yet interestingly, it has been suggested that intrauterine positional effects are inconsequential in inbred strains (70,71). However, mice and gerbils that do not have littermates reproduce poorly and have abnormal sexual maturity (reviewed in Ref. 69).
It has also been postulated that intrauterine position and endocrine disruption act via similar mechanisms, suggesting that intrauterine position may influence an individual’s sensitivity to an exogenous hormone and may alter its effects (65). Only a few studies have examined both BPA and intrauterine position, although other studies have taken intrauterine position into account to reduce variability of responses to BPA (72). One study indicates that intrauterine position influences the sensitivity of mice to BPA and that 0M females exposed to BPA were heavier than controls at weaning, whereas 2M females were unaffected (73). BPA also significantly reduced the number of days between vaginal opening and first estrus, but again only in 0M females. In another study, intrauterine position was shown to affect morphological aspects of the fetal mammary gland, but exposure to BPA in utero abolished these differences (74). Collectively, these results are indicative of the potential for low doses of both natural and exogenous hormones to alter organ morphology and reproductive development.
B. Uterine environments in other animal models and humans
Intrauterine effects may occur in other species, including wild house mice, rats, ferrets, swine, hamsters, voles, and sheep (reviewed in Ref. 65). An interesting and probably related phenomenon is the freemartin in cattle, sheep, goats, and other farm animals; freemartins arise during twin pregnancies when vascular connections link the placentas of opposite-sex twins (75). These shared blood vessels lead to XX/XY chimerism and subsequent masculinization of the reproductive tract in the female twin. Thus, a female cow born with a male twin is often sterile; the male twin is also often sterile or has a decreased fertility (reviewed in Refs. 65 and 75). Although an exact mechanism for explaining freemartins is unavailable, the phenotypes of affected female twins (i.e., shortened vaginal tracts, failure to show estrous behavior, infertility, low estradiol production, a diminished or absent surge of LH after estrogen challenge, etc.) suggest an endocrine basis for this phenomenon (75,76). Attempts to generate freemartin-like phenotypes by treating pregnant dams with testosterone have produced female pups with masculinized reproductive tracts in dogs and female sheep that fail to have regular estrous cycles or become pregnant (75). However, similar treatment of pregnant cows with testosterone did not affect the reproductive performance of exposed female offspring (77). Thus, farm animals may provide interesting insights into the complexities of a shared intrauterine environment, although the exact mechanisms for the observed phenotypes are still not completely understood.
A question remains: how relevant are findings in rodents and farm animals to human fetal development? A few studies indicate that the intrauterine environment may also have effects on human phenotypes, especially as multichild births increase due to fertility treatments. Human female fetuses have higher estradiol concentrations in the amniotic fluid than male fetuses (78). These fetal estrogens are derived from androgens produced by the fetal adrenals; the enzyme aromatase converts these androgens to estrogen in the placenta. Interestingly, however, estradiol levels are higher in umbilical cord blood of male fetuses compared with females (78). Several studies have found differences in same-sex and opposite-sex twins in parameters such as dental asymmetries, levels of sensation seeking, and production of otoacoustic emissions (reviewed in Ref. 65). These studies suggest that variations in the uterine environment can affect human development. Somewhat similar to the findings in freemartins, a recent study suggests that human females with a male co-twin have significantly reduced reproductive success compared with females with a female co-twin (79). Finally, epidemiological studies comparing human dizygotic twins and single births revealed that the propensity to breast cancer is enhanced in female twins, and this outcome was attributed to excess estrogen exposure in dizygotic twins during gestation (80).
In summary, the complex intrauterine environment of rodents and farm animals illustrates that even small variation in the fetal environment can have far-ranging consequences for later development and may have implications for multichild pregnancies in humans. Although the mechanisms for these intrauterine environmental effects are not completely understood, hormones play a prominent role in them (61,62,76) and suggest that minute differences in hormone exposure during fetal life can affect behaviors, organ morphology, and physiology (64,65).
V. Critical Periods of Exposure during Development
Hormones can have very different effects on development based on the period in which they are administered. For instance, whereas women exposed to DES during pregnancy were relatively unharmed, their daughters who were exposed in utero (so-called DES daughters) had significantly increased rates of uterine, cervical, and vaginal malformations including clear cell adenocarcinoma of the vagina (81). Breast cancer incidence in DES daughters older than 40 yr of age is significantly increased when compared with unexposed women of the same age (82).
Although exposure of rodents to BPA in adulthood requires relatively large doses to induce a uterotrophic response (in the milligrams per kilogram of body weight range), in utero exposure to much lower doses (in the nanograms per kilogram of body weight range) can induce alterations in estrogen-target organs of the fetuses that are observed later in life (32,83). After puberty, hormones can induce transient effects on target organs, whereas hormone withdrawal then results in the cessation of the hormone- induced effects. These have been termed “activational” effects (84). Alternatively, exposure to hormones during perinatal development tends to have permanent “organizational” effects on the developing individual.
As stated by vom Saal and Moyer: “In fact, it does seem that the period of greatest sensitivity to the organizing effect of steroids is the perinatal period, and the farther in time that one gets from this period, the less sensitive are tissues to the organizing effects of steroids” (84).
Thus, the timing of exposures is critical to the study of endocrine disruptors. Although adult exposures to BPA and other endocrine disruptors have been shown to affect a wide range of endpoints (discussed in detail in Ref. 60), these studies are not reflective of the damage a chemical can do during the period of organogenesis (85).
It has been proposed that different receptors are likely represented in different cell types at different developmental times and response stages (25,27). Coregulators and signaling partners may also be different for each cell type (86). Thus, it is not unexpected that xenoestrogens such as BPA could have diverse effects not only throughout the body of the organism, but also at different stages of life.
The “critical window” of exposure differs depending upon the time at which specific tissues or organs develop. For example, clear cell adenocarcinomas of the vagina and other vaginal and cervical pathologies were observed in DES daughters with higher prevalence among those exposed during the first 15 wk gestation (87). Women exposed during the first 7 wk gestation have a higher risk compared with those exposed later on (88). In the case of the mammary gland, this organ develops both prenatally and postnatally (89), and its critical period of development is different for diverse hormones and environmental toxicants. For instance, the critical period of the mouse mammary gland in response to the herbicide atrazine was narrowed down to gestational d 17–19 (90), although animals exposed during both gestation and lactation had more severe phenotypes than those exposed during gestation alone (91). In studies of animals exposed neonatally to 17β-estradiol, exposures that began after PND 1 led to phenotypes that were much less severe than exposures that commenced on PND 1 (92). It has therefore been proposed that the first day after birth is a “critical window” for the mouse mammary gland’s sensitivity to estrogen exposure.
Although regrettable, accidental exposures to chemicals have illustrated how an organ can have multiple periods of sensitivity. For instance, the 1976 explosion at a chemical plant near Seveso, Italy, led to the deposition of up to 30 kg of dioxin on the surrounding land (reviewed in Ref. 93). Blood samples from exposed children, adolescents, and women collected shortly after the exposure and in follow-up studies correlated serum dioxin levels with adverse outcomes including increased breast cancer risk and endometriosis (93,94). Interestingly, neonates and teenagers were most at risk of exposure, confirming findings that suggest that mammary tissue is especially sensitive to perturbations during the perinatal and pubertal periods and during pregnancy (95,96).
VI. Controversies Specific to BPA
In this section, we will address six major issues that have been raised by BPA experts, regulatory agencies, and the press (97,98). It is not our intent to solve each of the controversies, but merely to highlight them and offer alternative interpretations to those suggested by industry and some government agencies (98,99,100).
A. Controversy 1: What is the mechanism for low-dose BPA action?
Endogenous estrogens have effects on many levels of biological organization, from the whole individual to organ systems, cells, and gene expression. From an organismal and organ system perspective, estrogens are involved in several aspects of female sexual development including the development and maintenance of the reproductive tract and secondary sexual characteristics, and the regulation of reproductive cyclicity, pregnancy, and lactation. At the cellular level of complexity, estrogens mediate cell proliferation in cells containing ERs. Three alternative hypotheses have been proposed to explain the link between estrogens and cell proliferation. They are: 1) estrogens directly induce cell proliferation; 2) estrogens mediate proliferation by regulating growth factors; or 3) estrogens induce cell proliferation by canceling the actions of a proliferation inhibitor (101). Only the third is consistent with evolutionary theory, given that proliferation is the default state of cells. Thus, the proliferative activity of epithelial cells is prevented by an inhibitory protein in plasma (102), the three-dimensional context of tissues, or both (103). Therefore, whereas estrogens are said to “stimulate” proliferation of cells or target tissues, “stimulation” represents an operational term based on the ultimate effect observed, and not on an actual mechanism.
One proposed mechanism to explain the complex effects of low-dose BPA exposure in rodents is the impact of estrogen exposure on sensitive organs during a time when endogenous estrogens are low or nonexistent. In the rodent, the fetal ovary is thought to be quiescent until several days after birth, and circulating estrogens from either the fetus or the dam are expected to bind to α-fetoprotein, a glycoprotein made by the fetal liver that binds estrogens present in the serum and amniotic fluid of the developing fetus (104,105). BPA does not bind to α-fetoprotein, thus exposure of fetuses and neonates to even low doses could alter organogenesis and histogenesis (106).
Because of its relatively low affinity for the nuclear ERs compared with estradiol, BPA has often been referred to as a weak environmental estrogen. However, functional assays measuring the induction of specific indigenous genes, reporter gene assays, and the increase of cell number in the E-SCREEN assay are carried out in conditions that may enhance the potency of hormones because they are not metabolized rapidly in vitro. This would affect the determination of relative potencies when comparing hormones that are rapidly inactivated in the living animal with those that are not. Thus, these in vitro measurements do not represent a measure of a hormone’s true potency in a complex biological system such as the fetal and adult organism.
Moreover, recent studies have revealed a variety of pathways through which BPA can “stimulate” cellular responses at very low concentrations, below the levels where BPA is expected to bind to the classical nuclear or genomic ERs (reviewed in Ref. 23). Thus, low levels of BPA appear to act via mER, GPR30, ERs positioned in nonclassical locations such as the cytosol and mitochondria, as well as other receptors (reviewed in Ref. 25). Because these receptors are likely to be present in different cell types at various developmental times and response stages, low-dose BPA exposure could have profoundly diverse effects on the same organ at different life stages (13,19,23).
