Abstract
Lung cancer is the leading cause of cancer-related deaths. The morbidity and mortality of lung cancer have markedly increased in the past decade with at least 75% of patients with lung cancer having evidence of metastases at the time of diagnosis. It frequently metastasizes to bone resulting in osteolytic lesions with unknown mechanisms. The aim of this study was to identify factors that mediate lung cancer-induced osteoclast activity in vivo. Using a human cytokine antibody array, we first determined cytokine levels in a conditioned medium collected from non-small cell lung cancer A549 and H1299 cells and the non-neoplastic human bronchial epithelial BEAS2B cells. Both A549 and H1229 cells produced significantly higher amount of several cytokines including monocyte chemotactic protein 1 (MCP-1) and interleukin 8 (IL-8) compared with BEAS2B cells. These findings were confirmed by ELISA. From clinical serum specimens, we also observed that MCP-1 and IL-8 levels were increased in lung cancer patients with bone metastases compared with the patients with localized tumor. Next, we investigated the effects of MCP-1 on osteoclast formation in vitro using murine bone marrow-derived monocytes. A549 conditioned medium induced osteoclast formation that was inhibited by neutralizing antibodies against MCP-1. Finally, A549 cells were stably transfected with MCP-1 short hairpin RNA. The MCP-1 knockdown A549 cells were implanted into the tibia of severe combined immunodeficient mice for 4 weeks. The MCP-1 knockdown significantly diminished A549 cell growth. We conclude that MCP-1 promotes lung cancer-induced osteoclast activity and thus bone resorptive lesions in vivo.
Introduction
Lung cancer is the leading cause of cancer-related deaths and is the second most common malignancy in both men and women [1]. Epidemiological study has particularly documented that morbidity and mortality of lung cancer have markedly increased in the past decade in China [2], with at least 75% of patients with lung cancer having evidence of metastases at the time of diagnosis [3]. Lung cancer frequently metastasizes to bone resulting in osteolytic (bone resorptive) lesions through unknown mechanisms. It has been widely accepted that the cross talks between tumor cells and the bone microenvironment may play a key role for tumor cell homing to bone and tumor development [4]. The factors responsible for lung cancer-induced osteolysis, however, have not been well characterized.
Human bronchial epithelial cells produce a number of proinflammatory cytokines such as monocyte chemotactic protein 1 (MCP-1), interleukin 8 (IL-8), and growth-related oncogene α (GROα) that contributes to the recruitment of inflammatory cells, mucin secretion, tissue remodeling, and pathogen-killing processes [5]. Monocyte chemotactic protein 1 has been demonstrated as a member of the CC chemokine superfamily that plays a critical role in the recruitment and activation of monocytes during acute inflammation and angiogenesis [6–9]. In human lungs, MCP-1 is expressed in a variety of cell types, namely, macrophages, endothelial, bronchial epithelial, and smooth muscle cells [10–12]. Recently, it was reported that cancer cells such as prostate cancer, breast cancer, and multiple myeloma that preferentially home to bone produce MCP-1 and express its receptor CCR2 [9–15]. In patients with breast and ovarian cancers, MCP-1 levels are increased in their sera, which correlated with the tumor stage [16,17]. We and other researchers have reported that MCP-1, produced by prostate cancer epithelial cells, stroma/osteoblasts, and bone marrow endothelial cells (HBME), is chemotactic for prostate cancer cells [15,18]. We observed that MCP-1 also increases prostate cancer cell proliferation and induces osteoclast formation in vitro through its receptor CCR2 [18,19]. Importantly, we reported that CCR2 expression levels correlate with prostate cancer progression as determined by immunohistochemistry [20]. In support of our findings, Loberg et al. [21] recently reported similar results that MCP-1, derived from human HBMEs, induces prostate cancer cell proliferation and migration. More recently, we found that parathyroid hormone-related protein-induced MCP-1 production by HBME cells and osteoblasts promotes osteoclast differentiation and prostate cancer cell proliferation and invasion in vitro [18]. These results were mostly from studies in prostate cancer. Whether the roles of MCP-1 may be extended to lung cancer has become a critical question, and importantly, there is a lacking of in vivo studies on the roles of MCP-1 on lung cancer development. Our hypothesis for this study was that MCP-1 promotes lung cancer-induced bone resorptive lesions in vivo. Accordingly, we first determined the production of cytokines in poorly differentiated non-small cell lung cancer cell lines A549 and H1299 and in the human bronchial epithelial cell line BEAS2B. Furthermore, we investigated the effects of MCP-1 on osteoclast formation in vitro using murine bone marrow-derived monocytes and the effects of deleting MCP-1 expression in lung cancer cells on the tumor growth in bone.
