Abstract
Background: Hypertriglyceridemia is a risk factor for cardiovascular disease. Variation in the apolipoprotein A5 (APOA5) and glucokinase regulatory protein (GCKR) genes has been associated with fasting plasma triacylglycerol.
Objective: We investigated the combined effects of the GCKR rs780094C→T, APOA5 −1131T→C, and APOA5 56C→G single nucleotide polymorphisms (SNPs) on fasting triacylglycerol in several independent populations and the response to a high-fat meal and fenofibrate interventions.
Design: We used a cross-sectional design to investigate the association with fasting triacylglycerol in 8 populations from America, Asia, and Europe (n = 7730 men and women) and 2 intervention studies in US whites (n = 1061) to examine postprandial triacylglycerol after a high-fat meal and the response to fenofibrate. We defined 3 combined genotype groups: 1) protective (homozygous for the wild-type allele for all 3 SNPs); 2) intermediate (any mixed genotype not included in groups 1 and 3); and 3) risk (carriers of the variant alleles at both genes).
Results: Subjects within the risk group had significantly higher fasting triacylglycerol and a higher prevalence of hypertriglyceridemia than did subjects in the protective group across all populations. Moreover, subjects in the risk group had a greater postprandial triacylglycerol response to a high-fat meal and greater fenofibrate-induced reduction of fasting triacylglycerol than did the other groups, especially among persons with hypertriglyceridemia. Subjects with the intermediate genotype had intermediate values (P for trend <0.001).
Conclusions: SNPs in GCKR and APOA5 have an additive effect on both fasting and postprandial triacylglycerol and contribute to the interindividual variability in response to fenofibrate treatment.
INTRODUCTION
Accumulating evidence suggests that elevated plasma triacylglycerol concentrations, in both the fasting and the postprandial states, may pose a significant independent risk for cardiovascular disease (CVD) (1). Both fasting and postprandial lipoprotein concentrations vary substantially among individuals, and this interindividual variability is driven by a combination of nongenetic and genetic factors (2). Specific knowledge of these factors is key for developing dietary and pharmacologic approaches to CVD prevention and treatment (3).
Regarding the genetic component, multiple candidate genes have been shown to have significant associations with both fasting and postprandial plasma triacylglycerol concentrations (4); however, the level of replication of such associations has been very low, consistent with those observed for other common traits (5). In addition, the percentage of the variability explained by a few loci showing consistent associations is rather small (4), which supports the need to identify additional genes implicated in the regulation of fasting and postprandial lipoprotein metabolism and, potentially, CVD risk.
Along these lines, a new candidate gene for triacylglycerol metabolism recently emerged from the results of a genome-wide association study carried out on 2 independent populations from northern Europe (6). This study showed that a common genetic variant single nucleotide polymorphism (SNP) rs780094, within intron 16] at the glucokinase regulatory protein (GCKR) locus (2p23) was significantly and strongly associated with fasting triacylglycerol concentrations.
In liver and pancreatic islet cells, GCKR regulates glucokinase, which functions as a glucose sensor responsible for glucose phosphorylation in the first step of glycolysis. The mechanism responsible for the association with plasma triacylglycerols has not yet defined in humans; however, in a murine experimental model, adenoviral-mediated overexpression of GCKR in liver increased glucokinase activity, which led to lowered blood glucose and increased triacylglycerol concentrations (7–9).
On the basis of the current evidence, we hypothesized that genetic variation affecting the activity or expression of GCKR in humans would be associated with both fasting and postprandial triacylglycerol concentrations. Moreover, we proposed that genetic variation at the GCKR locus would have an additive or synergistic effect with genetic variation at the gene encoding apolipoprotein A5 (APOA5), another candidate for plasma triacylglycerol concentrations (10). Therefore, our primary aim was to examine these effects by analyzing the contribution of the combination of GCKR rs780094, APOA5 −1131T→C, and APOA5 56C→G SNPs on fasting triacylglycerol concentrations in 6 independent cohorts including ethnically diverse populations from America, Asia, and Europe. Moreover, we examined the effect of the combined genotypes on postprandial lipoprotein metabolism and their responses to fenofibrate treatment (11) in US non-Hispanic whites participating in one of the American cohorts.
SUBJECTS AND METHODS
Study participants
The genetic association studies were performed in 6 independent cohorts consisting of 8 different populations as described in detail below:
Boston Puerto Rican Center on Population Health and Health Disparities
The longitudinal Boston Puerto Rican Health Study includes 1200 free-living Puerto Rican (Hispanics of Caribbean origin) men and women, aged 45–75 y, in the greater Boston area. As 1 of 8 nationally funded National Institutes of Health Centers on Population Health and Health Disparities, this study is investigating health disparities in a Puerto Rican population. Enrolled subjects completed a baseline home interview as previously detailed (12). Written informed consent was obtained from each participant, and the protocol was approved by the Institutional Review Board at Tufts University.
Genetics of Lipid Lowering Drugs and Diet Network Study
The Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study sample consists of 1061 adults and is part of the PROGENI (Program for Genetic Interactions) Network. All subjects were of European ancestry. They were recruited from 2 National Heart, Lung, and Blood Institute Family Heart Study field centers (Minneapolis, MN, and Salt Lake City, UT). In addition to baseline determination, in this specific study, a postprandial fat-load test and a fenofibrate intervention study were carried out as previously described (13, 14). In brief, the GOLDN study has been conducted in several visits. Visit 1 entailed one blood draw for the baseline. One day after visit 1, visit 2 was conducted for the first postprandial fat challenge before the fenofibrate trial. After the fenofibrate intervention, visit 3 was performed for one blood draw for the baseline after drug trial (3–4 wk after visit 2). The study protocol was approved by the Institutional Review Boards at the University of Alabama, the University of Minnesota, the University of Utah, and Tufts University.
Harokopio Study
A total of 379 Greek white women without a known history of diabetes, cardiovascular disease, or cancer were enrolled in this study, which was approved by the Ethics Committee at Harokopio University, Athens, Greece. Fasting glucose concentrations >126 mg/dL, cortisol treatment, or lipid-lowering medication were criteria for exclusion from the analysis. Thus, our analysis was restricted to 351 women without diabetes (15).