Another consideration when examining the potency of BPA or any other endocrine disruptor is the presence of other estrogens in the system being studied. Humans are thought to be exposed to dozens, if not hundreds of chemicals having hormonal activity. Even in controlled animal experiments, exposures to detergents, feed, and plastics with estrogenic activities can occur. In a recent study examining mixtures of 11 xenoestrogens, including BPA, it was found that the presence of these chemicals at levels below their no-observed-effect concentrations significantly increased the effects of estradiol (107). Thus, the low-dose effects of BPA may also be due to its additivity with other (endogenous and exogenous) estrogens present, either in the organism or in cell culture conditions.
Although not specific to low-dose exposures, insights into the possible mechanisms of BPA action may be extrapolated from studies with DES. The effects of DES exposure on target organs are believed to be mediated by regulation of the expression of estrogen-target genes involved in tissue patterning, histodifferentiation, and cytodifferentiation. For example, neonatal exposure to DES exerts an estrogenic effect through repression of the Wnt7a signaling pathway in the female reproductive tract (108). Prenatal exposure to DES also altered the expression of several Hox genes in the mouse Müllerian duct and uterus (109). Thus, we and others have proposed that exposure to BPA and other xenoestrogens cause changes in the expression of estrogen-sensitive genes (110,111,112).
Alterations in a developing fetus’ environment result in developmental changes in metabolism, physiology, and organ structure that manifest in adulthood as an increased susceptibility to diseases (113,114,115). Studies conducted in our labs have specifically focused on the effects of perinatal BPA exposure on the tissue organization of the mammary gland, and they indicate that BPA exposure alters stromal-epithelial interactions in the fetal mammary gland during the period of exposure (74). BPA-induced alterations of the mammary stroma are revealed in the epithelium at puberty and adulthood as changes in cell proliferation and apoptotic patterns, abnormal development of epithelial structures at puberty, reduced stromal penetration, increased lateral branching, and advanced alveolar bud development (reviewed extensively in Refs. 110 and 116).
B. Controversy 2: Are humans exposed to truly significant levels of BPA?
Since 1999, more than a dozen studies using a variety of different analytical techniques have measured free, unconjugated BPA concentrations in human serum at levels ranging from 0.2–20 ng/ml serum (see Ref. 9). The relatively high levels of BPA in the serum of pregnant women, umbilical cord blood, and fetal plasma (9) indicate that BPA crosses the maternal-fetal placental barrier. BPA has also been measured in human urine from several populations around the world. These studies confirm widespread human exposure to BPA, as suspected from the studies of BPA in blood. A 2005 study conducted by the U.S. Centers for Disease Control and Prevention (CDC) detected BPA in 95% of urine samples from a reference population of 394 American adults using isotope dilution gas chromatography/mass spectrometry with average levels of total BPA in male and female urine of 1.63 and 1.12 ng/ml (μg/liter), respectively (117). A more recent CDC study of over 2500 Americans extends this finding, with BPA detected in 92.6% of participants (118). Measured urine concentrations ranged from 0.4–149 μg/liter with a geometric mean of 2.6 μg/liter and were significantly higher in children and adolescents compared with adults. Importantly, in some cases, the concentrations of total BPA (unconjugated and conjugated) in human blood and other tissues and fluids were higher than those that stimulated a number of molecular endpoints in cells cultured in vitro and appeared to be within the range of the levels of BPA in animal studies (10,19,56).
There has been much discussion regarding the methods used in biomonitoring studies (9,119); specifically, the use of ELISA to measure BPA concentrations in blood, urine, or other bodily tissues has been challenged because of its relative insensitivity and possibilities of cross-reactivity (120). It has also been suggested that the detection of low levels of unconjugated BPA in bodily tissues and fluids was due to contamination from collection materials and/or deconjugation of BPA metabolites during storage (100,119,121). However, the repeated finding of BPA in a variety of fluids using several methods, all with a similar range of detected levels, suggests otherwise (9).
At this time, only one large and well-controlled study of the possible health effects of BPA exposure on humans has been conducted, revealing positive correlations between urinary BPA concentrations and the prevalence of diabetes, heart disease, and liver toxicity (122). This cross-sectional study was performed using samples and information collected for the CDC National Health and Nutrition Examination Survey (NHANES) study and includes 1455 American adults. However, additional studies are needed to determine whether the associations between BPA concentrations in urine and disease prevalence are causal.
Several smaller studies have examined the effects of BPA exposure on other health outcomes. For instance, BPA levels in blood have been associated with a variety of conditions in women including obesity, endometrial hyperplasia, recurrent miscarriages, sterility, and polycystic ovarian syndrome (91,92,93,94,95). High BPA exposure was associated with chromosomal abnormalities, including higher maternal serum BPA among women carrying fetuses with an abnormal karyotype (123), and correlations were suggested between high urinary BPA concentrations and sister chromatid exchange measured in peripheral lymphocytes (124). These epidemiology studies have several limitations, including small sample sizes, limited details on subject selection criteria, and cross-sectional designs that include limited control for potential confounders. These limitations in design prevent accurate assessments regarding the potential health risks of BPA (9).
C. Controversy 3: Does human exposure occur exclusively through the oral route?
Few studies have estimated total BPA exposure. Using data from environmental (water, air, soil) and food (can inner surfaces, plastic containers) contamination, Kang et al. (125) estimated the daily human intake of BPA at less than 1 μg/kg BW · d. Alternatively, the European Commission’s Scientific Committee on Food estimated BPA exposure to be 0.48–1.6 μg/kg BW · d from food sources alone (126). Two additional studies were conducted to estimate BPA exposure levels in young children. The first examined their potential exposures at home and in daycare (127). BPA was detected in indoor and outdoor air samples, floor dust, and play area soil, and in liquid and solid foods in both locations at similar levels. Based on these environmental levels, the average BPA exposure level for young children was estimated at 42.98 ng/kg BW · d. A second observational study examining BPA exposures in 257 preschool children verified that BPA could be found in more than 50% of indoor air, hand wipe, solid food, and liquid food samples and suggested that 99% of exposures of preschool children originated in the diet; the estimated exposure from dietary sources was 52–74 ng/kg BW · d, and the estimated inhalation exposure was 0.24–0.41 ng/kg BW · d (128).
Additional studies have shown that BPA can be found in dust samples, indoor and outdoor air, sewage leachates, and water samples from around the world (reviewed in Ref. 9). Thus, humans are potentially exposed to low doses of BPA through routes other than the verified oral exposures.
D. Controversy 4: Is BPA inactivated by conjugation in the digestive system? Are animal studies using other modes of exposure relevant?
The liver plays an essential role in BPA metabolism in both animals and humans. Through glucuronidation the liver metabolizes and facilitates excretion of both endogenous and exogenous compounds. Liver enzymes responsible for glucuronidation of BPA and other xenoestrogens (UDP-glucuronosyltransferases, e.g., UGT2B1) produce BPA glucuronide, the major BPA metabolite in animals and humans that has little or no estrogenic activity (129). BPA is also conjugated in vivo to BPA sulfate by phenol sulfotransferases found in the liver (e.g., ST1A3) (130,131); sulfation of BPA abolishes its estrogenic activity (132). Detailed, systematic studies have not yet determined the proportion of BPA that is metabolized to BPA glucuronide and BPA sulfate. A small study suggests there may be gender differences in the concentrations of BPA metabolites in urine, with women having higher levels of BPA sulfate and men having higher levels of BPA glucuronide, but studies with larger sample sizes should verify this finding (133).
It has been assumed that oral intake leads to complete inactivation of BPA. However, pharmacokinetic studies indicate that not all BPA is conjugated by the liver (134). In rodents, conjugated BPA is deconjugated by enzymes in the lower intestine and colon (135). Studies also indicate that humans produce glucuronidases in their digestive tracts, with increasing production throughout infancy until adult levels are reached at 4 yr of age (136); thus, conjugated BPA may be deconjugated and activated by infants during the digestive process. Neonatal rodents also have limited ability to conjugate BPA to an inactive form, regardless of the mode of administration (sc vs. oral) (137). This may be true for human fetuses and neonates as well.
Finally, there is a possibility that conjugates may be deconjugated locally in other body tissues that release biologically active BPA. For instance, treatment of human breast cells with BPA sulfate and disulfate leads to desulfation via estrone sulfatases and uptake of unconjugated BPA (138). Because these arylsulfatases are ligand- and organ-specific, deconjugation of BPA is likely to be different throughout the body. Further studies are needed to determine the localization and activity of organ-specific glucuronidases in the human body, especially because studies indicate that UDP-glucuronosyltransferases in both adult and fetal rat testes can metabolize BPA to BPA glucuronide (139).
A few studies have addressed the issue of BPA bioaccumulation. In one study, pregnant rats were given a single large dose of BPA (1 g/kg BW) to allow for accurate measures of BPA in tissues long after administration (140). Forty minutes after administration, the concentration measured in fetuses was greater than that measured in maternal blood. Additionally, BPA retention times were higher in fetuses than in dams, indicating that the fetus may act as a “depot” for BPA. One possible explanation for these higher fetal BPA levels is that UGT2B1 activity was low or absent in fetal livers (129). UGT2B1 levels were also decreased in liver microsomes from pregnant and lactating dams compared with nonpregnant adult animals, indicating that both mother and fetus may be particularly sensitive to BPA exposure during the pregnant and lactational/neonatal periods (141).
These studies were criticized because BPA was either injected sc or administered through an osmotic pump, suggesting that only oral exposures were relevant to the human condition where BPA is expected to undergo extensive first-pass conjugation (98,100). However, any route of exposure that allows BPA to circulate in the maternal blood is closely “replicating the human condition.” A fetus is exposed to BPA through its mother’s blood, and studies of humans indicate that low levels of active BPA are regularly detected in blood. Moreover, several studies have indicated that BPA can cross the human placental barrier (9,142).
Additionally, a quantitative study in mice examined metabolism in pregnant females administered BPA sc (134). Twenty-four hours after administration, residual blood BPA levels averaged 2.2 ng/ml, with 85% of this BPA associated with the plasma fraction. At this time point, fetuses accounted for 4% of the administered radioactivity, with an average of 3.7 ng/g. The placenta maintained 0.55% of the administered BPA (3.14 ng/g), and the amniotic fluid contained 0.34% (4.85 ng/ml) (134).