Materials and Methods
Reagents
Recombinant human and murine macrophage colony-stimulating factor, receptor activator of nuclear factor κB ligand (RANKL), IL-8, MCP-1, IL-8 antibody,MCP-1 antibody, RANKL antibody, and isotype control antibodies were purchased from R&D Systems (Minneapolis, MN). All chemical reagents were purchased from Sigma (St. Louis, MO).
Cell Culture
The human non-small cell lung adenocarcinoma A549 and H1299 cell lines and a nonneoplastic bronchi epithelial BEAS2B cell line were kindly provided by Dr. Xue Wang (University of Pittsburgh, Pittsburgh, PA). A529 and BEAS2B cells were cultured with Dulbecco's modified Eagle's medium. H1299 cells were cultured in RPMI 1640 media (Invitrogen, Carlsbad, CA). The media were supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin.
Conditioned Media
BEAS2B, H1299, and A549 cells at 2 x 106 were grown in 100-mm tissue culture dishes overnight in cell culture media, then washed twice with PBS and changed the media to 1% FBS in RPMI 1640. After 48 hours, the conditioned media (CM) were collected. To normalize for differences in cell density because of proliferation during the culture period, cells from each plate were collected and total DNA content/plate was determined (spectrophotometric absorbance, 260 nm). Conditioned medium was then normalized for DNA content between samples by adding RPMI.
Cytokine Antibody Array
Human Cytokine Antibody Array kit was purchased from Ray-Biotech (Norcross, GA). This array consisted of 174 different antibodies spotted in duplicate. Experiments were performed as previously described [19]. Briefly, array membranes were incubated for 30 minutes in blocking buffer and then incubated for 2 hours with 1.5 ml of the CM collected from BEAS2B, H1299, and A549 or medium of RPMI 1640 with 1% FBS as control (control media). The membranes were washed and a diluted cocktail of biotinylated antibodies was added for 90 minutes. Membranes were then washed again, and the sandwiched antigens were detected by incubation for 2 hours with a peroxidase-labeled streptavidin solution diluted to 1:1000. Proteins were detected finally by enhanced chemiluminescence, and signals were captured on x-ray films. Array images were acquired at a resolution of 300 ppi on an MICROTEK scanner and were quantified with Software ImageQuant TL (Amersham Biosciences Inc, Piscataway, NJ). The fold changes among samples were analyzed by using RayBio Antibody Array Analysis Tool provided by the company. For each spot, the net density gray level was determined by subtracting the background gray level from the total raw density gray levels. The data after background subtraction were normalized according to positive control densities.
Measurement of Cytokines Levels by ELISA
The cytokine levels of the conditioned media collected from BEAS2B, H1299, and A549 cells and serum from lung cancer patients and healthy donors were measured by appropriate ELISA kits. Sera from patients with localized lung cancer (n = 18, median age at the time of diagnosis was 66.61 years, range 58–88 years), patients with bone metastases (n = 7, median age at the time of diagnosis was 61.71 years, range 41–80 years), and healthy age-matched donors (n = 20, median age at the time of diagnosis was 61.85 years, range 39–79 years) were obtained retrospectively from the Department of Clinical Chemistry, Tianjin Chest Hospital. The hospital institutional review board approved the sample collection and experimental procedures. Quantikine human MCP-1, IL-8, growth-related oncogene α (GROα), epithelial neutrophil-activating peptide-78 (ENA-78), regulated upon activation normal T-cell expressed and secreted (RANTES), chemokine (C-X-C motif) ligand 16 (CXCL16), matrix metalloproteinase 9 (MMP-9), tissue inhibitor of metalloproteinase 4 (TIMP-4), IL-4, and IL-5 ELISA kits were purchased from R&D Systems. ELISAs were performed according to the instructions from the manufacturer.
Mouse Bone Marrow-Derived Monocyte Culture
Primary mouse bone marrow cells were obtained by flushing femora from 4- to 6-week-old C57BL/6 mice. The animal protocol was approved by the Institutional Animal Care and Use Committee, University of Pittsburgh. Briefly, the bone marrow cells were incubated in α-minimum essential medium with 20% FBS overnight at 37°C in 100-mm tissue culture plates to separate nonadherent and adherent cells. The nonadherent cells were collected and used in cultures as the source of osteoclast formation assay as described below.
Osteoclast Formation from Mouse Bone Marrow-Derived Monocyte
Osteoclast formation assays were performed by culturing 1 x 105 nonadherent cells per well in 96-well plates in 0.1 ml of α-minimum essential medium with 10% FBS for 7 to 10 days. The cells were incubated with recombinant mouse M-CSF (10 ng/ml) and/or RANKL (50 ng/ml) and 10% CM collected from the BEAS2B, H1299, or A549 cells in the presence of various doses of neutralizing antibody against MCP-1, IL-8, and RANKL or isotype control antibodies. Half of the media was changed at day 4. After 7 days of culture, the cells were fixed with 2% formaldehyde and stained with K-ASSAY TRAP staining kit (Kamiya Biomedical, Seattle, WA). The positive staining cells that contained three or more nuclei were counted as osteoclastlike cells. Analysis of all osteoclast formation experiments included data from three independent experiments.