EPIGEM Study
The EPIGEM (Epidemiología Genética y Molecular) Study was designed to estimate the prevalence of genetic and environmental cardiovascular disease risk factors in the Valencia Region on the Eastern Mediterranean coast of Spain. Here, we included 1622 white (768 men and 854 women aged 18–70 y) randomly selected subjects from the general population as previously reported (16). The Ethics Committee on Human Research of the Valencia University approved the study protocol, and all subjects provided informed consent for participation.
PREDIMED-Valencia
The PREDIMED (Prevención con Dieta Mediterránea) Study is a parallel-group, multicenter, controlled, randomized clinical trial aimed at assessing the effects of the Mediterranean diet on the primary prevention of cardiovascular disease in subjects with high cardiovascular disease risk. Details of this study have been reported elsewhere (17). Here, we included data from the first cross-sectional examination of participants recruited in the Valencia region. Briefly, high-risk participants were selected by physicians in primary care centers. Eligible subjects were elderly community-dwelling persons who fulfilled at least 1 of 2 criteria: type 2 diabetes or 3 or more cardiovascular disease risk factors (current smoking, hypertension, dyslipidemia, overweight, or a family history of premature cardiovascular disease). Thus, we included 537 white subjects (184 men and 353 women) who had complete data for all the variables examined. The Institutional Review Board of Valencia University approved the study protocol. All subjects provided written informed consent to participate in the study.
Singapore National Health Survey
The Singapore National Health Survey 1988 is a national survey based on models and protocols recommended by the World Health Organization (18). In brief, 11,200 persons representing the distribution of the Singapore housing population were selected from the National Database on Dwellings. A process of disproportionate stratified and systematic sampling was used to select individuals between 18 and 69 y of age from this data set with oversampling of the minority groups to allow statistical comparison between ethnic groups. The ethnic composition of the sample was 64% Chinese, 21% Malays, and 15% Indians. Here we included 3351 subjects who participated in this study and had their genetic polymorphism determined. The study was approved by the Ministry of Health in Singapore, and informed consent was obtained from all participants.
Anthropometric and lipid determinations at baseline
Anthropometric variables including height and weight were measured in all cohorts by use of standard techniques. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Fasting blood samples were collected by venipuncture in all study populations, and fasting glucose and fasting triacylglycerol, total cholesterol, and HDL cholesterol were measured at baseline by standard methods (12–18). The exclusion criteria for all studied populations was fasting triacylglycerol >1500 mg/dL.
Postprandial study
The postprandial study was carried out in 1005 participants (484 men and 521 women) in the GOLDN cohort who underwent the oral fat-load test. The postprandial fat challenge consisted of a meal formulated according to the protocol of Patsch et al (19). The meal, which participants were instructed to consume within 15 min, had 700 calories/m2 body surface area (2.93 MJ/m2 body surface area); 3% of calories were derived from protein, 14% from carbohydrate, and 83% from fat sources. Cholesterol content was 240 mg and the ratio of polyunsaturated to saturated fat was 0.06. The average person consumed 175 mL heavy whipping cream (39.5% fat) and 7.5 mL powdered, instant, nonfat dry milk blended with ice and 15 mL chocolate- or strawberry-flavored syrup to increase palatability. We drew blood samples immediately before (time 0) and 3.5 and 6 h after the high-fat meal. High-fat meals were administered in the early morning, usually between 0630 and 0900. For each subject, the concentration of the measured parameter was plotted against time, and the area between the 6-h concentration curve and zero level was determined by the trapezoid rule (20). This area under the curve measures the entire triacylglycerol load to which the subject was exposed during the test.
Fenofibrate intervention
After visit 2, 844 GOLDN participants were given a supply of fenofibrate as 160-mg tablets (TriCor; Abbott Laboratories, Abbott Park, IL) and were informed to take one fenofibrate tablet with a breakfast meal once daily for 3 wk. Baseline and after-treatment samples from each individual for this and the postprandial study were stored until the completion of the subject's participation and were then analyzed together.
Additional biochemical determinations in the postprandial study and fenofibrate intervention
In the GOLDN Study, we additionally measured postprandial triacylglycerol by the glycerol-blanked enzymatic method on a Roche COBAS FARA centrifugal analyzer (Roche Diagnostics, Indianapolis, IN) and fasting and postprandial lipoprotein particle concentrations and size by proton nuclear magnetic resonance spectroscopy (21, 22). We measured the concentrations of the following subclasses: small LDL (diameter: 18.0–21.2 nm), large LDL (21.2–23.0 nm), intermediate-density lipoprotein (23.0–27.0 nm), large HDL (8.8–13.0 nm), medium HDL (8.2–8.8 nm), small HDL (7.3–8.2 nm), large VLDL (>60 nm), medium VLDL (35.0–60.0 nm), and small VLDL (27.0–35.0 nm). The small LDL subclass encompassed both intermediate small (19.8–21.2 nm) and very small (18.0–19.0 nm) particles.
Genetic analysis
DNA extraction was performed by standard procedures. Genotyping was performed with the Applied Biosystems 7900HT Fast Real-Time PCR (Foster City, CA) based on TaqMan allelic discrimination SNP genotyping technology as we described previously for the APOA5 −1131T→C (rs662799) and 56C→G (rs3135506) SNPs (9). The genotyping assay of rs780094 in the GCKR gene centered on the target sequence TGATCAGCAAA[C/T]ATGTGTCAGG was also carried out based on TaqMan allelic discrimination SNP genotyping technology. Genetic analysis for all study cohorts was carried out in the Nutrition and Genomics Laboratory at the Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging. Internal controls and repetitive experiments were used. In addition, 20% of samples were repeated at random to verify the reproducibility. Genotype error rate was <1%.
Statistical analysis
Statistical analyses were performed with SPSS version 15.0 (SPSS Inc, Chicago, IL). The chi-square test was used to determine whether the genotype distribution followed Hardy-Weinberg equilibrium. Plasma triacylglycerol and LDL were log-transformed, and chylomicrons and nonchylomicrons, including VLDL and subclasses of VLDL, were square root–transformed to approximate a normal distribution.
