Abstract
Background: Metabolic abnormalities and targeted treatment trials have been reported for several neurobehavioral disorders but are relatively understudied in autism.
Objective: The objective of this study was to determine whether or not treatment with the metabolic precursors, methylcobalamin and folinic acid, would improve plasma concentrations of transmethylation/transsulfuration metabolites and glutathione redox status in autistic children.
Design: In an open-label trial, 40 autistic children were treated with 75 μg/kg methylcobalamin (2 times/wk) and 400 μg folinic acid (2 times/d) for 3 mo. Metabolites in the transmethylation/transsulfuration pathway were measured before and after treatment and compared with values measured in age-matched control children.
Results: The results indicated that pretreatment metabolite concentrations in autistic children were significantly different from values in the control children. The 3-mo intervention resulted in significant increases in cysteine, cysteinylglycine, and glutathione concentrations (P < 0.001). The oxidized disulfide form of glutathione was decreased and the glutathione redox ratio increased after treatment (P < 0.008). Although mean metabolite concentrations were improved significantly after intervention, they remained below those in unaffected control children.
Conclusion: The significant improvements observed in transmethylation metabolites and glutathione redox status after treatment suggest that targeted nutritional intervention with methylcobalamin and folinic acid may be of clinical benefit in some children who have autism. This trial was registered at clinicaltrials.gov as NCT00692315.
INTRODUCTION
Autism is a behaviorally defined neurodevelopmental disorder characterized by significant impairment in reciprocal social interaction and communication as well as restricted interests and repetitive behaviors. The metabolic pathology of autism has been less explored than broad-scale genomic approaches despite metabolic abnormalities implicated in the pathogenesis of many other neurologic disorders (1–5). We have used a targeted approach to autism metabolomics by focusing on the dynamics of an integrated metabolic pathway that is important for the regulation of normal redox homeostasis and cellular methylation potential. In a recent case-control study, we reported that the metabolic profile of children diagnosed with autism was abnormal compared with that of unaffected control children (6, 7). Briefly, the mean ratio of plasma S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) was reduced significantly, and the mean concentration of reduced glutathione (GSH), the major intracellular antioxidant and mechanism for detoxification, also was decreased significantly. The oxidized disulfide form of GSH (GSSG) was increased significantly, which resulted in a 2-fold reduction in the mean GSH:GSSG redox ratio. Several metabolic precursors for GSH synthesis also were lower in the autistic children, suggesting that GSH synthesis may be insufficient. These new findings are of clinical relevance because they suggest a significant decrease in methylation capacity (↓SAM:SAH) and antioxidant/detoxification capacity (↓GSH:GSSG) and an increase in oxidative stress (↑GSSG) in autistic children.
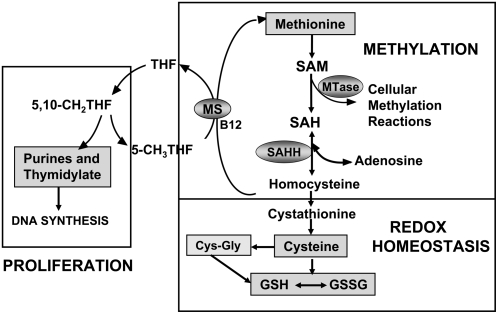
A diagram of the 3 interconnected pathways of folate, methionine, and GSH metabolism that are abnormal in many autistic children is shown in Figure 1. The metabolic interdependency between these pathways translates into broader impact on 1) DNA synthesis or repair and proliferation, 2) cellular methylation, and 3) GSH redox homeostasis, as indicated in Figure 1. GSH is a tripeptide of cysteine, glycine, and glutamate that is synthesized de novo in all cells and serves as the major intracellular antioxidant and redox buffer. The intracellular GSH-GSSG redox status provides the essential intracellular reducing environment required for normal immune function, detoxification capacity, redox-sensitive enzyme activity, and membrane redox signaling (8–12). Oxidative stress occurs when antioxidant defense mechanisms fail to counterbalance and control reactive oxygen species generated from endogenous oxidative metabolism or from pro-oxidant environmental exposures. Several recent reviews and research studies lend support to the hypothesis that redox imbalance and oxidative stress may be a contributing factor to autism pathology (6, 7, 13–19).