Animal Studies
Male severe combined immunodeficient mice (SCID) mice (Charles River, Wilmington, MA), 6 weeks of age, were housed under pathogen-free conditions in accordance with the National Institutes of Health guidelines. The animal protocol was approved by the Institutional Animal Care and Use Committee, University of Pittsburgh. Thirty SCID mice were used in these experiments. Single-cell suspension of A549 (3 x 105 cells in 20-µl volume of Dulbecco's modified Eagle's medium with 1% FBS) cells that MCP-1 had been knocked down, at different proportions, by short hairpin RNA (shRNA) technique or the control cells were injected into the right tibia of SCID mice (n = 10 per group) as described previously [22]. The tumor cells were allowed to grow for 4 weeks, at which time the mice were killed. Evidence of tumor-induced bone changes was evaluated using radiography (Faxitron X-ray Corp., Wheeling, IL), histology, and bone histomorphometry. To obtain MCP-1 knockdown cells, the Block-it RNAi designer (Invitrogen) was used to design shRNA specific to MCP-1 (Accession No. NM_002982; position 150–197; 5′-TGC AAT CAA TGC CCC AGT CAC CTG CTG TTA TAA CTT CAC CAATAG GA-3′). The MCP-1 shRNA controls were generated by inverting the bases at positions 9 to 13 within the MCP-1 sequences and were used as scrambled shRNA. Resulting sequences were cloned into the RNA expression vector pENTR/H1/TO (Invitrogen) and sequences were confirmed. Either the MCP-1 shRNA or the scrambled shRNA was transfected into A549 cells using lipofectamine reagents, and individual clones were selected using 100 µg/ml Zeocin (Invitrogen). Seven clones were evaluated for MCP-1's functional knockdown. Specifically, CM was collected from two selected clones. These clones were used for the in vivo experiment.
Cell Proliferation Assay
Cell proliferation was measured with the use of a CellTiter 96 AQeous Nonradioactive Cell Proliferation Assay (Promega, Madison, WI). Briefly, the cells of selected A549 clones were plated in 96-well plates at a density of 3000 cells per well, respectively, in 200 µl of RPMI 1640 plus 0.5% FBS. Ten nanograms per milliliter of rhMCP-1 was added into the cultures. The cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 24, 48, 72, and 96 hours, then 20 µl of combined MTS/PMS solution was added. After incubation for 2 hours at 37°C, the absorbance of each well at 490 nm was recorded by using an ELISA plate reader.
Histopathology and Bone Histomorphometry
Histopathology was performed as previously described [23]. Briefly, bone specimens (tibiae) were fixed in 10% formalin for 24 hours then decalcified using 10% EDTA for 6 days. The specimens were then paraffin-embedded, sectioned (4 µM), and stained with hematoxylin and eosin (H&E) to assess histology or used for TRAP activity staining (acid phosphatase kit, Model 387-A; Sigma Diagnostics, St. Louis, MO). For TRAP staining, the specimens were stained with acid phosphatase and tartrate solution for 1 hour at 37°C. Four discontinuous random regions of interest were examined within each tibia. Histomorphometric analysis was performed on H&E-stained sections, and tumor volume was quantified using Bioquant Osteo II (Bioquant Image Analysis Corp, Nashville, TN) as previously described [23]. The terminology used was that recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research.
Statistical Analysis
Statistical analysis was performed using Statview software (Abacus Concepts, Berkley, CA). Analysis of variance was used for initial analyses, followed by Fisher's protected least significant difference for post hoc analyses. Student's t test was used for comparisons between two groups. Differences with a P < .05 were determined as statistically significant.
Results
Lung Cancer Cells Produced High Amounts of MCP-1 and IL-8
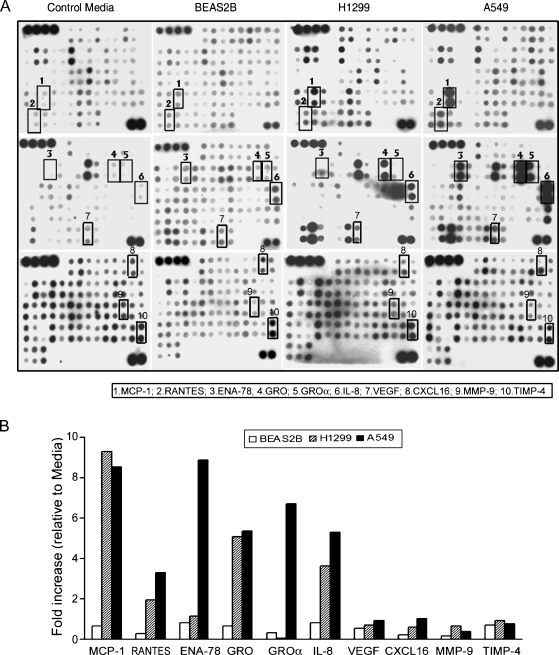
To identify factors that mediate lung cancer-induced bone lesions, a human cytokine antibody array was used to screen cytokine production by BEAS2B, H1299, and A549 cells using CM collected from these cells. Lung cancer cell lines H1299 and A549 produced high amounts of MCP-1, RANTES, ENA-78, GRO, GROα, IL-8, VEGF, CXCL16, and MMP-9 compared with nonmalignant BEAS2B cells (Figure 1, A and B). In addition, levels of GROα and ENA-78 were greater in A549 cells compared with those in H1299 cells. We did not observe significant differences in production of TIMP-4 (Figure 1, A and B), IL-4, and IL-5 (data not shown).