Analysis of covariance (ANCOVA) was used to test the association between genotype groups and fasting plasma triacylglycerol concentrations as a continuous variable. Models were stratified by population and were adjusted for sex, age, and BMI. A combined genotype variable including the GCKR rs780094, APOA5 −1131T→C, and APOA5 56C→G SNPs was created as detailed in the Results. Additive or interactive (synergistic or antagonistic) effects of the individual SNPs were first tested by examining the statistical significance of the main effects and interaction terms among the 3 SNPs. Homogeneity of genotypic effects according to sex was also tested by introducing the interaction term between the combined genotype variable and sex in the corresponding ANCOVA model. Moreover, analyses were performed for men and women separately to verify the homogeneity and the internal replication of associations. Adjusted means were estimated for men and women. The Tukey test was applied to compare adjusted means among genotypes. P values for lineal trends across genotype categories were computed in the ANCOVA models. We further stratified populations according to fasting triacylglycerol using 150 mg/dL as the cutoff (guidelines of the Adult Treatment Panel III for clinical diagnosis of hypertriglyceridemia). The percentage of subjects with hypertriglyceridemia (triacylglycerol > 150 mg/dL) was compared among combined genotypes and populations by use of chi-square tests. To compare postprandial lipid responses across genotype groups, we fitted general linear models with repeated measurements in which we tested overall genotype effects independent of time and the interaction between genotype and time. Covariates included age, sex, and BMI. Homogeneity of the genetic effect by sex was tested after checking the statistical significance of the corresponding interaction term in the statistical model. A 2-tailed P value of <0.05 was considered statistically significant. For the fenofibrate assay, we also fitted the general linear model with repeated measures (baseline and posttreatment) including interactions term and control for covariates.
RESULTS
Demographic, biochemical, and genetic characteristics of the study participants
Baseline demographic and biochemical characteristics of the 6 study cohorts consisting of 7730 persons from 8 different populations are presented in Table 1. The genotype frequencies for the GCKR rs780094 (C→T), APOA5 −1131T→C, and APOA5 56C→G SNPs across each population are shown in Table 2. All 3 SNPs were in Hardy-Weinberg equilibrium in these populations (P > 0.05), except the GCKR rs780094 in the Singaporean Chinese (P = 0.000063) despite repeated genotyping of this sample population. Taking into account that Chinese were the population with a higher sample size, this fact could contribute to the higher statistical significance of the small differences between observed and expected frequencies.
TABLE 1.
Demographic, anthropometric, and biochemical characteristics of the study cohorts1
| Greece | Spain |
United States |
Singapore |
|||||
| Harokopio Study, general population | EPIGEM, general population | PREDIMED Valencia, high CVD risk | GOLDN Study, white non-Hispanics | BPR-CPHHD, Puerto Rican Hispanics | SNHS-98, Chinese | SNHS-98, Malays | SNHS-98, Asian Indians | |
| n (M/F) | 0/351 | 768/854 | 184/353 | 508/553 | 226/582 | 1018/1240 | 309/315 | 225/244 |
| Age (y) | 47.7 ± 122 | 41.9 ± 14 | 67.1 ± 6 | 48.4 ± 16 | 58 ± 7 | 37.8 ± 12 | 38.7 ± 12 | 40.5 ± 12 |
| BMI (kg/m2) | 29.1 ± 5 | 26.1 ± 4 | 31.6 ± 5 | 28.2 ± 5 | 32.2 ± 8 | 22.6 ± 4 | 25.5 ± 5 | 25.1 ± 5 |
| Triacylglycerol (mg/dL) | 98.4 ± 49 | 111.0 ± 64 | 130.7 ± 67 | 132.1 ± 81 | 150 ± 78 | 117.6 ± 68 | 139.8 ± 83 | 141.6 ± 78 |
| HDL-C (mg/dL) | 51.7 ± 11 | 50.6 ± 11 | 51.3 ± 12 | 47.2 ± 13 | 45.3 ± 12 | 55.2 ± 14 | 50.2 ± 13 | 44.4 ± 12 |
| LDL-C (mg/dL) | 148.3 ± 35 | 131.2 ± 34 | 131.0 ± 31 | 121.6 ± 31 | 107.2 ± 35 | 130.5 ± 36 | 148.3 ± 38 | 142.9 ± 40 |
| Fasting blood glucose (mg/dL) | 97.3 ± 17 | 95.7 ± 23 | 128.6 ± 43 | 101.3 ± 18 | 122.8 ± 52 | 100.9 ± 22 | 109.3 ± 38 | 112.2 ± 38 |
EPIGEM, Epidemiología Genética y Molecular; PREDIMED, Prevención con Dieta Mediterránea; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; BPR-CPHHD, Boston Puerto Rican Health Study–Centers on Population Health and Health Disparities; SNHS-98, Singapore National Health Survey of 1998; CVD, cardiovascular disease; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
Mean ± SD (all such values).
TABLE 2.
Prevalence of the rs780094 at the GCKR gene, the −1131T→C at the APOA5 gene, and the 56C→G at the APOA5 gene single nucleotide polymorphisms in the different populations1
| Greece | Spain |
United States |
Singapore |
|||||
| Harokopio Study, general population | EPIGEM, general population | PREDIMED Valencia, high CVD risk | GOLDN Study, white non-Hispanics | BPR-CPHHD, Puerto Rican Hispanics | SNHS-98, Chinese | SNHS-98, Malays | SNHS-98, Asian Indians | |
| GCKR rs780094, CC/CT/TT (%) | 24/47/29 | 28/49/23 | 29/47/34 | 36/50/13 | 51/38/10 | 31/46/23 | 36/45/18 | 60/34/5 |
| APOA5 –1131T→C rs662799, TT/TC/CC (%) | 83/16/1 | 88/11/1 | 88/12/0.4 | 87/12/0.1 | 78/20/2 | 51/39/9 | 51/31/9 | 59/36/5 |
| APOA5 56C→G rs3135506, CC,CG,GG (%) | 87/12/1 | 89/10/1 | 87/12.5/0.2 | 89/10/0.4 | 81/18/1 | 99.7/0.2/0 | 96.3/3.37/0 | 94/5.6/0.2 |
EPIGEM, Epidemiología Genética y Molecular; PREDIMED, Prevención con Dieta Mediterránea; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; BPR-CPHHD, Boston Puerto Rican Health Study–Centers on Population Health and Health Disparities; SNHS-98, Singapore National Health Survey of 1998; CVD, cardiovascular disease. Genotype error rate was <1%.