FIGURE 1.
Diagram of tetrahydrofolate (THF)-dependent pathway of methionine transmethylation to homocysteine and the transsulfuration pathway from homocysteine to GSH synthesis. MS, methionine synthase; SAH, S-adenosylhomocysteine; SAHH, SAH hydrolase; GSSG, oxidized glutathione disulfide; SAM, S-adenyosylmethionine; MTase, methyltransferase.
The investigation was prompted by the observed decrease in GSH redox status and methylation capacity in many autistic children and by the plausible potential for micronutrient deficiencies in these children that result from inadequate dietary intake or gastrointestinal pathology (20–22). The primary goal of this study was to show whether or not targeted nutritional intervention designed to provide cofactors for methionine remethylation and GSH synthesis would be effective in improving methylation capacity and redox status in a cohort of autistic children.
SUBJECTS AND METHODS
Participants
Forty children who had an autistic disorder completed an open-label trial to test whether or not supplementation with the metabolic cofactors, methylcobalamin and folinic acid, for 3 mo would improve plasma concentrations of transmethylation metabolites or the synthesis of GSH. Inclusion criteria included a diagnosis of autistic disorder defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria (299.0) and a Childhood Autism Rating Scale score >30. Exclusion criteria included Asperger's disorder; pervasive developmental disorder, not otherwise specified; genetic disorders with comorbid autism; chronic seizures; severe gastrointestinal symptoms; recent infection; and use of high-dose vitamin or mineral supplements.
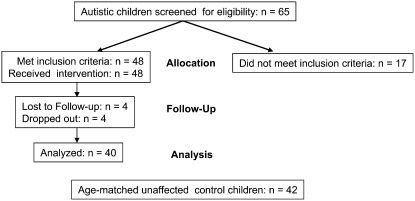
A flow diagram of the intervention study design is presented in Figure 2. Sixty-five autistic children met the inclusion criteria and were initially screened for metabolic evidence of reduced methylation capacity (SAM:SAH) or reduced GSH redox ratio (GSH:GSSG). Of these, 48 (75%) children met these metabolic qualifications and 17 (25%) were excluded because their baseline metabolic profile was within normal range. Four children dropped out during the study and another 4 children were lost to follow up. Of the 40 remaining children who completed the study, 87% were white, 8% were African American, and 5% were Asian. There were 33 boys (82%) and 7 girls (18%) ranging in age from 2 to 7 y (mean age: 4.8 ± 0.8 y). The control group for the baseline metabolite concentrations consisted of 42 apparently healthy age-matched children who had no previous history of developmental delay or neurologic symptoms (mean age: 4.5 ± 0.9 y). All parents signed informed consent approved by the institutional review board at the University of Arkansas for Medical Sciences and were instructed not to introduce any new interventions during the treatment period.
FIGURE 2.
Study design and patient follow-up.
Nutritional supplements
Methylcobalamin was obtained as a sterile injectable liquid from Hopewell Pharmacy and Compounding Center (Hopewell, NJ). The subcutaneous injectable route of administration was selected on the basis of empiric observations of clinical improvement in speech and cognition in a recent pilot study (6) and the possibility that it might enhance methionine synthase activity under conditions of oxidative stress by substituting for oxidized inactive coenzyme B12. Tuberculin syringes fitted with a 0.25-inch 31-gauge needle were prefilled with 75 μg/kg methylcobalamin and prepared individually on the basis of each child's weight in kilograms. Parents were given a demonstration and instructions for the sterile subcutaneous injection of methylcobalamin in the fatty tissue of the buttocks, which was given every third day for 3 mo. Folinic acid (400 μg) obtained from Custom Compounding Pharmacy (Little Rock, AR) was administered orally twice each day as a powder mixed with a convenient food. Folinic acid (5-formyl tetrahydrofolate) is absorbed as the reduced metabolite, is rapidly polyglutamated, and is more readily available for folate-dependent reactions than folic acid.