Figure 1.
Lung cancer cells produced high amounts of MCP-1 and IL-8. The cytokines' production in CM collected from lung cancer cells was measured using a human cytokine antibody array. (A) Images of the array that were assayed with CM obtained from control media, BEAS2B, H1299, and A549 cells. Details of the procedure are described in Materials and Methods. The signals of MCP-1, RANTES, ENA-78, GRO, GROα, IL-8, VEGF, CXCL16, MMP-9, and TIMP-4 were labeled with rectangles. (B) Radiographs of the arrays were scanned to determine the density of each cytokine. Data are presented as a mean value of fold increase relative to control media. The value for each scan was adjusted based on the intensity of positive control spots on each membrane.
Confirmation of Cytokine Array Data by ELISA
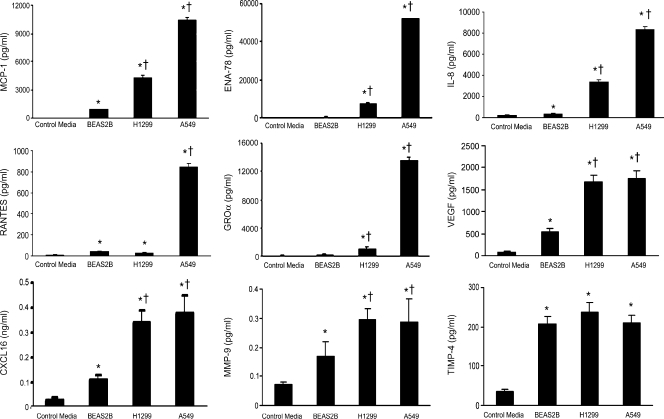
To validate the findings in cytokine array experiment, we measured, by ELISA, the levels of MCP-1, RANTES, ENA-78, GROα, IL-8, VEGF, CXCL16, MMP-9, TIMP-4, IL-4, and IL-5 in the CM used for the cytokine array. Consistent with the array observation, levels of MCP-1, ENA-78, GROα, IL-8, VEGF, CXCL16, and MMP-9 except for RANTES in H1299 and A549 cells were significantly greater than BEAS2B cells, whereas A549 produced the highest amount of these soluble factors (Figure 2). As expected, we did not observe significant differences in the production of TIMP-4 (Figure 2), IL-4, and IL-5 (data not shown).
Figure 2.
Confirmation of cytokine array data by ELISA. ELISA assays were performed with the CM used for the cytokine antibody array. Data are presented as mean ± SD from triplicates. *P < .001 compared with the control media. †P < .001 compared with BEAS2B.
Serum MCP-1 and IL-8 Levels Were Elevated in Lung Cancer Patients with Bone Metastases Compared with the Localized Cancer
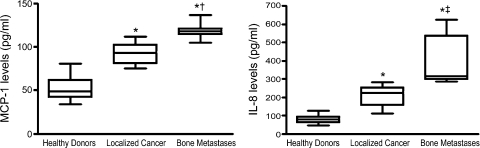
Both MCP-1 and IL-8 were reported to be produced by lung cancer A549 cells [24,25]; however, there are few reports on the clinical roles of both factors in lung cancer progression, although IL-8 was suggested as one of the important angiogenic factors in non-small cell lung cancer [25–29]. Therefore, serum MCP-1 and IL-8 levels in lung cancer patients and healthy age-matched donors were examined by ELISA. As shown in Figure 3, MCP-1 levels were significantly elevated in patients with localized lung cancer (mean ± SD was 92.28 ± 11.19 pg/ml) compared with healthy donor (mean ± SD was 52.95 ± 14.3 pg/ml). Importantly, MCP-1 levels were significantly increased in lung cancer patients with bone metastases (mean ± SD was 118.71 ± 9.60 pg/ml) compared with those in patients with localized cancer (P < .001, t test). IL-8 levels, in a similar manner, were significantly elevated in patients with localized lung cancer (mean ± SD was 206.33 ± 55.87 pg/ml) compared with those in healthy donors (mean ± SD was 79.25 ± 23.08 pg/ml). IL-8 levels were significantly increased in lung cancer patients with bone metastases (mean ± SD was 392.29 ± 134.23 pg/ml) compared with those in patients with localized cancer (P < .01, t test).