GCKR and APOA5 variants and fasting triacylglycerols
The GCKR rs780094C→T, APOA5 −1131T→C, and APOA5 56C→G SNPs were independent of each other in terms of linkage disequilibrium. Carriers of the variant allele were grouped, and 2 categories were considered for each SNP. When we first tested additive or interactive effects of these 3 SNPs in ANCOVA models (results not shown), we did not find statistically significant interaction terms for any population, which suggested additive effects.
Because the statistical significance of the main effects for the APOA5 −1131T→C and APOA5 56C→G SNPs varied depending on the population, we decided to create a mixed genotype for the APOA5 gene. The 2 categories considered in this mixed APOA5 genotype were homozygous for both major alleles (TT and CC for −1131T→C and 56C→G, respectively) compared with carriers of one of the minor alleles. No statistically significant interaction effects were found between this mixed APOA5 genotype and the GCKR rs780094C→T SNP in any population. Hence, we defined 4 combined genotype groups based on these 3 SNPs: 1) Homozygous for the wild-type alleles for each SNP (CC rs780094 at the GCKR gene and TT −1131T→C APOA5 + CC 56C→G APOA5): this group (n = 1679) was considered to be a protective combination and was termed the P group. 2) Homozygous for the wild-type allele at the GCKR rs780094 (CC) and carriers of the variant allele at one of the APOA5 SNPs (TC or CC at −1131T→C or CG or GG at 56C→G): this group (n = 981) was considered to be intermediate in effects and was named M1. 3) Carriers of the variant allele at the GCKR rs780094 (CT or TT) and homozygous for the common alleles at the APOA5 SNPs (TT −1131T→C + CC 56C→G): this group (n = 3314) was also considered intermediate in effect and was named M2. 4) Carriers of the variant allele for the GCKR rs780094 (CT or TT) and carriers of the variant alleles at one of the APOA5 SNPs (TC or CC at −1131T→C, or CG or GG at 56C→G): this group (n = 1756) was considered as a risk genotype and was termed the R group.
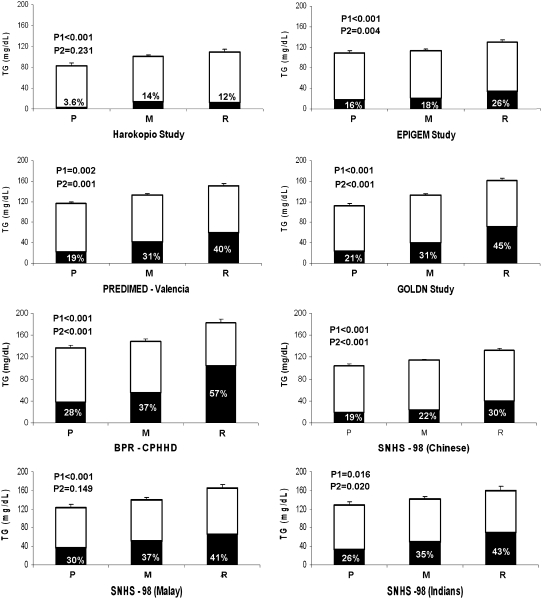
Fasting triacylglycerol concentrations by these 4 combined genotypes in the 7730 men and women from the 8 populations studied are shown in Table 3. We found highly consistent associations with fasting triacylglycerol concentrations across the 4 genetic categories, with statistically significant P values for lineal trend. Male carriers of the R genotype had significantly greater fasting triacylglycerol concentrations than did men with the P genotype, independently of American, Asian, or European origin. This significant effect was consistently observed among women from all populations except in the Asian-Indian group, perhaps because of the low sample size included in the R genotype. Multiple mean comparisons among genotype categories were also carried out by use of Tukey tests (Table 3). Because the behavior of the intermediate groups M1 and M2 was similar and no significant differences were observed between these 2 groups, we pooled these 2 groups into one and renamed the genotype group M. Thus, 3 combined genotype groups were considered in the further analyses: P, M, and R. In addition, because no heterogeneity by sex was observed, men and women were analyzed together in further analyses. The adjusted means of fasting triacylglycerol in both men and women for the 8 studied populations depending on the P, M, and R combined genotypes are shown in Figure 1. Statistically significant P values for lineal trend (P1) across genotype categories showed an additive effect of the variants considered in all 8 populations. Moreover, we also studied whether these genotypes were associated with hypertriglyceridemia (triacylglycerol > 150 mg/dL). Interestingly, the percentage of hypertriglyceridemic subjects (Figure 1) was significantly higher in carriers of the R genotype than in those carrying the P genotype. Despite the same trend in percentages, we did not find statistically significant differences for Greeks and Malays because of the lower sample size.
TABLE 3.