The importance of exposure route remains a highly contested issue because several studies suggest that there may be differences in metabolic pharmacokinetics after oral, sc, and iv exposures (9,23,100). The routes by which adult animals are exposed can affect the resulting circulating levels of BPA (reviewed in Ref. 9). However, several studies suggest that there are fewer differences in metabolism and excretion of BPA based on route of exposure. For instance, a quantitative study compared the metabolism of a low dose of radioactive BPA (25 μg /kg BW) administered orally or sc to mice (134). No qualitative differences in the concentrations of BPA and BPA metabolites or their bodily distribution were detected. A second study compared the metabolism and excretion of BPA by oral or iv exposure in rats dosed with 0.10 mg radiolabeled BPA/kg BW (143). The iv and oral dosing led to a urinary excretion of 8.4 and 6.3% of the radioactivity, respectively, within 24 h of treatment. Fecal excretion from the iv and oral dosing was 77.6 and 81.6% of the administered dose, respectively. BPA was also detected in the blood up to 48 h after administration. Levels were highest within the first 6 h of exposure, with slightly higher levels observed in animals exposed through the iv route. These results indicated that the metabolic kinetics were similar regardless of the mode of exposure. Finally, several studies suggest that humans are exposed to BPA through nonoral routes; BPA exposure may occur by bathing in BPA- contaminated water, by inhalation of BPA-contaminated air, or via implanted medical devices and tubing (23). For these reasons, it is unwarranted to use the route of exposure as an argument to discount relevant data from animal studies when evaluating the risk of human exposure to BPA.
No single exposure paradigm is without problems. Critics who suggest that only oral dosing paradigms should be used (100) have failed to address important issues with these methods, including stress associated with oral gavage, the inability to assess actual exposure levels from food and water consumption, and the use of oils as a delivery vehicle, which are often contaminated with other estrogens (summarized in Ref. 144).
One major controversy remains: are rodent models applicable for understanding the pharmacokinetics of BPA metabolism in humans? Some data suggest that BPA metabolism in rodents differs from metabolic endpoints in primate models. For instance, in rodents, most BPA is excreted in the feces, but in the monkey, BPA is excreted via urine (145). However, mice and Japanese monkeys dosed with 100 mg/kg BPA during pregnancy showed that BPA could be detected in several fetal tissues, including serum, liver, brain, uterus, and testes within 30 min (in mice) and 1 h (in monkeys) of treatment (146). Thus, regardless of the mode of exposure or the major route of BPA excretion (urinary vs. fecal), the fetus is exposed to BPA.
Studies that have attempted to determine directly the pharmacokinetics of BPA metabolism in human subjects have used relatively insensitive methods, leading to additional controversy (9,119,147,148). For instance, 5 mg radioactive BPA/person (54–90 μg/kg BW) was administered orally, and elimination of BPA was complete within 24 h of dosing. Maximal plasma concentrations were reached 80 min after dosing and rapidly declined over the next 6 h. BPA was detected only in its glucuronidated form, indicating that in humans, BPA is absorbed from the gastrointestinal tract quickly, conjugated with glucuronic acid in the liver, and BPA glucuronide is rapidly filtered from the blood by the kidneys and excreted in urine. However, it is likely that unconjugated BPA was simply not detected because it fell below the detection limit. In a second metabolic study, 25 μg BPA/person was administered, and then unconjugated BPA and BPA conjugates were measured in urine (148). In the three men examined, 85% of the applied BPA dose was recovered in urine after 5 h, mostly as BPA glucuronide. In the three women examined, 75% of BPA was recovered as BPA glucuronide after the same period of time. In two of six individuals, unconjugated BPA was detected in the urine at levels of approximately 1 ng/ml; because of its hydrophobic properties, this unconjugated BPA was likely a degradation product of conjugated BPA. Again, this study was limited by a relatively high detection limit (1.14 ng/ml for unconjugated BPA; 10.1 ng/ml for BPA glucuronide). This detection was 10 times to more than 100 times less sensitive than methods used in biomonitoring studies (9,23); thus, contrary to the assertion that toxicokinetics in humans are already well understood (100,119), human pharmacokinetic studies using sensitive methods are still lacking.
An indication that BPA may bioaccumulate in humans stems from the comparisons of estimated BPA exposure levels, the pharmacokinetic studies available, and the measured BPA levels in human tissues and fluids. Because BPA exposures are estimated at 40 ng to 5 μg/kg BW · d, studies indicating that BPA is rapidly metabolized and excreted from the body suggest that BPA should be undetectable in human samples (23). Instead, BPA is detected in the nanogram per milliliter range in blood and tissue samples (reviewed in Ref. 9,23). These results suggest that: 1) BPA intake is higher than estimated; 2) metabolism of BPA after chronic, low-level exposure does not follow any current metabolic model; and/or 3) BPA bioaccumulates in the body (reviewed in Ref. 9). Thus, suggestions that BPA is rapidly metabolized and removed from the body (100,119,147,148) ignore the fact that unconjugated BPA has been repeatedly detected in human fluids and tissues (reviewed in Refs. 9 and 23). When sensitive methods are used, unconjugated BPA is detected in most human tissues, including the fetal-placental unit (142).
The use of rodents to explain the effects of BPA exposure has been criticized regarding the relevance of the pharmacokinetic parameters for humans (see Ref. 100, for instance). However, critics of the rodent models have yet to acknowledge the severe deficiencies in human studies:
1) Humans are not exposed to a single chemical at a time; thus, studies examining the effects of BPA alone are not feasible. Humans are thought to be exposed to dozens, if not hundreds of chemicals with hormonal activity. As mentioned previously, even in controlled animal experiments, exposure to detergents, feed, and plastics with estrogenic activities can occur. Previous studies indicate that the low-dose effects of BPA may be due to its additivity with other estrogens present. This is an important consideration (107,149).
2) With studies repeatedly showing that BPA is detectable in more than 90% of humans, there is no identifiable negative control group (117,118). Humans are exposed to BPA inadvertently through their food and beverages, but they are also likely to be exposed via air, drinking and bathing water, dust, and soil (9). Eliminating all BPA exposures in test subjects becomes improbable.
3) Pediatricians promote the idea that children are not little adults; the pharmacokinetics of chemicals and drugs are very different in fetuses and neonates compared with adults (12,23). Men and pregnant and nonpregnant women also differ in their metabolism of chemicals including BPA, suggesting that pharmacokinetics from normal adults cannot be extrapolated to models of metabolism in pregnant women (134,141). To conduct experiments on the population of greatest concern, i.e., human fetuses and neonates, pregnant and lactating mothers would have to be treated with controlled doses of BPA; informed consent would be required for such an experiment, and most researchers in the field have enough concern about human exposures that this experiment would likely be viewed as unethical.
Although not directly addressing the issues that can be examined with controlled rodent studies, noninvasive biomonitoring studies can and should be performed with human infants. At this time, the most extensive biomonitoring study, the CDC’s NHANES study, examined children as young as age 6 and found that urinary BPA concentrations were highest in children (118). However, infants are expected to have the highest levels of exposure and thus should be examined in depth (9,98).
In summary, there is extensive evidence for the kinetics of BPA metabolism in rodent models after acute exposures to relatively high doses. Acute studies in both animals and humans indicate rapid metabolism and clearance. However, acute studies do not reflect the situation in humans, where exposure is more likely chronic and low level. Therefore, additional studies of chronic, low-level exposure to BPA are needed in both animal models and human subjects.
E. Controversy 5: Are there any definitive patterns to the effects seen in BPA-exposed animals?
Estrogens bind ERs, and they in turn bind to estrogen responsive elements and induce the expression of genes containing these elements in their target cells. These cells include those in the female reproductive organs (vagina, uterus, oviduct, ovary, and cervix), the mammary gland, the brain (including the hypothalamus and pituitary), male reproductive organs (testis and epididymus), the thyroid gland, and the skeletal and cardiovascular systems, among others (150). As a xenoestrogen with the capability of binding to ERs, BPA also has the potential to alter development at various levels of organization. A brief summary of some of these results is described later in Section E. We have focused only on studies examining the organizational effects of BPA exposure during development. Additionally, because of differing effects of exposure to low and high doses of hormones (discussed in Section III as NMDR curves), we will only refer to studies that used low doses of BPA, i.e., doses at or below the EPA reference dose of 50 μg/kg BW · d (60). Finally, because significant bias has been attributed to studies that were conducted by researchers funded by the chemical industry (151), we will focus on research performed in laboratories supported by governmental funding agencies. Over 100 additional studies, including those that used adult exposures and higher doses of BPA, were reviewed previously (60).
Differential hormone exposure during the fetal or perinatal period is important for brain sexual differentiation. Testosterone from the developing testes plays an essential role in the masculinization and/or defeminization of the brain. In rodents, testosterone secreted by the fetal and neonatal testes (the ovaries are not capable of steroid synthesis at this time) is converted to estradiol by aromatase in specific brain regions during critical periods of development (reviewed in Ref. 152), and it is estradiol that is responsible for many of the actions of testosterone in brain sexual differentiation. Therefore, perinatal exposure to estrogen-like chemicals, including BPA, has the potential to alter the development of sexually dimorphic pathways in the rodent brain. Perinatally, BPA-exposed females showed evidence of defeminization as demonstrated by a decrease in the number of dopamine neurons in the sexually dimorphic anteroventral periventricular nucleus of the hypothalamus; thus, BPA exposure led to the loss of a documented sexual dimorphism in this brain region (152).
The organizational effects of gonadal hormones also influence sexually dimorphic behaviors. Studies of social and sexual behaviors in rodents have shown that exposure to low doses of BPA obliterated expected sex differences (reviewed in Ref. 60). Perinatal BPA exposure has also been associated with aggressive behavior in adulthood (153,154). Behaviors shown to be affected by low-dose perinatal BPA exposure include timing of the copulatory sequence in male rats, play behaviors, and other sociosexual behaviors (154,155,156). Female rodents exposed to BPA during the perinatal period also displayed decreased maternal behaviors and loss of responsiveness to amphetamines (157,158).
Many, but not all, of the sex differences influenced by early BPA exposure in rodents are dependent on estrogen signaling in the male during development. The very limited data available for primates, including humans, suggest that the organizational effects of testosterone on brain sexual differentiation are mediated primarily through androgen receptors and therefore might be less susceptible to influence by the estrogenic actions of BPA (for review, see Ref. 159). However, in utero DES exposure has been reported to alter some sexually dimorphic behaviors of females (reviewed in Ref. 160), suggesting the potential for in utero effects of estrogenic compounds in humans.
Low-dose BPA exposure during perinatal development led to alterations of the organs of the male reproductive tract, including changes in testis weight at puberty and in adulthood (153,161). BPA exposure in utero also resulted in increased prostate size in adults (162), as well as changes in the periductal stroma and alterations in glandular cell function of this organ (163). Further studies revealed that increases in prostate size could be detected in the fetus and correlated with increases in proliferation of basal epithelial cells located in the primary prostate ducts (72).