Figure 3.
Box plot of serum MCP-1 and IL-8 levels in lung cancer patients and healthy donors. Serum levels of MCP-1 and IL-8 in lung cancer patients with localized cancer (n = 18) and bone metastases (n = 7) and in healthy donors (n = 20) were measured by ELISA. The minimum detectable level of MCP-1 is typically less than 5.0 pg/ml. Statistical significance was determined using Student's t test. *P < .001 compared with healthy donors. †P < .001 compared with the patients with localized cancer. ‡P < .01 compared with the patients with localized cancer. Horizontal lines in the box represent the first, second (the median), and third quartiles; whiskers (vertical lines) represent extension of data up and down.
Neutralizing Antibodies against MCP-1 Inhibited the Lung Cancer CM-Induced Osteoclast Formation
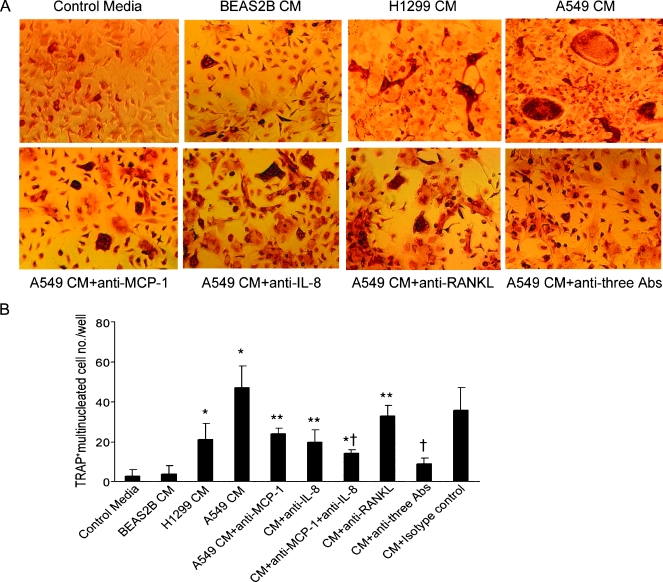
Next, to test the functional role of MCP-1 in lung cancer-induced bone resorptive lesions in vitro, we determined lung cancer A549 CM-induced osteoclast formation using mouse bone marrow-derived monocytes (MBMCs) in the presence of neutralizing antibodies for MCP-1, IL-8, RANKL, or combination of the two or three antibodies. A549 CM induced multinucleated osteoclast-like cell formation, and this induction was significantly diminished by each individual antibody, whereas the adding of three antibodies blocked more than 80% of osteoclast formation (Figure 4, A and B). Neutralizing antibody against MCP-1 efficiently blocked the osteoclast fusion. A549 CM-induced osteoclast formation and bone resorption from MBMC were dose-dependently inhibited by adding anti-MCP-1-neutralizing antibodies and were further synergistically inhibited with the addition of anti-IL-8 and anti-RANKL (data not shown). These results indicate that MCP-1 may play a key role in tumor-induced osteoclast formation in vitro.
Figure 4.
Neutralizing antibodies for MCP-1 inhibited lung cancer A549 CM-induced osteoclast formation. Nonadherent MBMC was cultured at 1 x 105 per well in 96-well plates for 7 to 10 days. The cells were incubated with recombinant mouse M-CSF (10 ng/ml) and/or RANKL (50 ng/ml) and 10% CM collected from the BEAS2B, H1299, or A549 cells in the presence of various doses of neutralizing antibodies against MCP-1 (1 µg/ml), IL-8 (200 ng/ml), and RANKL (1 µg/ml). (A) Representative micrographs of MBMC cell cultures stained for TRAP. (B) The number of osteoclast-like multinucleated cells per well was quantified. Samples were evaluated in quadruplicate. Results are reported as mean (±SD). Data were analyzed using one-way analysis of variance. *P < .001 compared with BEAS2B CM-treated group. **P < .01 compared with A549 CM-treated group. †P < .001 compared with A549 CM-treated group.