Triacylglycerol concentrations in the studied populations comprising a total of 7730 individuals according to the 4 combined genotype groups for the rs780094 at the GCKR gene and −1131T→C and 56C→G single nucleotide polymorphisms (SNPs) at the APOA5 gene by sex1
| Protective genotype (P) |
Intermediate (M1) |
Intermediate (M2) |
Risk genotype (R) |
P for trend, males | P for trend, females | |||||
| Males | Females | Males | Females | Males | Females | Males | Females | |||
| mg/dL | mg/dL | mg/dL | mg/dL | |||||||
| Greece | ||||||||||
| Harokopio Study | — | 80 ± 62,3 | — | 90 ± 8 | — | 90 ± 32 | — | 95 ± 53 | <0.001 | |
| (55) | (30) | (197) | (69) | |||||||
| Spain | ||||||||||
| EPIGEM | 107 ± 52 | 79 ± 42 | 111 ± 10 | 83 ± 7 | 112 ± 33 | 85 ± 2 | 129 ± 62,3 | 91 ± 42 | 0.003 | 0.016 |
| (general population) | (147) | (189) | (46) | (63) | (452) | (474) | (123) | (128) | ||
| PREDIMED-Valencia | 99 ± 102 | 108 ± 72 | 124 ± 17 | 110 ± 13 | 119 ± 73 | 117 ± 4 | 142 ± 122,3 | 126 ± 82 | 0.006 | 0.021 |
| (high-CVD risk) | (43) | (76) | (15) | (24) | (98) | (196) | (28) | (57) | ||
| United States | ||||||||||
| GOLDN Study | 101 ± 62–4 | 96 ± 62–4 | 137 ± 122 | 119 ± 112 | 125 ± 44,5 | 100 ± 44,5 | 142 ± 83,5 | 129 ± 83,5 | <0.001 | <0.001 |
| (whites) | (148) | (153) | (38) | (41) | (247) | (277) | (73) | (84) | ||
| BPR-CPHHD | 130 ± 92 | 119 ± 62,3 | 131 ± 11 | 133 ± 72,4 | 141 ± 9 | 129 ± 65 | 162 ± 112 | 165 ± 73–5 | 0.030 | <0.001 |
| (Puerto Rican) | (64) | (178) | (51) | (116) | (66) | (181) | (45) | (107) | ||
| Singapore | ||||||||||
| SNHS-98 | 112 ± 52,3 | 83 ± 42,3 | 124 ± 52,4 | 90 ± 52 | 115 ± 35 | 83 ± 34 | 142 ± 33–5 | 93 ± 33,4 | <0.001 | 0.003 |
| (Chinese) | (156) | (192) | (156) | (178) | (361) | (452) | (345) | (418) | ||
| SNHS-98 | 137 ± 13 | 88 ± 112 | 145 ± 10 | 109 ± 12 | 142 ± 8 | 103.5 ± 9 | 157 ± 9 | 112 ± 82 | 0.022 | 0.003 |
| (Malays) | (50) | (62) | (55) | (50) | (105) | (98) | (99) | (105) | ||
| SNHS-98 | 137 ± 92,3 | 97 ± 8 | 159 ± 102 | 112 ± 9 | 145 ± 11 | 97 ± 10 | 174 ± 133 | 95 ± 12 | 0.012 | 0.806 |
| (Asian Indians) | (73) | (93) | (62) | (56) | (56) | (54) | (34) | (41) | ||
Values are adjusted means ± SEs; number of subjects within each subgroup in parentheses. Combined genotypes are as follows: P, homozygous subjects for the wild-type alleles for each SNP (CC rs780094 at the GCKR gene and TT −1131T→C APOA5 + CC 56C→G APOA5), n = 1679; M1, homozygous subjects for the wild-type allele at the GCKR rs780094 (CC) and carriers of the variant allele at one of the APOA5 SNPs (TC or CC at −1131T→C or CG or GG at 56C→G), n = 981; M2, carriers of the variant allele at the GCKR rs780094 (CT or TT) and homozygous for the common alleles at the APOA5 SNPs (TT −1131T→C + CC 56C→G), n = 3314; R, carriers of the variant allele for the GCKR rs780094 (CT or TT) and carriers of the variant alleles at one of the APOA5 SNPs (TC or CC at −1131T→C or CG or GG at 56C→G), n = 1756. EPIGEM, Epidemiología Genética y Molecular; PREDIMED, Prevención con Dieta Mediterránea; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; BPR-CPHHD, Boston Puerto Rican Health Study–Centers on Population Health and Health Disparities; SNHS-98, Singapore National Health Survey of 1998; CVD, cardiovascular disease. Statistical analyses were carried out after a log transformation and adjustment for covariates including age and BMI. P values for linear trend are shown for all cohorts. Post hoc Tukey's test was applied to compare means.
Same superscripts indicate P < 0.05 within each group (males or females) and population.
FIGURE 1.
Comparison of fasting triacylglycerol (TG) across 3 combined genotype groups in the 8 studied populations. Values are means (±SEMs) and percentages. P1: P values for lineal trend of triacylglycerol means among genotypes (adjusted for age, sex, and BMI) in men and women. P2: P values obtained in chi-square test for percentages. The black bars represent the percentage of hypertriglyceridemic (triacylglycerol > 150 mg/dL) subjects within each genotype group. P, homozygous subjects for the wild-type alleles for each single nucleotide polymorphism (SNP; CC rs780094 at the GCKR gene and TT −1131T→C APOA5 + CC 56C→G APOA5), n = 1679; M, any mixed genotype not included in groups P and R, n = 4295; R, carriers of the variant allele for the GCKR rs780094 (CT or TT) and carriers of the variant alleles at one of the APOA5 SNPs (TC or CC at −1131T→C or CG or GG at 56C→G), n = 1756; EPIGEM, Epidemiología Genética y Molecular; PREDIMED, Prevención con Dieta Mediterránea; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; BPR CPHHD, Boston Puerto Rican Health Study–Centers on Population Health and Health Disparities; SNHS, Singapore National Health Survey.
GCKR and APOA5 variants and postprandial lipemia
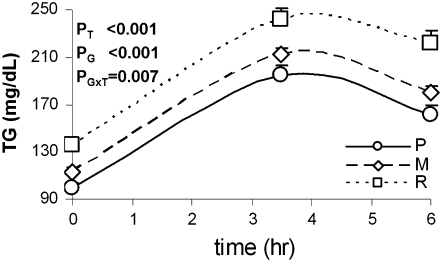
Next, we examined the potential additive effect of the combined APOA5 and GCKR SNPs on postprandial lipemia response to an oral fat overload as detailed in the Subjects and Methods. Because we did not observe any significant interaction between sex and genotype, men and women were combined for further analyses. After adjustment for sex, age, and BMI, the genotype-specific triacylglycerol difference persisted throughout the postprandial phase (Figure 2). Subjects included in the R genotype group showed a higher postprandial response of triacylglycerol than did those included in the P group (P < 0.001). Subjects in the M group showed an intermediate response.
FIGURE 2.