Female reproductive endpoints were affected by perinatal BPA exposure as well. Low doses of BPA induced both earlier vaginal opening and earlier first estrus (62). Alterations were also observed in adult estrous cycles after perinatal exposure (62,164). In the ovaries of perinatally exposed females, a significant increase in antral follicles was observed at 3 months of age (164). Exposed animals also showed an increase in the number of blood-filled ovarian bursae at 6 months of age; these were thought to be indicative of advanced reproductive aging. Females exposed to BPA in utero had a significant increase in the number of oocytes with gross aberrations; when these females were mated, there was a significant increase in the number of aneuploid eggs and embryos (165). Low-dose BPA exposure altered the weight of the vagina, the volume of the uterine lamina propria, and receptor expression and cell proliferation in multiple compartments of the uterus (83,164).
In our lab, we have examined the effects of perinatal exposure to low doses of BPA on the developing mouse mammary gland. We found that BPA altered patterns of tissue organization at several stages, including embryonic development (embryonic d 18), peripuberty, and adulthood (164,166,167). At puberty, we observed an increased sensitivity to estradiol (168). We also detected intraductal hyperplasias, manifested as ducts with a “beaded” appearance, in adult females that were perinatally exposed to BPA (169).
A few studies have also examined the effects of perinatal BPA exposure on other estrogen-sensitive organs and systems (60). For instance, the immune system was affected in exposed male offspring; these mice produced increased IgG2a antibodies in adulthood (170). BPA exposure altered differentiation of adipocytes as well as body weight (reviewed in Ref. 60).
The influence of developmental exposure to BPA on body weight is not fully understood; some studies have found that BPA decreased body weight, and others have shown no effect of exposure (reviewed in Ref. 60). Experimental design, differences in exposure level, and vastly different composition and estrogenic activity of feed could account for at least some of these findings (171,172). However, preimplantation mouse embryos cultured in 1 nm BPA that were transplanted into unexposed females and allowed to develop were significantly heavier at weaning compared with control embryos cultured with vehicle only (173).
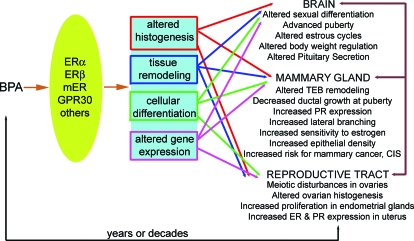
Taken together, these data indicate that animals exposed to BPA during gestation or the perinatal period show a wide variety of endocrine-related pathologies (reviewed in Refs. 60 and 174). We have previously proposed a model for the action of BPA in the reproductive system of the developing female (110,174) (Fig. 4). A similar model is likely to be relevant in the developing male as well. In the female model, we propose that BPA acts via ER (ERα, ERβ, mER, etc.) present in estrogen-target organs altering organ histogenesis, tissue remodeling, and cellular differentiation. The phenotypes described previously in this section, observed generally at puberty and in adulthood, are thus consequences of these induced early changes. Different actions (complementary and opposing) may occur via different ERs, and these receptors may be present in different concentrations in diverse cell types at various developmental stages, contributing to the complex phenotypes observed after BPA exposure.
Figure 4.
Proposed mechanisms for the endpoints affected in perinatally BPA-exposed females. BPA binds ERs, including the classical ERs (ERα and ERβ) and mERs. This causes alterations at several levels of organization including tissues, cells, and gene expression. These alterations lead to diverse changes in estrogen-target organs including the brain, mammary gland, ovary, and uterus, among others. Additionally, changes in one target organ can lead to secondary alterations in other organs. In addition to these classical targets, other nonclassical targets of BPA action include bone, cardiovascular tissue, the pancreas, adipose tissue, and the immune system (not pictured).
Because much of the work from our own lab has examined the mammary gland as a target of BPA exposure, our proposed model is best illustrated using this organ. Reciprocal stromal-epithelial interactions are essential for the proper formation, growth, and hormone responsiveness of the fetal mammary gland, supporting the idea that alterations in the stroma modify the phenotype of the epithelium (175,176). Due to its estrogenic activity, BPA likely binds to ERα and ERβ, both of which are localized primarily in the stromal compartment from embryonic d 12.5 through 18. By binding to ERs, BPA causes changes in the stroma, including accelerated maturation of fat cells and production of a collagen-rich extracellular matrix, which alter development of the epithelial compartment including growth parameters of the ductal tree, cell shape, size, and organization (i.e., lumen formation) (74). Thus, the phenotypes observed at puberty and in adulthood, long after exposure to BPA has ended, may be due to alterations in the mammary stroma during an early period of exposure. Future studies using tissue recombination techniques might further clarify the tissue targets of BPA.
A more detailed model is needed to incorporate “nontraditional” target organs. Given that development is a highly integrative process, it will not suffice to use only bottom-up approaches, namely, from receptors to genes, genes to cells, etc., but additionally and simultaneously a top-down approach must be used, asking which effects are due to direct action in the organ exhibiting an effect and which ones are due to indirect effects (for example, through actions in other organs).
It is a separate controversy to ask whether the endpoints examined in animal models are relevant for human diseases. For instance, is the increase in the number of aneuploid eggs and embryos observed in BPA-exposed female mice (165) related to recurrent miscarriages observed in women with higher concentrations of BPA in their blood (177)? This is a difficult issue to address experimentally and will likely remain debated by scientists and government officials charged with determining risk assessments for BPA and other endocrine disruptors.
F. Controversy 6: Could low doses of BPA affect cancer incidence?
In 1982, the National Toxicology Program (NTP) undertook a large-scale chronic feed study to assess the carcinogenicity of BPA in Fischer 344 rats and B6C3 F1 hybrid mice (reviewed in Ref. 178). Rats of both sexes were fed chow containing 0, 1000, or 2000 ppm BPA. Male mice were fed chow containing 0, 1,000, or 5,000 ppm, and female mice were fed chow containing 0, 5,000 or 10,000 ppm beginning at 5 wk of age for more than 100 wk. Based on food consumption in rodents, these doses correspond to greater than 50 mg/kg BW · d. BPA induced slight increases in hematological cancers in rats and male mice. Male rats also had a significant increase in testicular interstitial cell tumors and mammary fibroadenomas. No carcinogenic events were detected in female mice. Although this study is often cited as evidence that there is no link between BPA and cancer, its experimental design is subject to criticism. First, the animals were given excessive doses of BPA, well above the human exposure range, under the assumption that BPA would follow a threshold-model dose response curve. Second, different dosing paradigms were set by species and sex. Finally, this study did not examine the effects of perinatal (organizational) BPA exposure on long-term health outcomes.
A recent study examined the effects of neonatal BPA exposure on prostate cancer (179). Male Sprague-Dawley rats were injected with 10 μg BPA/kg BW on PND 1, 3, and 5. At PND 90, half were treated with testosterone and estrogen, whereas the other half were untreated. In animals treated neonatally with BPA and then untreated during adulthood, there were no significant changes in prostatic lesions. However, in animals treated neonatally with BPA and then treated with hormones in adulthood, there was a significant increase in the incidence and severity of prostatic intraepithelial neoplasias. These lesions had a significant increase in their cell proliferative index.
The connection between perinatal BPA exposure and mammary cancer in rodents is currently strengthening. First, the results described in Section E mice indicate that BPA caused changes in the organization of the mammary gland at puberty and in adulthood. Some of these changes are similar to known risk factors for breast cancer in humans (Table 1). For instance, at puberty, alterations in apoptosis leads to an increase in the number of terminal end buds that persist in the mammary gland and an increase in terminal ducts, the structures where cancers are thought to arise (166,180); similar structures are retained in nulliparous women, who are at a higher risk for developing breast cancer. An increase in the number of epithelial cells expressing progesterone receptor leads to an increase in lateral branching and eventually to increased epithelial density (166,167), which may be equivalent to the human breast cancer risk factor of increased mammographic density (181).
Table 1.
Observations made in the mammary glands of perinatally BPA-exposed mice and plausible associated risk factors for human breast cancer
| Alteration observed in BPA-exposed rodents | Related human risk factor/disease |
|---|---|
| Increased density of terminal ends and terminal end budsa | Similar to relatively undifferentiated gland structures found in nulliparous women, who are at higher risk for breast cancer. Also may be the structures where cancers arise |
| Increased sensitivity to estrogensa | Longer lifetime exposure to estrogen |
| Increased epithelial densitya | Increased mammographic density |
| Preneoplastic intraductal hyperplasiasa,b | Precancerous epithelial lesions |
| Carcinomas in situb | Carcinomas in situ |
| Palpable tumors after subcarcinogenic doses of chemical carcinogensb | Tumors |
Observed in mice.
Observed in rats.
Because the rat model of carcinogenesis better mimics human breast cancer than the available mouse models (182), the link between BPA and mammary cancer was further pursued using inbred Wistar-Furth rats. These were exposed to BPA from embryonic d 9 through PND 1 (0, 2.5, 25, 250, or 1000 μg/kg BW · d) (183). At PND 50, animals exposed to all doses of BPA had a 3- to 4-fold increase in the number of hyperplastic ducts when compared with controls. Surprisingly, at PND 90, only those animals exposed to the lowest dose of BPA had a significant increase in the number of these structures compared with controls. These hyperplastic ducts were Ki67-positive and ER-positive, indicating that these lesions were both estrogen sensitive and proliferating. Additionally, cribiform-like structures, identified as carcinomas in situ, were observed at both PND 50 and PND 90 in animals exposed perinatally to the two highest doses of BPA.
A second study exposed Wistar rats to either 0 or 25 μg/kg BW · d from embryonic d 8 through birth, and then challenged these rats with a subcarcinogenic dose of the chemical carcinogen N-nitroso-N-methylurea at puberty (184). Rats exposed to BPA in utero and then challenged with N-nitroso-N-methylurea developed significantly more hyperplastic ducts at 110 and 180 d of age compared with animals exposed to vehicle in utero. At 180 d of age, the BPA-exposed animals also developed mammary malignancies. Together, these studies indicate that BPA alone can induce carcinomas in prenatally exposed rats and that early BPA exposure can sensitize the mammary gland to carcinogenic insults experienced later in life.