Knockdown of MCP-1 in A549 Cells Diminished the Tumor Growth in Bone
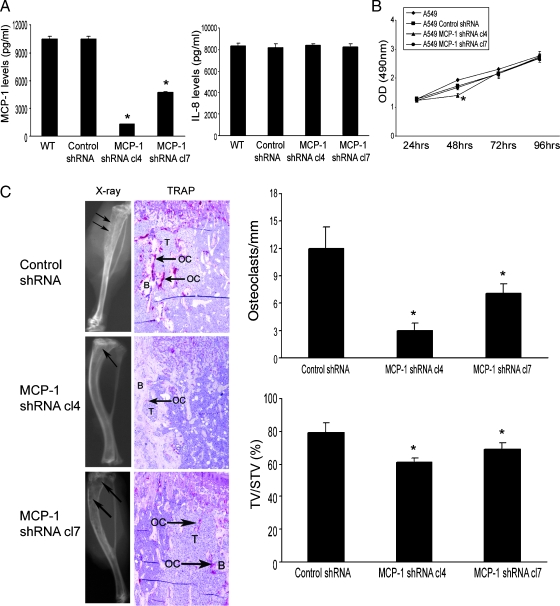
To determine the role of MCP-1 in lung cancer-induced bone resorptive lesions in vivo, the A549 cells were stably transfected with shRNA targeting MCP-1 or a scrambled shRNA. Production of MCP-1 in the knockdown cells was examined by ELISA. We confirmed 88% and 56% knockdown of the MCP-1 production in the selected clone 4 and clone 7, respectively (Figure 5A). We found that IL-8 levels were not altered in these selected clones. Using the selected A549 clones, we determined the cell proliferation in vitro by an MTS assay to rule out a possibility of off-target effects of stable siRNA expression. As shown in Figure 5B, we found minimal inhibition of cell proliferation in the selected clone 4 only at 48 hours but not at 72 and 96 hours. We did not observe significant inhibition in clone 7 at any time point. Then the two clones that stably transfected with shRNA targeting MCP-1 or the control cells that stably transfected with scrambled shRNA were injected into the tibiae of SCID mice (n = 10 per group). The tumors were allowed to grow for 4 weeks. The MCP-1 knockdown in A549 cells significantly diminish the tumor volume compared with the control cells determined by bone histomorphormetry (Figure 5C). Furthermore, TRAP staining at the tumor-bone interface demonstrated that the tumor-induced osteoclast activity was significantly reduced by MCP-1 knockdown A549 cells compared with the control cells (Figure 5C). The proportion of the MCP-1 knockdown for a given cell line was correlated with the proportion of ablation of the bone lesions and osteoclast activity at the tumor-bone interface. These results demonstrate a direct effect of MCP-1 produced by lung cancer cells on osteoclast activity in vivo.
Figure 5.
Knockdown MCP-1 in A549 cells diminished the tumor growth in bone. (A) ELISA was performed in the CM from the selected A549 cells that were stably transfected with shRNA targeting of either MCP-1 or a scrambled control. *P < .001 compared with wild type (WT) or control shRNA cells. (B) MCP-1 knockdown in A549 cells minimally decreased the tumor cell proliferation as determined by MTS assay. *P < .01, significant difference from the control cells by t test. (C) Single-cell suspension of the selected A549 cells was injected into the right tibia of SCID mice. The tumor cells were allowed to grow for 4 weeks, at which time the mice were killed. Evidence of tumor-induced bone osteolysis was evaluated by x-ray, H&E staining, and TRAP staining. On the x-ray film, arrows point to the tumor-induced osteolysis. On TRAP staining, T represents tumor cells, B represents bone, OC represents osteoclast, and arrows point to the increased osteoclast activities. Osteoclast number per millimeter bone surface is determined by bone histomorphometry. Tumor volume versus non-bone soft tissue volume was quantified by bone histomorphometry. Results are reported as mean ± SD. *P < .001 compared with the control cells.
Discussion
Chemokines comprise a family of structurally related small proteins that are involved in the inflammatory responses through their ability to recruit leukocytes. In cancer development, specifically in cancer bone metastases, the expression of certain chemokines, such as SDF-1/CXCL12, fractalkine/CX3CL1, IL8/CXCL8, and MCP-1/CCL2, and their receptors, CXCR4, CXCR1, CXCR2, and CCR2, respectively, has been identified in cancer cells and/or other cell types in the tumor growth microenvironment [15,21,30–37]. These cancers include various types of cancer, such as prostate and breast cancers, which are the tumors that preferentially metastasize to bone [15,30,34,35,37,38]. Although very frequent, bone lesions due to lung cancer have been neglected research-wise compared with the analog condition caused by other neoplasms. In this report, using a human cytokine antibody array, we found that both lung cancer cell lines A549 and H1229 produced significantly higher amounts of MCP-1, IL-8, RANTES, ENA 78, GROα, VEGF, CXCL16, and MMP-9 compared with BEAS2B cells. These results are similar to that we have reported in prostate cancer [19]. Although the roles of RANTES, ENA 78, GROα, VEGF, CXCL16, and MMP-9 in lung cancer development are currently ongoing studies, our results from this report suggest that MCP-1 mediates lung cancer-induced osteoclast activity both in vitro and in vivo.