Sample treatment and HPLC method for metabolite analysis
Fasting blood samples were collected into EDTA-evacuated tubes and immediately chilled on ice before centrifuging at 4000 × g for 10 min at 4°C. To prevent metabolite interconversion, the ice-cold samples were centrifuged within 15 min of the blood collection and the plasma stored at −80°C until HPLC quantification ≤2 wk after receipt. Details of the method for HPLC with electrochemical detection and metabolite quantitation are described elsewhere (23, 24).
Statistics
Metabolic data are presented as the means ± SD. The data were prospectively collected and analyzed using SigmaStat (version 2.0) and Excel software (Microsoft Office 2003; Microsoft Corp, Redmond, WA). Statistical differences between plasma metabolites before and after intervention were determined using the paired Student's t test with significance set at 0.05. Comparisons between case and control children were done using the Student's t test.
RESULTS
Participants
Of the 48 children who qualified metabolically for the study, 40 children completed the 3-mo intervention trial with methylcobalamin and folinic acid. Two children dropped out of the study because the parents were uncomfortable giving the methylcobalamin injections and 2 children dropped out because of hyperactivity and reduced sleep. Four families moved during the study or were lost to follow-up after the baseline visit. Most parents (80%) reported no increase in hyperactivity, although 10% (4 children) reported moderate hyperactivity that was reduced when the dose of folinic acid was decreased to 400 μg/d. Other potential adverse effects (one child each) included sleep disruption, difficulty getting to sleep, increased impulsiveness, and irritability. On completion of the study, 78% of the parents expressed the desire to continue independently with the supplements, one parent chose not to continue, and 8 parents did not provide this information.
Metabolic profile
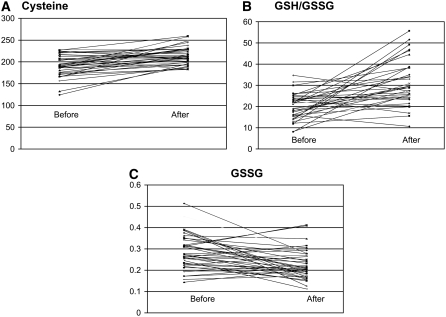
The primary outcome measure for this open-label trial was impact of the intervention on the pretreatment baseline metabolic profile. Methionine transmethylation metabolites all were significantly different from those in the control children (P < 0.005) at baseline with the exceptions of homocysteine and SAH (Table 1). The 3-mo intervention did not alter methionine, SAM, and SAH concentrations significantly even though methylcobalamin and folinic acid provide methyl groups for the methionine cycle. In contrast, mean concentrations of cysteine and cysteinylglycine were increased significantly after the intervention and no longer were statistically different compared with those in the healthy control children. Although total and free GSH concentrations and the GSH:GSSG redox ratios were increased significantly by the intervention, they remained below those in control subjects (P < 0.01). The mean concentration of oxidized GSSG was decreased significantly after intervention (P < 0.001) but remained above the mean concentration in the control children (P < 0.01). In Figure 3, scatterplots showing the distribution of the individual data points before and after intervention are presented for cysteine, GSSG, and GSH:GSSG ratio (Figure 3A).
TABLE 1.