Finally, a recent study of ER-positive and ER-negative human breast cancer cell lines suggests that BPA can antagonize the cytotoxicity of several chemotherapy drugs independent of classical ERs (185). Although this study was conducted in vitro, it highlights the possibility that exposure of human patients to BPA during cancer treatments may decrease the efficacy of chemotherapeutic drugs.
Estrogen exposure throughout a woman’s life is a major risk factor for the development of breast cancer, as demonstrated by the increased risk associated with early age of menarche and late age of menopause (186). The positive correlation between increased intrauterine levels of estrogens (a phenomenon observed in dizygotic twin births) and breast cancer in daughters born from such pregnancies also supports this link (187). It is still unknown whether exposure to nonendogenous estrogens could have the same impact on breast cancer risk. Evidence that gestational exposure to the very potent xenoestrogen DES leads to a higher incidence of breast cancer in adulthood supports this hypothesis (82). The multitude of environmental chemicals with hormonal activities to which we are all exposed involuntarily and unknowingly, in addition to prescribed hormones (hormonal contraceptives or hormone replacement therapy), might contribute to the increased breast cancer incidence that has been observed during the last 50 yr in the industrialized world.
VII. Expert Opinions and Government Decisions
The chemical industry maintains that BPA is a component of consumer products that “make our lives easier, healthier and safer” (188). However, the BPA industry has acknowledged low-level migration of BPA from baby bottles, water bottles, tableware, and food cans (188). Industry publications state that “the potential human exposure to BPA is more than 400 times lower than the US EPA reference dose” of 0.05 mg/kg BW · d and state that these low levels of exposure do not pose any risk to human health, under the premise that health effects that do not occur at high levels of exposure cannot be induced by much lower levels of a chemical. Notwithstanding, significant effects of low doses of BPA have been observed by numerous different independent researchers using various endpoints, some of which were discussed in Section E.
The field of endocrine disruption, and particularly BPA research, has been influenced by social issues, legislation, and the public press. In the fall of 2006, a meeting of approximately 45 experts in the field of BPA research was organized by the National Institutes of Environmental Health Sciences (NIEHS).
The scientists at this meeting generated the Chapel Hill Consensus Statement, signed by 38 authors stating: “The published scientific literature … reveals that human exposure to BPA is within the range that is predicted to be biologically active in over 95% of people sampled. The wide range of adverse effects of low doses of BPA in laboratory animals exposed both during development and in adulthood is a great cause for concern with regard to the potential for similar adverse effects in humans … There is extensive evidence that outcomes may not become apparent until long after BPA exposure during development has occurred … These developmental effects are irreversible and can occur due to low-dose exposure during brief sensitive periods in development, even though no BPA may be detected when the damage or disease is expressed” (97).
At the same time, the NTP’s Center for the Evaluation of Risks to Human Reproduction (CERHR) established a committee to evaluate the scientific evidence for the effects of BPA on human reproductive health (98). The CERHR panel concluded that BPA has no effect on “changes in prostate weight, age at puberty (rat), pathology or tumors in any tissue, or reproductive tract malformations.” The panel did express “some” concern for the effects of BPA exposure on neural and behavioral endpoints. The report generated by the CERHR committee was challenged by a number of scientists because of the use of arbitrary criteria to evaluate animal studies, the unbalanced use of these criteria, and the lack of BPA experts on the appointed panel (144). In April 2008, after extensive review of the CERHR panel report and its criticisms, the NTP released its own draft report, in large part agreeing with the Chapel Hill Consensus Statement.
The NTP report stated: “The NTP concurs with the conclusion of the CERHR Expert Panel on bisphenol-A that there is some concern for neural and behavioral effects in fetuses, infants, and children at current human exposures. The NTP also has some concern for Bisphenol A exposure in these populations based on effects in the prostate gland, mammary gland, and an earlier age for puberty in females” (189).
In June 2008, the NTP held a public hearing to draw final conclusions about BPA risk assessment. The panel held a deciding vote to concur that there is some concern for neural and behavioral effects and the prostate gland in fetuses, infants, and children at current human exposures (190). However, the levels of concern for effects on the mammary gland and an earlier age for puberty were downgraded to minimal.
Finally, the stance of the U.S. Food and Drug Administration (FDA) on BPA safety cannot be ignored. In April 2008, the FDA created a task force to address the possible health effects of human BPA exposure. The FDA’s statement said: “Based on our ongoing review, we believe there is a large body of evidence that indicates that FDA-regulated products containing BPA currently on the market are safe and that exposure levels to BPA from food contact materials, including for infants and children, are below those that may cause health effects” (191).
Yet, the FDA relied primarily on two animal studies to make this decision. In the first, the authors concluded that there was no effect of BPA exposure (192). However, this study used the Sprague-Dawley rat, which is insensitive to estrogens and lacked a positive control; thus, it is impossible to conclude from this study whether BPA truly had no effect on the multiple generations of animals exposed or whether the rats were simply insensitive to estrogens (193). In the second study, low-dose effects of BPA were also not detected (99). Again, this study had questionable positive controls that required large doses of estradiol to reveal any effects, suggesting that for some reason sensitivity to estrogen was severely diminished in these studies (194).
The studies used by the FDA were designed to detect changes in the incidence of gross pathologies and were not sensitive enough to observe quantitative or qualitative differences in sensitive endpoints that have been shown by others to be affected by BPA exposure (60). Great concern was expressed over the use of only two published studies, ignoring more than 100 that show effects of low-dose exposure (194). Of particular concern was the use of good laboratory practices (GLP) as a criterion for selecting adequate studies. GLP standards imply that a particular form of good record keeping was performed, not that studies are well designed; these standards were created to promote transparency in industry-funded laboratories and do not apply to research conducted by universities and other National Institutes of Health-funded institutes (194). In response to the concerns that were voiced, the FDA stated, “We will continue to consider new research and information as they become available.”
In the fall of 2008, these concerns and others were addressed by a subcommittee of scientists that were asked to provide advice and make preliminary recommendations for safety assessments to the FDA (195). This subcommittee found that the FDA’s initial report had significant limitations, including an inadequate number of infant formula samples used to make exposure estimates, a lack of scientific support for the rejection of non-GLP studies, deviations from the assessments of adequacy made by the CERHR panel, and the need for inclusion of the most recently published studies.
Finally, the FDA subcommittee wrote: “Coupling together the available qualitative and quantitative information (including application of uncertainty factors) provides a sufficient scientific basis to conclude that the margins of safety defined by FDA as ‘adequate’ are, in fact, inadequate” (195).
The FDA’s Commissioner of Food and Drugs, Andrew von Eschenbach, praised the work of the subcommittee and the members of the science board, suggesting that the subcommittee’s report was “a strong affirmation of [the FDA’s] process—a process to identify information that will better inform our regulatory decision making” (196).
VIII. Conclusions
The data collected thus far in the field of environmental toxicology are sufficiently robust to raise concerns about the potentially deleterious impact of endocrine-disrupting chemicals on human development. To extrapolate evidence from animal studies to humans should be done cautiously because differences among species and strains have been reported regarding a variety of parameters. However, the mouse and rat have been shown to be excellent models for the understanding of the sad episode of the human DES syndrome. Importantly, recent studies indicate that in both rats and nonhuman primates, BPA abolishes estrogen-dependent spine synapse formation in the hippocampus and prefrontal cortex, lending additional support to the use of rodent models (197,198). Thus, it would be unwise to ignore the incremental evidence stemming from rigorously controlled laboratory experiments and from chemically exposed wildlife, alongside the increasing incidence of comparable issues in human populations exposed to these same chemicals during different developmental stages. All of this evidence should encourage regulatory agencies to apply the precautionary principle and thus ban and/or substitute those chemicals that are likely to be harmful to the normal development of humans and wildlife. The NTP report, the most recent statement by the FDA’s commissioner, and a report from Health Canada classifying BPA as a human and environmental toxin all suggest a potential change in the perception of the regulatory community toward recognizing the risk posed by BPA exposure.
Although scientific inquiry is a dynamic give-and-take among researchers with different opinions and viewpoints, the so-called controversies surrounding low-dose effects and NMDR curves should be put to rest, given that they now affect public health decisions. These phenomena have been demonstrated time and again for a sufficient number of endocrine-related endpoints, and they no longer merit being considered “controversial” topics. It is time to span the great divides that exist in this field.
Acknowledgments
The authors are grateful to Cheryl Schaeberle for assistance in preparing this manuscript.
Footnotes
The authors are supported by grants ES08314, ES013884, and ES015182 from the National Institutes of Environmental Health Sciences.
Conflict of Interest Statement: L.N.V., M.V.M., B.S.R., and C.S. have nothing to declare. A.M.S. was compensated as an advisor to the National Science Foundation.
First Published Online December 12, 2009
Abbreviations: AhR, Aryl hydrocarbon receptor; BPA, bisphenol-A; BW, body weight; DES, diethylstilbestrol; ER, estrogen receptor; ERR-γ, estrogen-related receptor-γ; GLP, good laboratory practices; GPR30, G protein-coupled receptor 30; mER, membrane-bound ERα; NMDR, nonmonotonic dose response; PND, postnatal day.