From clinical serum specimens, we further observed that MCP-1 levels, as well as IL-8 levels, were elevated in patients with localized lung cancer compared with those in healthy donors. Importantly, we found that serum MCP-1 and IL-8 levels were increased in lung cancer patients with bone metastases compared with those in patients with localized cancer. These results suggest that MCP-1 and IL-8 could be used as biomarkers for the tumor progression, specifically for bone metastases. Consistent with this report, it has been reported that various cancer cells that preferentially home to bone, including prostate cancer, breast cancer, and myeloma cells, express MCP-1 and its receptor CCR2 [9,13–15]. In patients with breast and ovarian cancers, it has been also reported that increased serum MCP-1 levels are associated with the tumor stages [16,17,39].
Osteoclast activities are important to the development of bone metastases in solid cancer types. Soluble factors, for example, IL-8, were recently reported to promote tumor-induced osteolysis in breast cancer bone metastatic animal model [40]. In the current report, we extended these findings to lung cancer. We showed that A549 CM induced osteoclast formation that was inhibited by neutralizing antibodies against MCP-1. This effect was additive to adding neutralizing antibodies against IL-8 and RANKL, two of the key regulators in osteoclastogenesis. We postulate that a few cytokines, in a manner of network, may play key roles in the interaction between the tumor cells and the cells in the bone microenvironment.
To determine the effects of deleting MCP-1 expression on lung cancer A549 cells-induced bone resorptive lesions in animals, we used an intratibial injection murine model. The selected MCP-1 knockdown A549 cells were injected into SCID mice. We showed that MCP-1 knockdown A549 cells significantly diminished the tumor growth in bone determined by histopathology and bone histomorphometry. We also observed reduction in numbers of tumor-induced osteoclasts at the tumor and bone interface, which is consistent with the observed inhibition of osteoclast formation in vitro. Furthermore, our in vivo data have demonstrated the intrinsic nature of A549 cells to be osteoclastic. Thus, a decrease in A549 growth in bone is associated with a reduction in osteoclast activity. Therefore, we conclude that MCP-1, produced by human lung cancer cells, promotes the tumor-induced resorptive lesions in vivo. In support of our findings, it was very recently reported that systemic delivery of neutralizing antibodies against MCP-1 induced prostate cancer tumor regression in murine models [21].
Critically, we observed that the proportion of the MCP-1 knockdown in A549 cells was correlated with the proportion of ablation of the bone lesions. These results suggest that MCP-1 is indeed one of the key mediators in the lung cancer cell-induced bone lesions. To consider off-target effects of stable siRNA expression, we performed an in vitro cell proliferation assay to test whether MCP-1 knockdown affects tumor cell proliferation. Interestingly, we observed minimal, if there is any, inhibition of A549 cell proliferation. These results excluded the possibility that MCP-1 knockdown in A549 cells that diminished the tumor growth in bone was solely due to the decreased cell proliferation. Taken together, the most interesting significance of the current study is the increased MCP-1 in the circulation in lung cancer patients with bone metastases compared with the localized cancer. Future studies should address the origin of MCP-1 and address whether it could be used as a prognostic biomarker for cancer development. Nevertheless, the results from this study warrant future clinical exploration of targeting MCP-1 that could eventually be used in a variety of cancer types.
Acknowledgments
The authors thank G. David Roodman for his helpful discussion.
Footnotes
This work was supported in part by funds from the Department of Defense (PC061231 to J.Z.) and from the University of Pittsburgh Cancer Institute (CCSG to J.Z.) and by Key Project of the National Science Foundation of China (30430300 to Q.Z.).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Parkin DM, Li LD, Chen YD, Bray F. Estimation and projection of the national profile of cancer mortality in China: 1991–2005. Br J Cancer. 2004;90:2157–2166. doi: 10.1038/sj.bjc.6601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulshine JL, Sullivan DC. Clinical practice. Lung cancer screening. N Engl J Med. 2005;352:2714–2720. doi: 10.1056/NEJMcp042630. [DOI] [PubMed] [Google Scholar]
- 4.Keller ET, Dai J, Escara-Wilke J, Hall CL, Ignatoski K, Taichman RS, Keller J. New trends in the treatment of bone metastasis. J Cell Biochem. 2007;102:1095–1102. doi: 10.1002/jcb.21540. [DOI] [PubMed] [Google Scholar]
- 5.Becker S, Quay J, Koren HS, Haskill JS. Constitutive and stimulated MCP-1, GRO alpha, beta, and gamma expression in human airway epithelium and bronchoalveolar macrophages. Am J Physiol. 1994;266:L278–L286. doi: 10.1152/ajplung.1994.266.3.L278. [DOI] [PubMed] [Google Scholar]