Postprandial triacylglycerol (TG) response in the GOLDN (Genetics of Lipid Lowering Drugs and Diet Network) study participants who underwent the oral fat-load test, according to the 3 GCKR-APOA5 combined genotype groups. P, homozygous subjects for the wild-type alleles for each single nucleotide polymorphism (SNP; CC rs780094 at the GCKR gene and TT −1131T→C APOA5 + CC 56C→G APOA5), n = 281; M, any mixed genotype not included in groups P and R, n = 583; and R, carriers of the variant allele for the GCKR rs780094 (CT or TT) and carriers of the variant alleles at one of the APOA5 SNPs (TC or CC at −1131T→C or CG or GG at 56C→G), n = 141. Adjusted TG means (for sex, age, and BMI) and SEMs, immediately before (time 0) and 3.5 and 6 h after the high-fat meal are compared. Adjusted P values for the effect of the genotype (PG), the time (PT), and the interaction of genotype and time (PGxT) obtained in the statistical model for repeated measures are shown.
We further examined the association of these genotype groups with postprandial responses of chylomicrons and nonchylomicron fractions, including total VLDL, large, medium, and small VLDL. Subjects within the R group had greater postprandial concentrations of chylomicron particles (P < 0.001), total VLDL (P = 0.028), large VLDL (P < 0.001), and medium VLDL (P < 0.001) than did subjects in the P group (results not shown). Marginal differences were observed for small VLDL (P = 0.086). To further illustrate genotype-specific differences in the postprandial triacylglycerol response, we compared the area under the curve for postprandial triacylglycerol across 3 genotype groups. Subjects in the R group had higher area under the curve for triacylglycerol than did subjects in the P group. Intermediate values were observed for the M group [P: 1139 ± 604 arbitrary units (AU); M: 1285 ± 749 AU; R: 1514 ± 870 AU, P < 0.001].
Fenofibrate intervention
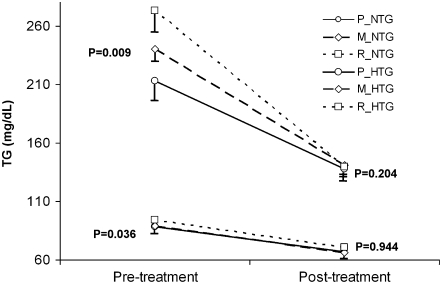
To understand the nature of the differential response to fenofibrate intervention, which potentially could be influenced by the combined GCKR and APOA5 genotypes, we compared triacylglycerol responses to a 3-wk fenofibrate treatment across the 3 previously defined genotype groups. The allele-specific differences in fasting triacylglycerol observed at baseline in normotriglyceridemic subjects (P = 0.036) or hypertriglyceridemic subjects (P = 0.009) according to the P, M, and R genotype groups (Figure 3) were no longer significant after fenofibrate treatment in both normotriglyceridemic (P = 0.944) and hypertriglyceridemic (P = 0.204) groups. When this interaction was formally tested in a repeated-measures model adjusted for sex, age, BMI, and baseline triacylglycerols, we obtained a statistically significant interaction term between treatment and the combined genotype (P = 0.003). The genotype-specific reduction of triacylglycerol concentrations was more evident in the hypertriglyceridemic group. For example, subjects within the R group showed a greater reduction of triacylglycerol concentrations than did subjects in the P and M groups after fenofibrate treatment. Further analysis with exclusion of subjects with triacylglycerol concentrations >500 mg/dL did not change the statistical significance of these results.
FIGURE 3.
Fasting means (±SEMs) of plasma triacylglycerol (TG) concentrations after (pretreatment) and before (posttreatment) the fenofibrate intervention (3 wk, 160 mg/d) in the GOLDN (Genetics of Lipid Lowering Drugs and Diet Network) study participants (n = 844) according to the 3 GCKR-APOA5 combined genotype groups and hypertriglyceridemic (TG > 150 mg/dL) status. P, homozygous subjects for the wild-type alleles for each single-nucleotide polymorphism (SNP; CC rs780094 at the GCKR gene and TT −1131T→C APOA5 + CC 56C→G APOA5), n = 236; M, any mixed genotype not included in groups P and R, n = 490; and R, carriers of the variant allele for the GCKR rs780094 (CT or TT) and carriers of the variant alleles at one of the APOA5 SNPs (TC or CC at −1131T→C or CG or GG at 56C→G), n = 118. TG means were adjusted for sex, age, and BMI at pretreatment and additionally for baseline TG at the posttreatment analysis. P values indicate the global mean comparison among the 3 genotype groups in each category and time. NTG, normotriglyceridemia; HTG, hypertriglyceridemia.
DISCUSSION
Most published claims of gene-biomarker and gene-disease associations have not been replicated when studied in independent samples (5, 23). Moreover, even for those SNPs at well-established candidate genes and showing consistent replication, the contribution of each allele to trait variability is rather small. Consequently, it is unclear whether single genetic markers may add information on disease risk above and beyond that provided by the measurement of traditional biochemical and behavioral risk factors. However, recent reports (24–27) support the notion that significant predictive value from genetic markers can be gained by combining genotype information from multiple loci. The same concept applies to the ability to predict individual responses to factors such as diet, smoking, alcohol, and drugs (28).
In this context, we have shown in a large-scale collaborative study encompassing 6 independent cohorts and including a total of 7730 persons from different ethnic backgrounds and 3 geographical locations (America, Asia, and Europe) that genetic variants at both GCKR and APOA5 loci have an additive effect on fasting triacylglycerol concentrations. Thus, we obtained a gradient of triacylglycerol concentrations based on a defined combination of genotype groups. Subjects carrying simultaneously triacylglycerol-raising alleles at both the GCKR and APOA5 genes (R group) had higher fasting and postprandial triacylglycerol concentrations than did subjects homozygous for the wild type allele (P group). Moreover, persons carrying triacylglycerol-raising alleles at either one of these loci (M group) had intermediate triacylglycerol concentrations that were significantly different from both P and R subjects. The consistency of our findings is remarkable considering that the cohorts studied included subjects from multiple ethnic backgrounds, cultures, and dietary habits. Therefore, in view of the association of plasma triacylglycerol concentrations with CHD risk, subjects within the R or M groups maybe at higher CHD risk than subjects in the P group as a result of their impaired triacylglycerol metabolism. This hypothesis needs to be tested in studies properly designed to address this question.