Mean plasma metabolite concentrations (±SD) in age-matched control children, children who had autism at baseline before intervention, and children with autism after 3-mo intervention with methylcobalamin and folinic acid1
| Children with autism |
||||
| Plasma metabolite concentration | Control children (n = 42) | Pretreatment (n = 40) | Posttreatment (n = 40) | P value2 |
| Methionine | 24 ± 3 | 21 ± 43 | 22 ± 34 | NS |
| SAM (nmol/L) | 78 ± 22 | 66 ± 133 | 69 ± 124 | NS |
| SAH (nmol/L) | 14.3 ± 4.3 | 15.2 ± 5 | 14.8 ± 4 | NS |
| SAM:SAH (μmol/L) | 5.6 ± 2.0 | 4.7 ± 1.53 | 5.0 ± 2.0 | NS |
| Homocysteine (μmol/L) | 5.0 ± 1.2 | 4.8 ± 1.8 | 5.3 ± 1.1 | 0.04 |
| Cysteine (μmol/L) | 210 ± 18 | 191 ± 243 | 215 ± 19 | 0.001 |
| Cysteinylglycine (μmol/L) | 45 ± 6 | 40 ± 93 | 46 ± 9 | 0.002 |
| tGSH (μmol/L) | 7.5 ± 1.8 | 5.4 ± 1.33 | 6.2 ± 1.24 | 0.001 |
| fGSH (μmol/L) | 2.8 ± 0.8 | 1.5 ± 0.43 | 1.8 ± 0.44 | 0.008 |
| GSSG (μmol/L) | 0.18 ± 0.07 | 0.28 ± 0.083 | 0.22 ± 0.064 | 0.001 |
| tGSH:GSSG | 47 ± 18 | 21 ± 63 | 30 ± 94 | 0.001 |
| fGSH:GSSG | 17 ± 6.8 | 6 ± 23 | 9 ± 34 | 0.001 |
fGSH, free glutathione; SAH, S-adenosylhomocsyteine; SAM, S-adenosylmethionine; tGSH, total glutathione; GSSG, oxidized glutathione disulfide. NS, P > 0.05.
Pre- and posttreatment comparison.
Significantly different from control children, P < 0.005.
Significantly different from control children, P < 0.01.
FIGURE 3.
Scatterplots of individual data for plasma cysteine (A), GSH:GSSG ratios (B), and GSSG concentrations (C) from 40 autistic children before and after 3-mo treatment with methylcobalamin and folinic acid. GSH, glutathione; GSSG, oxidized disulfide form of glutathione.
DISCUSSION
This intervention trial was undertaken to determine whether or not treatment with metabolic precursors for methionine and GSH synthesis would improve plasma biomarkers of impaired methylation capacity (SAM:SAH) and GSH-dependent antioxidant-detoxification status (GSH:GSSG) in children who have autistic disorder. Children meeting the entrance criteria were initially screened for metabolic evidence of reduced SAM:SAH or reduced GSH:GSSG as an indication of potential benefit from the nutritional intervention. Measures of autistic behavior were assessed by a trained study nurse before and after treatment using the Vineland Adaptive Behavior Scales. Although significant improvement was observed after treatment, the scores remained significantly below standard normal scores. Because parent report in an open-label trial is subject to expectation bias, these results are not conclusive and therefore not presented. Nonetheless, the improvement in Vineland Adaptive Behavior Scale scores provided preliminary evidence for a double-blind placebo-controlled crossover study that currently is underway.
The 3-mo treatment was successful in increasing mean concentrations of the transsulfuration metabolites, cysteine, cysteinylglycine, and GSH, while reducing the concentration of the oxidized disulfide GSSG. However, plasma concentrations of methionine and SAM were unaffected and remained significantly below those in the control subjects (P < 0.005). This was an unexpected finding because methylcobalamin and folinic acid directly and indirectly provide methyl groups for synthesis of methionine and SAM and secondarily provide metabolic precursors for more distant transsulfuration reactions (Figure 1).