References
- Carson R 1987 Silent spring: 25th anniversary edition. New York: Houghton Mifflin Co. [Google Scholar]
- 1994 Toxic Substances Control Act: EPA’s limited progress in regulating toxic chemicals. http://archive.gao.gov/t2pbat3/151661.pdf [Google Scholar]
- Colborn T, Dumanoski D, Myers JP 1995 Our stolen future. New York: Penguin Books [Google Scholar]
- 1998 Fooling with nature. Interview with Theo Colborn, Ph.D. Frontline, Public Broadcasting Service. http://www.pbs.org/wgbh/pages/frontline/shows/nature/interviews/colborn.html [Google Scholar]
- 1992 Wingspread Consensus Statement. In: Colborn T, Clement C, eds. Chemically induced alterations in sexual and functional development: the human/wildlife connection. Princeton, NJ: Princeton Scientific Publishing; 1–8 [Google Scholar]
- Bern HA 1992 The fragile fetus. In: Colborn T, Clement C, eds. Chemically-induced alterations in sexual and functional development: the wildlife/human connection. Princeton, NJ: Princeton Scientific Publishing; 9–15 [Google Scholar]
- Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA 1996 Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect 104:715–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2001 National Toxicology Program’s Report of the Endocrine Disruptors Low Dose Peer Review. http://ntp.niehs.nih.gov/ntp/htdocs/liason/LowDosePeerFinalRpt.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV 2007 Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177 [DOI] [PubMed] [Google Scholar]
- Le HH, Carlson EM, Chua JP, Belcher SM 2008 Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett 176:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey RE, George JD, Price CJ, Tyl RW, Marr MC, Kimmel CA 1987 The developmental toxicity of bisphenol A in rats and mice. Fundam Appl Toxicol 8:571–582 [DOI] [PubMed] [Google Scholar]
- Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS 2003 Large effects from small exposures: I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 111:994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds EC, Lawson W 1936 Synthetic estrogenic agents without the phenanthrene nucleus. Nature 137:996 [Google Scholar]
- Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW 1998 Bisphenol A interacts with the estrogen receptor α in a distinct manner from estradiol. Mol Cell Endocrinol 142:203–214 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT, van der Burg B, Gustafsson JA 1998 Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 139:4252–4263 [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S 2005 Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 84:249–259 [DOI] [PubMed] [Google Scholar]
- Routledge EJ, White R, Parker MG, Sumpter JP 2000 Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) α and ER β. J Biol Chem 275:35986–35993 [DOI] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM 2007 In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 24:178–198 [DOI] [PubMed] [Google Scholar]
- Soto AM, Maffini MV, Schaeberle CM, Sonnenschein C 2006 Strengths and weaknesses of in vitro assays for estrogenic and androgenic activity. Best Pract Res Clin Endocrinol Metab 20:15–33 [DOI] [PubMed] [Google Scholar]
- Soto AM, Lin T-M, Justicia H, Silvia RM, Sonnenschein C 1992 An “in culture” bioassay to assess the estrogenicity of xenobiotics. In: Colborn T, Clement C, eds. Chemically induced alterations in sexual development: the wildlife/human connection. Princeton, NJ: Princeton Scientific Publishing; 295–309 [Google Scholar]
- Andersen HR, Andersson A-M, Arnold SF, Autrup H, Barfoed M, Beresford NA, Bjerregaard P, Christiansen LB, Gissel B, Hummel R, Jorgensen EB, Korsgaard B, Le Guevel R, Leffers H, McLachlan J, Moller A, Nielsen JB, Olea N, Oles-Karasko A, Pakdel F, Pedersen KL, Perez P, Skakkebaek NE, Sonnenschein C, Soto AM, Sumpter JP, Thorpe SM, Grandjean P 1999 Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect 107:89–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Tong W, Perkins R, Soto AM, Prechtl NV, Sheehan DM 2000 Quantitative comparisons of in vitro assays for estrogenic activities. Environ Health Perspect 108:723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS 2006 Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147:S56–S69 [DOI] [PubMed] [Google Scholar]
- Powell CE, Soto AM, Sonnenschein C 2001 Identification and characterization of membrane estrogen receptor from MCF7 estrogen-target cells. J Steroid Biochem Mol Biol 77:97–108 [DOI] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Alyea RA 2007 Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids 72:124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Dong J 2006 Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol 102:175–179 [DOI] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC 2005 Signaling from the membrane via membrane estrogen receptor-α: estrogens, xenoestrogens, and phytoestrogens. Steroids 70:364–371 [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, Nadal A 2005 Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic α-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect 113:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, Nef S, Stefani E, Nadal A 2008 Pancreatic insulin content regulation by the estrogen receptor ER α. PLoS ONE 3:e2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Nakatsuka M, Asagiri K, Habara T, Takata M, Konishi H, Kudo T 2002 Bisphenol A stimulates NO synthesis through a non-genomic estrogen receptor-mediated mechanism in mouse endothelial cells. Toxicol Lett 135:95–101 [DOI] [PubMed] [Google Scholar]
- Nadal A, Alonso-Magdalena P, Ripoll C, Fuentes E 2005 Disentangling the molecular mechanisms of action of endogenous and environmental estrogens. Pflugers Arch 449:335–343 [DOI] [PubMed] [Google Scholar]
- Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM 2001 The mouse uterotrophic assay: a re-evaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect 109:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N 1998 The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology 139:2741–2747 [DOI] [PubMed] [Google Scholar]
- Colerangle JB, Roy D 1997 Profound effects of the weak environmental estrogen-like chemical bisphenol A on the growth of the mammary gland of Noble rats. J Steroid Biochem Mol Biol 60:153–160 [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K 2002 Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab 87:5185–5190 [DOI] [PubMed] [Google Scholar]
- Kitamura S, Jinno N, Ohta S, Kuroki H, Fujimoto N 2002 Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem Biophys Res Commun 293:554–559 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C 2005 Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146:607–612 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Wayne NL 2008 Impact of bisphenol-A on early embryonic development and reproductive maturation. Reprod Toxicol 25:177–183 [DOI] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP 1998 Several environmental oestrogens are also anti-androgens. J Endocrinol 158:327–339 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K 2003 Antiandrogenic effects of bisphenol A and nonphenol on the function of androgen receptor. Toxicol Sci 75:40–46 [DOI] [PubMed] [Google Scholar]
- Sun H, Xu LC, Chen JF, Song L, Wang XR 2006 Effect of bisphenol A, tetrachlorobisphenol A and pentachlorophenol on the transcriptional activities of androgen receptor-mediated reporter gene. Food Chem Toxicol 44:1916–1921 [DOI] [PubMed] [Google Scholar]
- Kruger T, Long M, Bonefeld-Jorgensen EC 2008 Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology 246:112–123 [DOI] [PubMed] [Google Scholar]
- Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer S, Safe S 2000 Interaction of methoxychlor and related compounds with estrogen receptor α and β, and androgen receptor: structure-activity studies. Mol Pharmacol 58:852–858 [PubMed] [Google Scholar]
- Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP 2004 Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145:592–603 [DOI] [PubMed] [Google Scholar]
- Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y 2007 Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR γ. J Biochem 142:517–524 [DOI] [PubMed] [Google Scholar]
- Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y 2006 Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRγ) with high constitutive activity. Toxicol Lett 167:95–105 [DOI] [PubMed] [Google Scholar]
- Pocar P, Fischer B, Klonisch T, Hombach-Klonisch S 2005 Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction 129:379–389 [DOI] [PubMed] [Google Scholar]
- Sheehan DM, vom Saal FS 1997 Low dose effects of hormones: a challenge for risk assessment. Risk Policy Report 4:31–39 [Google Scholar]
- Vandenberg LN, Wadia PR, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM 2006 The mammary gland response to estradiol: monotonic at the cellular level, non-monotonic at the tissue-level of organization? J Steroid Biochem Mol Biol 101:263–274 [DOI] [PubMed] [Google Scholar]
- Conolly RB, Lutz WK 2004 Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol Sci 77:151–157 [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA 2001 The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci 62:330–338 [DOI] [PubMed] [Google Scholar]
- Medlock KL, Lyttle CR, Kelepouris N, Newman ED, Sheehan DM 1991 Estradiol down-regulation of the rat uterine estrogen receptor. Proc Soc Exp Biol Med 196:292–300 [DOI] [PubMed] [Google Scholar]
- Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM 1998 Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod 59:1143–1152 [DOI] [PubMed] [Google Scholar]
- Soto AM, Lin T-M, Sakabe K, Olea N, Damassa DA, Sonnenschein C 1995 Variants of the human prostate LNCaP cell line as a tool to study discrete components of the androgen-mediated proliferative response. Oncol Res 7:545–558 [PubMed] [Google Scholar]
- Sonnenschein C, Olea N, Pasanen ME, Soto AM 1989 Negative controls of cell proliferation: human prostate cancer cells and androgens. Cancer Res 49:3474–3481 [PubMed] [Google Scholar]
- Geck P, Maffini MV, Szelei J, Sonnenschein C, Soto AM 2000 Androgen-induced proliferative quiescence in prostate cancer: the role of AS3 as its mediator. Proc Natl Acad Sci USA 97:10185–10190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak AL, Bulayeva NN, Watson CS 2005 Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-α mediated Ca++ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect 113:431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Petre CE, Monk KR, Puga A, Knudsen KE 2002 The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol Cancer Ther 1:515–524 [PubMed] [Google Scholar]
- Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N 2008 Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect 116:1642–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS 2007 In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM 2001 Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 109:675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Igushi T 2002 Low dose effects of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol 16:117–122 [DOI] [PubMed] [Google Scholar]
- Gandelman R, vom Saal FS, Reinisch JM 1977 Contiguity to male foetuses affects morphology and behaviour of female mice. Nature 266:722–724 [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG 2004 Animal models and studies of in utero endocrine disruptor effects. ILAR Journal 45:438–442 [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG 2002 Intrauterine position effects. Neurosci Biobehav Rev 26:665–678 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Bronson FH 1980 Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Science 208:597–599 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Bronson FH 1978 In utero proximity of female mouse fetuses to males: effect on reproductive performance during later life. Biol Reprod 19:842–853 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Bronson FH 1980 Variation in length of estrous cycles in mice due to former intrauterine proximity to male fetuses. Biol Reprod 22:777–780 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Pryor S, Bronson FH 1981 Effects of prior intrauterine position and housing on oestrous cycle length in adolescent mice. J Reprod Fertil 62:33–37 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Richter CA, Ruhlen RR, Nagel SC, Timms BG, Welshons WV 2005 The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of bisphenol A. Birth Defects Res (Part A) 73:140–145 [DOI] [PubMed] [Google Scholar]