The use of nonfasting triacylglycerol concentrations in CHD risk assessment appears to have clinical relevance, because impaired postprandial lipemia may be a significant factor in the pathogenesis of atherosclerosis (29). Plasma concentrations of chylomicrons and their remnants reflect the working of postprandial lipid metabolism and correlate with both lesion progression and cardiovascular events. However, other factors during the postprandial phase, such as the absorption of oxidized cholesterol, may play a role in the development of atherosclerosis (30). One of our cohorts (the GOLDN study) contained information about postprandial lipemia after an oral fat load, and we showed that the reported differences across genotype groups during the fasting state were replicated in the postprandial phase, thus reinforcing the notion that subjects in the R group may be at increased CHD risk.
The mechanisms underlying the observed associations for these combinations of polymorphisms need to be elucidated. Current knowledge about the metabolic role of APOA5 and GCKR is primarily based on animal models. In the case of the most novel candidate gene (GCKR), we know that an increase in liver glucokinase activity of ≈2.5-fold in ad libitum–fed transgenic animals resulted in a 19% increase in triacylglycerol concentrations (7). Extrapolating these results to humans, overexpression of GCKR with subsequent increase in glucokinase activity would be expected to lower blood glucose and insulin concentrations but to increase hepatic glycolytic flux, malonyl CoA production, and triacylglycerol and VLDL synthesis. In this context, our findings support the hypothesis that the presence of the minor allele for the rs780094 SNP is related to increased expression, or alternatively increased activity, of the GCKR gene, leading to a higher postprandial response of triacylglycerol and VLDL particles and higher fasting triacylglycerol concentrations. In terms of the contribution of the APOA5 locus to the hypertriglyceridemic phenotype, both SNPs (APOA5 −1131T→C and APOA5 56C→G) examined in the current studies have been shown to be functional and associated with decreased APOA5 concentration or activity, which may result in decreased LPL-mediated clearance of postprandial chylomicrons and increased postprandial response. The combined effect of increased synthesis of triacylglycerol-rich lipoproteins driven by the GCKR gene variation, combined by their impaired catabolism, driven by the APOA5 gene variants, may explain the additive effects observed by their combination in all the populations studied in this work.
An additional finding from our study relates to the results of the pharmacologic arm of the GOLDN Study. To date, most clinical trials aimed at reducing CVD risk by targeting plasma triacylglycerol concentrations have been struggling with demonstrating significant reduction in mortality. This may be in part because of the wide interindividual variability in triacylglycerol responses associated with therapy based on fibric acid derivatives, the most common pharmacologic agents aimed at reducing plasma triacylglycerol concentrations.
Fenofibrate belongs to this class of lipid-modifying agents (14). As indicated above, its major therapeutic effect has been attributed to its effect on plasma triacylglycerol, although it may also be associated with a moderate decrease in LDL-cholesterol and an increase in HDL-cholesterol concentrations. Fibrates activate peroxisome proliferator-activated receptor-α, which results in transcriptional induction of synthesis of the major HDL apolipoproteins, apo A-I and apo A-II. Fibrates lower hepatic apo C-III production and increase lipoprotein lipase–mediated lipolysis via peroxisome proliferator-activated receptor. Fibrates stimulate cellular fatty acid uptake, conversion to acyl-CoA derivatives, and catabolism by the beta-oxidation pathways, which, combined with a reduction in fatty acid and triacylglycerol synthesis, results in a decrease in VLDL production. It has been shown that interindividual variations in the response to fenofibrate could be partially driven by genetic factors (31, 32). In this regard, our study shows that the fenofibrate-induced reduction of triacylglycerol concentrations was more evident in subjects within the higher risk group (R) than in those in the intermediate (M) and protective (P) genotype groups. Consistent with this finding, the statistically significant genotype-related triacylglycerol differences observed among genotype groups disappeared after fenofibrate intervention. Interestingly, this genotype-fenofibrate response interaction was magnified in those subjects with triacylglycerol above the established risk threshold of 150 mg/dL. This information could be used for the development of predictive tools to identify, in addition to those subjects with higher triacylglycerol concentrations, those who may benefit the most from fenofibrate therapy.
The mechanism of this interaction cannot be derived from our experimental approach, and the fact that the increased response of the R group remained after we adjusted for baseline triacylglycerol concentrations indicates that mechanisms beyond those involved in determining the baseline differences across genotype may exist for response to fenofibrate. We previously showed that the APOA5 gene is involved in the differential response to fenofibrate (14); however, the finding involving the GCKR gene is novel and adds to that observed for APOA5.
In summary, the present study showed that the combination of minor alleles at the APOA5 (ie, −1131T→C and 56G→C) and GCKR loci (rs780094) exert an additive effect on plasma fasting and postprandial triacylglycerol concentrations and showed the significant effect of GCKR and APOA5 on triacylglycerol-rich lipoprotein metabolism. Furthermore, we report an allele-specific response to fenofibrate intervention for the combined genotype, which suggests that subjects within the risk genotype group had a higher decrease in plasma triacylglycerol concentrations after treatment.
Acknowledgments
The authors' responsibilities were as follows—JMO: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, conception and design of the study: DKA, KLT, PP-M, and JMO: provision of study materials or subjects; DC, NY, EST, CSK, MT, and MG: collection and assembly of data; DC, JS, NY, EST, CSK, LDP, SK, C-QL, MP, IB, and JMO: analysis and interpretation of the data; DC, PP-M, and JMO: statistical expertise; JS, RJS, MP, IB, and DC: drafting of the manuscript; PP-M, DC and JMO: critical review of the manuscript for important intellectual content; DKA, NY, KLT, LDP, C-QL, MT, DC, MO-M, JL-M, EST, SK, FP-J, and JMO: obtained funding; DC, DKA, NY, KLT, EST, and JMO. None of the authors had any conflicts of interest.