Because supplements improved but did not normalize methionine, SAM and GSH concentrations may reflect ongoing metabolic adaptation to incompletely resolved oxidative stress. Within the methionine cycle, methionine synthase, betaine-homocysteine methyltransferase, and methionine adenosyltransferase are redox-sensitive enzymes that tend to be down-regulated with oxidative stress (25–28). The reciprocal regulation of transmethylation and transsulfuration under pro-oxidant conditions serves to promote GSH synthesis as the metabolic priority at the expense of methionine transmethylation. Adaptive up-regulation of cystathionine beta synthase (CBS) activity promotes cysteine and GSH synthesis by irreversibly diverting homocysteine away from methionine remethylation and down the transsulfuration pathway (29). The heme component of CBS is believed to serve as a redox sensor that increases enzyme activity with oxidation to the ferric state and truncation of the enzyme protein (30). Acutely, this metabolic adaptation provides a mechanism to restore GSH concentrations and to maintain intracellular redox status during oxidative stress. With chronic oxidative stress, however, the synthesis of methionine and its product, SAM, can progressively decline as a result of oxidative inactivation of the cobalamin cofactor of methionine synthase (31). In this way, unresolved oxidative stress can promote precursor depletion and a progressive decrease in cysteine and GSH synthesis. Treatment with methylcobalamin and folinic acid seems to have rescued GSH synthesis in this cohort of children at the expense of transmethylation metabolites.
Plasma metabolite concentrations provide only a static cross-sectional view of a highly dynamic homeostatic process that is constantly responding to the changing functional needs and metabolic priorities of the body (32). Thus, to obtain accurate, reproducible, and comparative results, it is imperative that sampling be done in the fasting state at the same time of day and that potential confounding factors, such as acute infection and seizures, are excluded (33). Because the complete transsulfuration of methionine to GSH occurs primarily in the liver, plasma concentrations of cysteine and GSH generally reflect hepatic synthesis and export (9, 34). Approximately 80% of GSH synthesized in the liver is exported to the plasma where it is hydrolyzed to cysteinylglycine and cysteine for uptake by tissues, such as the brain, that lack or weakly express the complete transsulfuration pathway (35). Thus, viral infection, nutritional deficiencies, impaired detoxification, and other factors that negatively affect GSH synthesis in the liver can indirectly affect peripheral redox status in brain and immune cells that require cyst(e)ine import from the liver to complete transsulfuration and GSH synthesis. Cysteine generally is considered a semiessential amino acid because it can be synthesized from methionine via the transsulfuration pathway. The consistent decrease in plasma methionine and cysteine concentrations observed in autistic children suggests that cysteine may be an essential amino acid for these children.
If increased vulnerability to pro-oxidant exposures and decreased GSH-dependent antioxidant capacity is a constitutive feature of autism, then autistic children should exhibit systemic evidence of oxidative stress. In addition to evidence of oxidative stress in immune cells (36), several clinical studies documented an increased prevalence of gastrointestinal inflammation and increased mucosal permeability in the upper and lower intestines in autistic children (21, 22, 37). A recent report documented the presence of chronic inflammation in the autistic brain that seems mediated by innate microglial activation and proinflammatory cytokines (18, 38). The inflammatory response is augmented when GSH concentrations are low, and chronic inflammation depletes GSH further and promotes a self-perpetuating cycle that could exacerbate gastrointestinal and central nervous system inflammation associated with autism. These reports and the results reported herein support the hypothesis that unresolved oxidative stress may contribute to the clinical pathology of autism.
The prevalence of nutritional intervention and complementary and alternative medicine (CAM) in children diagnosed with an autism spectrum disorder is estimated at ≈74% (39). Parents report that most nutritional and CAM-associated treatments are helpful or without effect but not harmful. The main reasons parents cite for choosing to use alternative therapies are concerns about the safety and side effects of available medications for autism and a need to be involved in decisions involving care of their child (40). Because the majority of CAM use is based on anecdotal evidence, there is a need for clinical trials to evaluate the efficacy of these treatments. The broad heterogeneity of clinical and behavioral symptoms in autistic children predicts that no single treatment will benefit every autistic child. Thus, definition and characterization of subgroups of children who respond positively or negatively to intervention are necessary to identify more clearly those children most likely to benefit from a given treatment or medication. In summary, the significant improvement in GSH-mediated redox status in some children who have autism provides evidence consistent with a moderate beneficial effect after 3-mo treatment with methylcobalamin and folinic acid.
Acknowledgments
We thank the Arkansas families affected by autism whose participation made this study possible. We also thank the pediatricians and nurses at the University of Arkansas for Medical Sciences Dennis Developmental Center for patient referrals and Hopewell Pharmacy and Compounding Center (Hopewell, NJ) for their generous donation of methylcobalamin for the study.