- vom Saal FS 1989 Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. J Anim Sci 67:1824–1840 [DOI] [PubMed] [Google Scholar]
- Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS 2005 Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci USA 102:7014–7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS 1999 Exposure to bisphenol A advances puberty. Nature 401:763–764 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM 2007 Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology 148:116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM 2005 The freemartin syndrome: an update. Anim Reprod Sci 87:93–109 [DOI] [PubMed] [Google Scholar]
- Satoh S, Hirata T-I, Miyake Y-I, Kaneda Y 1997 The possibility of early estimation for fertility in bovine heterosexual twin females. J Vet Med Sci 59:221–222 [DOI] [PubMed] [Google Scholar]
- Reiling BA, Berger LL, Faulkner DB, McKeith FK, Nash TG 1995 Effect of prenatal androgenization on performance, lactation, carcass, and sensory traits of heifers in a single-calf heifer system. J Anim Sci 73:986–992 [DOI] [PubMed] [Google Scholar]
- van de Beek C, Thijssen JHH, Cohen-Kettenis PT, van Goozen SHM, Buitelaar JK 2004 Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormone exposure? Horm Behav 46:663–669 [DOI] [PubMed] [Google Scholar]
- Lummaa V, Pettay JE, Russell AF 2007 Male twins reduce fitness of female co-twins in humans. Proc Natl Acad Sci USA 104:10915–10920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichopoulos D 1990 Is breast cancer initiated in utero? Epidemiology 1:95–96 [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC 1971 Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 284:878–881 [DOI] [PubMed] [Google Scholar]
- Palmer JR, Wise LA, Hatch EE, Troisi R, Titus-Ernstoff L, Strohsnitter W, Kaufman R, Herbst AL, Noller KL, Hyer M, Hoover RN 2006 Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 15:1509–1514 [DOI] [PubMed] [Google Scholar]
- Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM 2005 Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod 72:1344–1351 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Moyer CL 1985 Prenatal effects on reproductive capacity during aging in female mice. Biol Reprod 32:1116–1126 [DOI] [PubMed] [Google Scholar]
- Gilbert SF 2006 Developmental biology. 6th ed. Sunderland, MA: Sinauer Associates [Google Scholar]
- Hermanson O, Glass CK, Rosenfeld MG 2002 Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol Metab 13:55–60 [DOI] [PubMed] [Google Scholar]
- Mittendorf R 1995 Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology 51:435–445 [DOI] [PubMed] [Google Scholar]
- Hatch EE, Herbst AL, Hoover RN, Noller KL, Adam E, Kaufman RH, Palmer JR, Titus-Ernstoff L, Hyer M, Hartge P, Robboy SJ 2001 Incidence of squamous neoplasia of the cervix and vagina in women exposed prenatally to diethylstilbestrol (United States). Cancer Causes Control 12:837–845 [DOI] [PubMed] [Google Scholar]
- Richert MM, Schwertfeger KL, Ryder JW, Anderson SM 2000 An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 5:227–241 [DOI] [PubMed] [Google Scholar]
- Rayner JL, Enoch RR, Fenton SE 2005 Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol Sci 87:255–266 [DOI] [PubMed] [Google Scholar]
- Rayner JL, Wood C, Fenton SE 2004 Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol Appl Pharmacol 195:23–34 [DOI] [PubMed] [Google Scholar]
- Bern HA, Mills KT, Jones LA 1983 Critical period of neonatal estrogen exposure in occurrence of mammary gland abnormalities in adult mice. Proc Soc Exp Biol Med 172:239–242 [DOI] [PubMed] [Google Scholar]
- Warner M, Eskenazi B, Mocarelli P, Gerthoux PM, Samuels S, Needham L, Patterson D, Brambilla P 2002 Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study. Environ Health Perspect 110:625–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham LL, Patternson DGJ, Brambilla P, Gavoni N, Casalini S, Panazza S, Turner W, Gerthoux PM 2007 Serum dioxin concentrations and endometriosis: a cohort study in Seveso, Italy. Environ Health Perspect 110:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GW, Karpf ABC, Kratochwil K 1999 Regulation of mammary gland development by tissue interaction. J Mammary Gland Biol Neoplasia 4:9–19 [DOI] [PubMed] [Google Scholar]
- Medina D, Sivaraman L, Hilsenbeck SG, Conneely OM, Ginger M, Rosen JM, O'Malley BW 2001 Mechanisms of hormonal prevention of breast cancer. Ann NY Acad Sci 952:23–35 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Hauser R, Heindel JJ, Ho S-M, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill YB, Zoeller RT 2007 Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol 24:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2007 NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. http://cerhr.niehs.nih. gov/chemicals/bisphenol/BPAFinalEPVF112607.pdf [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, Seely JC, Dimond SS, Van Miller JP, Shiotsuka RN, Beyer D, Hentges SG, Waechter Jr JM 2008 Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol Sci 104:362–384 [DOI] [PubMed] [Google Scholar]
- Willhite CC, Ball GL, McLellan CJ 2008 Derivation of a Bisphenol A organ reference dose (RfD) and drinking-water equivalent concentration. J Toxicol Environ Health B Crit Rev 11:69–146 [DOI] [PubMed] [Google Scholar]
- Soto AM, Sonnenschein C 2001 The two faces of Janus: sex steroids as mediators of both cell proliferation and cell death. J Natl Cancer Inst 93:1673–1675 [DOI] [PubMed] [Google Scholar]
- Powell CE, Soto AM, Michaelson CL, Diba F, Mounier F, Verroust PJ, Sonnenschein C 2003 Plasma membrane-resident albumin binding protein associated with the proliferation of MCF7 serum-sensitive cells. Steroids 68:487–496 [DOI] [PubMed] [Google Scholar]
- Sonnenschein C, Soto AM 1999 The society of cells: cancer and control of cell proliferation. New York: Springer Verlag [Google Scholar]
- Mannan MA, O'Shaughnessy PJ 1992 Steroidogenesis during postnatal development in the mouse ovary. J Endocrinol 130:101–106 [DOI] [PubMed] [Google Scholar]
- Greco TL, Payne AH 1994 Ontogeny of expression of the genes for steroidogenic enzymes P450 side-chain cleavage, 3-β-hydroxysteroid-dehydrogenase, P450 17 α-hydroxylase/C17-20 lyase, and P450 aromatase in fetal mouse gonads. Endocrinology 135:262–268 [DOI] [PubMed] [Google Scholar]
- Nagel SC, vom Saal FS, Welshons WV 1999 Developmental effects of estrogenic chemicals are predicted by an in vitro assay incorporating modification of cell uptake by serum. J Steroid Biochem Mol Biol 69:343–357 [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A 2002 Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone activity. Environ Health Perspect 110:917–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Sassoon DA 2006 PCBs exert an estrogenic effect through repression of the Wnt7a signaling pathway in the female reproductive tract. Environ Health Perspect 114:898–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Benson GV, Lim H, Dey SK, Maas RL 1998 Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES). Dev Biol 197:141–154 [DOI] [PubMed] [Google Scholar]
- Soto AM, Vandenberg LN, Maffini MV, Sonnenschein C 2008 Does breast cancer start in the womb? Basic Clin Pharmacol Toxicol 102:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel P, Schulz T, Smolnikar K, Strunck E, Vollmer G, Michna H 2000 Ability of xeno- and phytoestrogens to modulate expression of estrogen-sensitive genes in rat uterus: estrogenicity profiles and uterotropic activity. J Steroid Biochem Mol Biol 73:1–10 [DOI] [PubMed] [Google Scholar]
- Naciff JM, Jump ML, Torontali SM, Carr GJ, Tiesman JP, Overmann GJ, Daston GP 2002 Gene expression profile induced by 17α-ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat. Toxicol Sci 68:184–199 [DOI] [PubMed] [Google Scholar]
- Barker DJP 2003 The developmental origins of adult disease. Eur J Endocrinol 18:733–736 [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJP 2004 Fetal programming and adult health. Public Health Nutr 4:611–624 [DOI] [PubMed] [Google Scholar]
- Barker DJP, Hanson MA 2004 Altered regional blood flow in the fetus: the origins of cardiovascular disease? Acta Paediatricia 93:1559–1560 [PubMed] [Google Scholar]
- Soto AM, Maffini MV, Sonnenschein C 2008 Neoplasia as development gone awry: the role of endocrine disruptors. Int J Androl 31:288–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham JL 2005 Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113:391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham JL 2008 Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekant W, Volkel W 2008 Human exposure to bisphenol A by biomonitoring: methods: results and assessment of environmental exposures. Toxicol Appl Pharmacol 228:114–134 [DOI] [PubMed] [Google Scholar]
- Fukata H, Miyagawa H, Yamazaki N, Mori C 2006 Comparison of ELISA- and LC-MS-based methodologies for the exposure assessment of bisphenol A. Toxicol Mech Methods 16:427–430 [DOI] [PubMed] [Google Scholar]
- Atkinson JC, Diamond F, Eichmiller F, Selwitz R, Jones G 2002 Stability of bisphenol A, triethylene glycol dimethylacrylate, and bisphenol A dimethylacrylate in whole saliva. Dent Mater 18:128–135 [DOI] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D 2008 Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300:1303–1310 [DOI] [PubMed] [Google Scholar]
- Yamada H, Furuta I, Kato EH, Kataoka S, Usuki Y, Kobashi G, Sata F, Kishi R, Fujimoto S 2002 Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol 16:735–739 [DOI] [PubMed] [Google Scholar]
- Yang M, Kim S-Y, Chang S-S, Lee I-S, Kawamoto T 2006 Urinary concentrations of bisphenol A in relation to biomarkers of sensitivity and effect and endocrine-related health effects. Environ Mol Mutagen 47:571–578 [DOI] [PubMed] [Google Scholar]
- Kang J-H, Kondo F, Katayama Y 2006 Human exposure to bisphenol A. Toxicology 226:79–89 [DOI] [PubMed] [Google Scholar]
- 2002 Opinion of the scientific committee on food on bisphenol A. http://europa.eu.int/comm/food/fs/sc/scf/out128_en.pdf [Google Scholar]
- Wilson NK, Chuang JC, Lyu C, Menton R, Morgan MK 2003 Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J Expo Anal Environ Epidemiol 13:187–202 [DOI] [PubMed] [Google Scholar]
- Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS 2007 An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res 103:9–20 [DOI] [PubMed] [Google Scholar]
- Yokota H, Iwano H, Endo M, Kobayashi T, Inoue H, Ikushiro S, Yuasa A 1999 Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem J 340:405–409 [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM 2005 Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2- hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 383:638–644 [DOI] [PubMed] [Google Scholar]
- Suiko M, Sakakibara Y, Liu MC 2000 Sulfation of environment estrogen-like chemicals by cytosolic sulfotransferases. Biochem Biophys Res Commun 267:80–84 [DOI] [PubMed] [Google Scholar]
- Shimizu M, Ohta K, Matsumoto Y, Fukuoka M, Ohno Y, Ozawa S 2002 Sulfation of bisphenol A abolished its estrogenicity based on proliferation and gene expression in human breast cancer MCF-7 cells. Toxicol in Vitro 16:549–556 [DOI] [PubMed] [Google Scholar]