REFERENCES
- 1.Le NA, Walter MF. The role of hypertriglyceridemia in atherosclerosis. Curr Atheroscler Rep 2007;9:110–5 [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr 2007;98:458–73 [DOI] [PubMed] [Google Scholar]
- 3.Brunzell JD. Clinical practice: hypertriglyceridemia. N Engl J Med 2007;357:1009–17 [DOI] [PubMed] [Google Scholar]
- 4.Pollex RL, Hegele RA. Genetic determinants of plasma lipoproteins. Nat Clin Pract Cardiovasc Med 2007;4:600–9 [DOI] [PubMed] [Google Scholar]
- 5.Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet 2001;29:306–9 [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research;Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316:1331–6 [DOI] [PubMed] [Google Scholar]
- 7.O'Doherty RM, Lehman DL, Telemaque-Potts S, Newgard CB. Metabolic impact of glucokinase overexpression in liver: lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes 1999;48:2022–7 [DOI] [PubMed] [Google Scholar]
- 8.Slosberg ED, Desai UJ, Fanelli B, et al. Treatment of type 2 diabetes by adenoviral-mediated overexpression of the glucokinase regulatory protein. Diabetes 2001;50:1813–20 [DOI] [PubMed] [Google Scholar]
- 9.Ferre T, Riu E, Bosch F, Valera A. Evidence from transgenic mice that glucokinase is rate limiting for glucose utilization in the liver. FASEB J 1996;10:1213–8 [DOI] [PubMed] [Google Scholar]
- 10.Pennacchio LA, Rubin EM. Apolipoprotein A5, a newly identified gene that affects plasma triglyceride levels in humans and mice. Arterioscler Thromb Vasc Biol 2003;23:529–34 [DOI] [PubMed] [Google Scholar]
- 11.Keating GM, Croom KF. Fenofibrate: a review of its use in primary dyslipidaemia, the metabolic syndrome and type 2 diabetes mellitus. Drugs 2007;67:121–53 [DOI] [PubMed] [Google Scholar]
- 12.Lai CQ, Tucker KL, Parnell LD, et al. PPARGC1A variation associated with DNA damage, diabetes, and cardiovascular diseases: the Boston Puerto Rican Health Study. Diabetes 2008;57:809–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corella D, Arnett DK, Tsai MY, et al. The −256T>C polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin Chem 2007;53:1144–52 [DOI] [PubMed] [Google Scholar]
- 14.Lai CQ, Arnett DK, Corella D, et al. Fenofibrate effect on triglyceride and postprandial response of apolipoprotein A5 variants: the GOLDN study. Arterioscler Thromb Vasc Biol 2007;27:1417–25 [DOI] [PubMed] [Google Scholar]
- 15.Yannakoulia M, Yiannakouris N, Melistas L, et al. Dietary factors associated with plasma high molecular and total adiponectin levels in apparently healthy women. Eur J Endocrinol 2008;159:R5–10 [DOI] [PubMed] [Google Scholar]
- 16.Qi L, Corella D, Sorli JV, et al. Genetic variation at the perilipin (PLIN) locus is associated with obesity-related phenotypes in white women. Clin Genet 2004;66:299–310 [DOI] [PubMed] [Google Scholar]
- 17.Estruch R, Martinez-Gonzalez MA, Corella D, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 2006;145:1–11 [DOI] [PubMed] [Google Scholar]
- 18.Ang LW, Ma S, Cutter J, Chew SK, Tan CE, Tai ES. The metabolic syndrome in Chinese, Malays and Asian Indians: factor analysis of data from the 1998 Singapore National Health Survey. Diabetes Res Clin Pract 2005;67:53–62 [DOI] [PubMed] [Google Scholar]
- 19.Patsch JR, Miesenbock G, Hopferwieser T, et al. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb 1992;12:1336–45 [DOI] [PubMed] [Google Scholar]
- 20.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab (Zaragoza) 2002;48:171–80 [PubMed] [Google Scholar]
- 22.Tsai MY, Georgopoulos A, Otvos JD, et al. Comparison of ultracentrifugation and nuclear magnetic resonance spectroscopy in the quantification of triglyceride-rich lipoproteins after an oral fat load. Clin Chem 2004;50:1201–4 [DOI] [PubMed] [Google Scholar]
- 23.Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med 2002;4:45–61 [DOI] [PubMed] [Google Scholar]
- 24.Yiannakouris N, Trichopoulou A, Benetou V, Psaltopoulou T, Ordovas JM, Trichopoulos D. A direct assessment of genetic contribution to the incidence of coronary infarct in the general population Greek EPIC cohort. Eur J Epidemiol 2006;21:859–67 [DOI] [PubMed] [Google Scholar]
- 25.Trichopoulou A, Yiannakouris N, Bamia C, Benetou V, Trichopoulos D, Ordovas JM. Genetic predisposition, nongenetic risk factors, and coronary infarct. Arch Intern Med 2008;168:891–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison AC, Bare LA, Chambless LE, et al. Prediction of coronary heart disease risk using a genetic risk score: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2007;166:28–35 [DOI] [PubMed] [Google Scholar]
- 27.Kathiresan S, Melander O, Anevski D, et al. Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med 2008;358:1240–9 [DOI] [PubMed] [Google Scholar]
- 28.Siest G, Jeannesson E, Berrahmoune H, et al. Pharmacogenomics and drug response in cardiovascular disorders. Pharmacogenomics 2004;5:779–802 [DOI] [PubMed] [Google Scholar]
- 29.Boquist S, Ruotolo G, Tang R, et al. Alimentary lipemia, postprandial triglyceride-rich lipoproteins, and common carotid intima-media thickness in healthy, middle-aged men. Circulation 1999;100:723–8 [DOI] [PubMed] [Google Scholar]
- 30.Staprans I, Pan XM, Rapp JH, Moser AH, Feingold KR. Ezetimibe inhibits the incorporation of dietary oxidized cholesterol into lipoproteins. J Lipid Res 2006;47:2575–80 [DOI] [PubMed] [Google Scholar]
- 31.Brisson D, Ledoux K, Bosse Y, et al. Effect of apolipoprotein E, peroxisome proliferator-activated receptor alpha and lipoprotein lipase gene mutations on the ability of fenofibrate to improve lipid profiles and reach clinical guideline targets among hypertriglyceridemic patients. Pharmacogenetics 2002;12:313–20 [DOI] [PubMed] [Google Scholar]
- 32.Smith JA, Arnett DK, Kelly RJ, et al. The genetic architecture of fasting plasma triglyceride response to fenofibrate treatment. Eur J Hum Genet 2008;16:603–13 [DOI] [PMC free article] [PubMed] [Google Scholar]