The authors' responsibilities were as follows—SJJ (principal investigator): conducted the study, interpreted the data, and wrote the manuscript; SM (laboratory director): provided HPLC expertise for metabolite analysis; GF and TR: provided clinical advice, assisted with the interpretation of data, and provided critical review of the manuscript; SJ: provided technical assistance and served as study coordinator; OP: provided technical assistance for the HPLC analysis; AH (study nurse): recruited patients and administered the Vineland behavior testing; and DWG: provided statistical support for data analysis. None of the authors had a conflict of interest to report.
REFERENCES
- 1.Miller AL. The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev 2003;8:7–19 [PubMed] [Google Scholar]
- 2.Muntjewerff JW, Van der Put N, Eskes T, et al. Homocysteine metabolism and B-vitamins in schizophrenic patients: low plasma folate as a possible independent risk factor for schizophrenia. Psychiatry Res 2003;121:1–9 [DOI] [PubMed] [Google Scholar]
- 3.Giordano V, Peluso G, Iannuccelli M, Benatti P, Nicolai R, Calvani M. Systemic and brain metabolic dysfunction as a new paradigm for approaching Alzheimer's dementia. Neurochem Res 2007;32:555–67 [DOI] [PubMed] [Google Scholar]
- 4.Serra JA, Dominguez RO, de Lustig ES, et al. Parkinson's disease is associated with oxidative stress: comparison of peripheral antioxidant profiles in living Parkinson's, Alzheimer's and vascular dementia patients. J Neural Transm 2001;108:1135–48 [DOI] [PubMed] [Google Scholar]
- 5.Pennington K, Beasley CL, Dicker P, et al. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry (Epub ahead of print 2007) [DOI] [PubMed] [Google Scholar]
- 6.James SJ, Cutler P, Melnyk S, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 2004;80:1611–7 [DOI] [PubMed] [Google Scholar]
- 7.James SJ, Melnyk S, Jernigan S, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet 2006;141:947–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Droge W, Breitkreutz R. Glutathione and immune function. Proc Nutr Soc 2000;59:595–600 [DOI] [PubMed] [Google Scholar]
- 9.Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 2003;333:19–39 [DOI] [PubMed] [Google Scholar]
- 10.Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol 2006;71:551–64 [DOI] [PubMed] [Google Scholar]
- 11.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001;30:1191–212 [DOI] [PubMed] [Google Scholar]
- 12.Reid M, Jahoor F. Glutathione in disease. Curr Opin Clin Nutr Metab Care 2001;4:65–71 [DOI] [PubMed] [Google Scholar]
- 13.Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology 2006;13:171–81 [DOI] [PubMed] [Google Scholar]
- 14.Kern JK, Jones AM. Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B Crit Rev 2006;9:485–99 [DOI] [PubMed] [Google Scholar]
- 15.Yorbik O, Sayal A, Akay C, Akbiyik DI, Sohmen T. Investigation of antioxidant enzymes in children with autistic disorder. Prostaglandins Leukot Essent Fatty Acids 2002;67:341–3 [DOI] [PubMed] [Google Scholar]
- 16.Zoroglu SS, Armutcu F, Ozen S, et al. Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. Eur Arch Psychiatry Clin Neurosci 2004;254:143–7 [DOI] [PubMed] [Google Scholar]
- 17.Zoroglu SS, Yurekli M, Meram I, et al. Pathophysiological role of nitric oxide and adrenomedullin in autism. Cell Biochem Funct 2003;21:55–60 [DOI] [PubMed] [Google Scholar]
- 18.Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry 2005;17:485–95 [DOI] [PubMed] [Google Scholar]
- 19.Ming X, Stein TP, Brimacombe M, Johnson WG, Lambert GH, Wagner GC. Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukot Essent Fatty Acids 2005;73:379–84 [DOI] [PubMed] [Google Scholar]