- Kim Y-H, Kim C-S, Park S, Han SY, Pyo M-Y, Yang M 2003 Gender differences in the levels of bisphenol A metabolites in urine. Biochem Biophys Res Commun 312:441–448 [DOI] [PubMed] [Google Scholar]
- Zalko D, Soto AM, Dolo L, Dorio C, Ratahao E, Debrauwer L, Faure R, Cravedi J-P 2003 Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect 111:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Yokota H, Kibe R, Sayama Y, Yuasa A 2002 Excretion of bisphenol A-glucuronide into the small intestine and deconjugation in the cecum of the rat. Biochemica et Biophysica Acta 1573:171–176 [DOI] [PubMed] [Google Scholar]
- Mykkanen H, Tikka J, Pitkanen T, Hanninen O 1997 Fecal bacterial enzyme activities in infants increase with age and adoption of adult-type diet. J Pediatr Gastroenterol Nutr 25:312–316 [DOI] [PubMed] [Google Scholar]
- Taylor JA, Welshons WV, Vom Saal FS 2008 No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reprod Toxicol 25:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell CL, Barvian KK, Young PCM, Bigsby RM, Verdugo DE, Bertozzi CR, Widlanski TS 2006 A role for sulfation-desulfation in the uptake of bisphenol A into breast tumor cells. Chem Biol 13:891–897 [DOI] [PubMed] [Google Scholar]
- Miyakoda H, Tabata M, Onodera S, Takeda K 2000 Comparison of conjugative activity, conversion of bisphenol A to bisphenol A glucuronide, in fetal and mature male rat. J Health Sci 46:269–274 [Google Scholar]
- Takahashi O, Oishi S 2000 Disposition of orally administered 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in pregnant rats and placental transfer to fetuses. Environ Health Perspect 108:931–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto J, Yokota H, Yuasa A 2002 Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect 110:193–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I 2002 Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect 110:A703–A707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi H, Betsui H, Ohno Y 2003 Disposition of a low dose of 14C-bisphenol A in male rats and its main bilary excretion as BPA glucuronide. Toxicol Sci 73:17–25 [DOI] [PubMed] [Google Scholar]
- 2008 Bisphenol A—public comments. http://cerhr.niehs.nih.gov/chemicals/bisphenol/pubcomm-bisphenol.html [Google Scholar]
- Tominaga T, Negishi T, Hirooka H, Miyachi A, Inoue A, Hayasaka I, Yoshikawa Y 2006 Toxicokinetics of bisphenol A in rats, monkeys and chimpanzees by the LC-MS/MS method. Toxicology 226:208–217 [DOI] [PubMed] [Google Scholar]
- Uchida K, Suzuki A, Kobayashi Y, Buchanan DL, Sato T, Watanabe H, Katsu Y, Suzuki J, Asaoka K, Mori C, Arizono K, Iguchi T 2002 Bisphenol-A administration during pregnancy results in fetal exposure in mice and monkeys. J Health Sci 48:579–582 [Google Scholar]
- Volkel W, Colnot T, Csanady GA, Filsner JG, Dekant W 2002 Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol 15:1281–1287 [DOI] [PubMed] [Google Scholar]
- Volkel W, Bittner N, Dekant W 2005 Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug Metab Dispos 33:1748–1757 [DOI] [PubMed] [Google Scholar]
- Soto AM, Fernandez MF, Luizzi MF, Oles Karasko AS, Sonnenschein C 1997 Developing a marker of exposure to xenoestrogen mixtures in human serum. Environ Health Perspect 105:647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C 2005 An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 113:926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM 2006 Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology 147:3681–3691 [DOI] [PubMed] [Google Scholar]
- Kawai K, Nozaki T, Nishikata H, Aou S, Takii M, Kubo C 2003 Aggressive behavior and serum testosterone concentration during the maturation process of male mice: the effects of fetal exposure to bisphenol A. Environ Health Perspect 111:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessi-Fulgheri F 2002 Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect 110:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Porrini S, Farabollini F 2002 Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect 110(Suppl):403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrini S, Belloni V, Della Seta D, Farabollini F, Giannelli G, Dessi-Fulgheri F 2005 Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull 65:261–266 [DOI] [PubMed] [Google Scholar]
- Palanza P, Howdeshell KL, Parmigiani S, vom Saal FS 2002 Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect 110:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Gioiosa L, Adriania W, Palanza P 2005 D-Amphetamine-related reinforcing effects are reduced in mice exposed prenatally to estrogenic endocrine disruptors. Brain Res Bull 65:235–240 [DOI] [PubMed] [Google Scholar]
- Wallen K, Baum MJ 2002 Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, eds. Hormones, brain and behavior. San Diego: Academic Press; 385–423 [Google Scholar]
- Heines M 2002 Sexual differentiation of human brain and behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, eds. Hormones, brain and behavior. San Diego: Academic Press; 425–485 [Google Scholar]
- Kabuto H, Amakawa M, Shishibori T 2004 Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci 74:2931–2940 [DOI] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, Thayer KA, Judy BM, vom Saal FS 1999 Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicol Ind Health 15:12–25 [DOI] [PubMed] [Google Scholar]
- Ramos JG, Varayoud J, Sonnenschein C, Soto AM, Munoz-de-Toro M, Luque EH 2001 Prenatal exposure to low doses of bisphenol A alters the periductal stroma and glandular cell function in the rat ventral prostate. Biol Reprod 65:1271–1277 [DOI] [PubMed] [Google Scholar]
- Markey CM, Coombs MA, Sonnenschein C, Soto AM 2003 Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev 5:67–75 [DOI] [PubMed] [Google Scholar]
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA 2007 Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genetics 3:e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Luque EH, Munoz-de-Toro M, Sonnenschein C, Soto AM 2001 In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod 65:1215–1223 [DOI] [PubMed] [Google Scholar]
- Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM 2005 Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology 146:4138–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadia PR, Vandenberg LN, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM 2007 Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ Health Perspect 115:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, Rubin BS, Soto AM 2008 Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol 26:210–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S, Yamaki K, Li X, Sai T, Yanagisawa R, Takano H, Taneda S, Hayashi H, Mori Y 2004 Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology 112:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Richter CA, Mao J, Welshons WV 2005 Commercial animal feed: variability in estrogenic activity and effects on body weight in mice. Birth Defects Res (Part A) 73:474–475 [DOI] [PubMed] [Google Scholar]
- Heindel JJ, vom Saal FS 2008 Meeting report: batch-to-batch variability in estrogenic activity in commercial animal diets—importance and approaches for laboratory animal research. Environ Health Perspect 116:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Yano T, Taketan Y 2001 Preimplantation exposure to bisphenol A advances postnatal development. Reprod Toxicol 15:71–74 [DOI] [PubMed] [Google Scholar]
- Soto AM, Rubin BS, Sonnenschein C 2007 Endocrine disruption and the female. In: Gore A, ed. Endocrine-disrupting chemicals. Totowa, NJ: Humana Press [Google Scholar]
- Clarke R 2000 Introduction and overview: sex steroids in the mammary gland. J Mammary Gland Biol Neoplasia 5:245–250 [DOI] [PubMed] [Google Scholar]
- Sakakura T, Nishizuka Y, Dawe CJ 1976 Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science 194:1439–1441 [DOI] [PubMed] [Google Scholar]
- Sugiura-Ogaswara M, Ozaki Y, Sonta S-I, Makino T, Suzumori K 2005 Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod 20:2325–2329 [DOI] [PubMed] [Google Scholar]
- Huff J 2001 Carcinogenicity of bisphenol-A in Fischer rats and B6C3F1 mice. Odontology 89:12–20 [DOI] [PubMed] [Google Scholar]
- Ho S-M, Tang WY, Belmonte de Frausto J, Prins GS 2006 Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66:5624–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Russo IH 1978 DNA labeling index and structure of the rat mammary gland as determinants of its susceptibility to carcinogenesis. J Natl Cancer Inst 61:1451–1459 [PubMed] [Google Scholar]
- McCormack VA, Dos Santos Silva I 2006 Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidem Biomar 15:1159–1169 [DOI] [PubMed] [Google Scholar]
- Nandi S, Guzman R, Yang J 1995 Hormones and mammary carcinogenesis in mice, rats, and humans: a unifying hypothesis. Proc Natl Acad Sci USA 92:3650–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM 2007 Induction of mammary gland ductal hyperplasias and carcinomas in situ following fetal bisphenol A exposure. Reprod Toxicol 23:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Munoz-de-Toro M 2007 Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect 115:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPensee EW, Tuttle TR, Fox SR, Ben-Jonathan N 8 October 2008 Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor α and negative breast cancer cells. Environ Health Perspect 10.1289/ehp.11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike MC, Spicer DV, Dahmoush L, Press MF 1993 Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev 15:17–35 [DOI] [PubMed] [Google Scholar]
- Ekbom A, Trichopoulos D, Adami HO, Hsieh CC, Lan SJ 1992 Evidence of prenatal influences on breast cancer risk. Lancet 340:1015–1018 [DOI] [PubMed] [Google Scholar]
- Bisphenol A. http://www.bisphenol-a.org [Google Scholar]
- 2008 Draft NTP brief on Bisphenol A. http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPADraftBriefVF_04_14_08.pdf [Google Scholar]
- 2008 NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. http://cerhr.niehs. nih.gov/chemicals/bisphenol/bisphenol.pdf [PubMed] [Google Scholar]
- Bisphenol A (BPA). http://www.fda.gov/oc/opacom/hottopics/bpa.html [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, Joiner RL, Butula JH, Dimond SS, Cagen SZ, Shitsuka RN, Stropp GD, Waechter JM 2002 Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci 68:121–146 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Welshons WV 2006 Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ Res 100:50–76 [DOI] [PubMed] [Google Scholar]
- Myers JP, vom Saal FS, Akingbemi BT, Arizono K, Belcher S, Colborn T, Chahoud I, Crain DA, Farabollini F, Guillette LJ, Hassold T, Ho S-M, Hunt PA, Iguchi T, Jobling S, Kanno J, Laufer H, Marcus M, McLachlan JA, Nadal A, Oehlmann J, Olea N, Palanza P, Parmigiani S, Rubin BS, Schonfelder G, Sonnenschein C, Soto AM, Talsness CE, Taylor JA, Vandenberg LN, Vandenbergh JG, Vogel S, Watson CS, Welshons WV, Zoeller RT 22 October 2008 Why public health agencies cannot depend upon ‘Good Laboratory Practices’ as a criterion for selecting data: the case of bisphenol-A. Environ Health Perspect 10.1289/ehp.0800173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2008 FDA Science Board Subcommittee on Bisphenol A: scientific peer-review of the draft assessment of bisphenol A for use in food contact applications. http://www.fda.gov/OHRMS/DOCKETS/ac/08/briefing/2008–4386b1–05.pdf [Google Scholar]
- 2008 Andy’s take: bisphenol A. http://www.fda.gov/oc/ vonEschenbach/andys_take/andys_take_103108.pdf [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ 2008 Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci USA 105:14187–14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C 2005 The environmental estrogen bisphenol A inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect 113:675–679 [DOI] [PMC free article] [PubMed] [Google Scholar]