- 20.Arnold GL, Hyman SL, Mooney RA, Kirby RS. Plasma amino acids profiles in children with autism: potential risk of nutritional deficiencies. J Autism Dev Disord 2003;33:449–54 [DOI] [PubMed] [Google Scholar]
- 21.Molloy CA, Manning-Court P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism 2003;7:165–71 [DOI] [PubMed] [Google Scholar]
- 22.Horvath K, Perman JA. Autism and gastrointestinal symptoms. Curr Gastroenterol Rep 2002;4:251–8 [DOI] [PubMed] [Google Scholar]
- 23.Melnyk S, Pogribna M, Pogribny I, Hine RJ, James SJ. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J Nutr Biochem 1999;10:490–7 [DOI] [PubMed] [Google Scholar]
- 24.Melnyk S, Pogribna M, Pogribny IP, Yi P, James SJ. Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection: alterations with plasma homocysteine and pyridoxal 5′-phosphate concentrations. Clin Chem 2000;46:265–72 [PubMed] [Google Scholar]
- 25.Olteanu H, Banerjee R. Redundancy in the pathway for redox regulation of mammalian methionine synthase—reductive activation by the dual flavoprotein, novel reductase 1. J Biol Chem 2003;278:38310–4 [DOI] [PubMed] [Google Scholar]
- 26.Castro C, Millian NS, Garrow TA. Liver betaine-homocysteine S-methyltransferase activity undergoes a redox switch at the active site zinc. Arch Biochem Biophys 2008;472:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 2000;39:13005–11 [DOI] [PubMed] [Google Scholar]
- 28.Avila MA, Carretero MV, Rodriguez EN, Mato JM. Regulation by hypoxia of methionine adenosyltransferase activity and gene expression in rat hepatocytes. Gastroenterology 1998;114:364–71 [DOI] [PubMed] [Google Scholar]
- 29.Prudova A, Bauman Z, Braun A, Vitvitsky V, Lu SC, Banerjee R. S-adenosylmethionine stabilizes cystathionine beta-synthase and modulates redox capacity. Proc Natl Acad Sci USA 2006;103:6489–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee R, Zou CG. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: a PLP-dependent hemesensor protein. Arch Biochem Biophys 2005;433:144–56 [DOI] [PubMed] [Google Scholar]
- 31.Zou CG, Banerjee R. Homocysteine and redox signaling. Antioxid Redox Signal 2005;7:547–59 [DOI] [PubMed] [Google Scholar]
- 32.Reed MC, Nijhout HF, Neuhouser ML, et al. A mathematical model gives insights into nutritional and genetic aspects of folate-mediated one-carbon metabolism. J Nutr 2006;136:2653–61 [DOI] [PubMed] [Google Scholar]
- 33.Liang LP, Patel M. Seizure-induced changes in mitochondrial redox status. Free Radic Biol Med 2006;40:316–22 [DOI] [PubMed] [Google Scholar]
- 34.Meister A. Metabolism and function of glutathione: an overview. Biochem Soc Trans 1982;10:78–9 [DOI] [PubMed] [Google Scholar]
- 35.Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol 1999;73:209–67 [DOI] [PubMed] [Google Scholar]
- 36.Jyonouchi H, Sun SN, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology 2002;46:76–84 [DOI] [PubMed] [Google Scholar]
- 37.White JF. Intestinal pathophysiology in autism. Exp Biol Med (Maywood) 2003;228:639–49 [DOI] [PubMed] [Google Scholar]
- 38.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2005;57:67–81 [DOI] [PubMed] [Google Scholar]
- 39.Hanson E, Kalish LA, Bunce E, et al. Use of complementary and alternative medicine among children diagnosed with autism spectrum disorder. J Autism Dev Disord 2007;37:628–36 [DOI] [PubMed] [Google Scholar]
- 40.Hyman SL, Levy SE. Introduction: novel therapies in developmental disabilities: hope, reason, and evidence. Ment Retard Dev Disabil Res Rev 2005;11:107–9 [DOI] [PubMed] [Google Scholar]