- 6.Bernardini G, Ribatti D, Spinetti G, Morbidelli L, Ziche M, Santoni A, Capogrossi MC, Napolitano M. Analysis of the role of chemokines in angiogenesis. J Immunol Methods. 2003;273:83–101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 7.Tangirala RK, Murao K, Quehenberger O. Regulation of expression of the human monocyte chemotactic protein-1 receptor (hCCR2) by cytokines. J Biol Chem. 1997;272:8050–8056. doi: 10.1074/jbc.272.12.8050. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda T, Kitadai Y, Tanaka S, Yang X, Mukaida N, Yoshihara M, Chayama K. Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res. 2005;11:7629–7636. doi: 10.1158/1078-0432.CCR-05-0798. [DOI] [PubMed] [Google Scholar]
- 9.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 10.Antoniades HN, Neville-Golden J, Galanopoulos T, Kradin RL, Valente AJ, Graves DT. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 1992;89:5371–5375. doi: 10.1073/pnas.89.12.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyonaga K, Takeya M, Saita N, Sakamoto O, Yoshimura T, Ando M, Takahashi K. Monocyte chemoattractant protein-1 in idiopathic pulmonary fibrosis and other interstitial lung diseases. Hum Pathol. 1994;25:455–463. doi: 10.1016/0046-8177(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 12.Car BD, Meloni F, Luisetti M, Semenzato G, Gialdroni-Grassi G, Walz A. Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Crit Care Med. 1994;149:655–659. doi: 10.1164/ajrccm.149.3.8118632. [DOI] [PubMed] [Google Scholar]
- 13.Vande Broek I, Asosingh K, Vanderkerken K, Straetmans N, Van Camp B, Van Riet I. Chemokine receptor CCR2 is expressed by human multiple myeloma cells and mediates migration to bone marrow stromal cell-produced monocyte chemotactic proteins MCP-1, -2 and -3. Br J Cancer. 2003;88:855–862. doi: 10.1038/sj.bjc.6600833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janis K, Hoeltke J, Nazareth M, Fanti P, Poppenberg K, Aronica SM. Estrogen decreases expression of chemokine receptors, and suppresses chemokine bioactivity in murine monocytes. Am J Reprod Immunol. 2004;51:22–31. doi: 10.1046/j.8755-8920.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, Melhem MF, Yao Z, Zhang J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 16.Lebrecht A, Hefler L, Tempfer C, Koelbl H. Serum cytokine concentrations in patients with cervical cancer: interleukin-4, interferon-gamma, and monocyte chemoattractant protein-1. Gynecol Oncol. 2001;83:170–171. doi: 10.1006/gyno.2001.6361. [DOI] [PubMed] [Google Scholar]
- 17.Hefler L, Tempfer C, Heinze G, Mayerhofer K, Breitenecker G, Leodolter S, Reinthaller A, Kainz C. Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br J Cancer. 1999;81:855–859. doi: 10.1038/sj.bjc.6690776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y, Xiao G, Galson DL, Nishio Y, Mizokami A, Keller ET, Yao Z, Zhang J. PTHrP-induced MCP-1 production by human bone marrow endothelial cells and osteoblasts promotes osteoclast differentiation and prostate cancer cell proliferation and invasion in vitro. Int J Cancer. 2007;121:724–733. doi: 10.1002/ijc.22704. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, Roodman GD, Zhang J. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao Z, Zhang J. CCR2 expression correlates with prostate cancer progression. J Cell Biochem. 2007;101:676–685. doi: 10.1002/jcb.21220. [DOI] [PubMed] [Google Scholar]
- 21.Loberg RD, Day LL, Harwood J, Ying C, St John LN, Giles R, Neeley CK, Pienta KJ. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107:1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Dai J, Yao Z, Lu Y, Dougall W, Keller ET. Soluble receptor activator of nuclear factor kappaB Fc diminishes prostate cancer progression in bone. Cancer Res. 2003;63:7883–7890. [PubMed] [Google Scholar]
- 24.Wang H, Yi T, Zheng Y, He S. Induction of monocyte chemoattractant protein-1 release from A549 cells by agonists of protease-activated receptor-1 and -2. Eur J Cell Biol. 2007;86:233–242. doi: 10.1016/j.ejcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med. 1994;179:1409–1415. doi: 10.1084/jem.179.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Serum vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) levels in small cell lung cancer. Cancer Invest. 2006;24:492–496. doi: 10.1080/07357900600814771. [DOI] [PubMed] [Google Scholar]
- 27.Orditura M, De Vita F, Catalano G, Infusino S, Lieto E, Martinelli E, Morgillo F, Castellano P, Pignatelli C, Galizia G. Elevated serum levels of interleukin-8 in advanced non-small cell lung cancer patients: relationship with prognosis. J Interferon Cytokine Res. 2002;22:1129–1135. doi: 10.1089/10799900260442557. [DOI] [PubMed] [Google Scholar]
- 28.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996;97:2792–2802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Strom SR, Burdick MD, Iannettoni MD, Strieter RM. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother. 2000;49:63–70. doi: 10.1007/s002620050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 31.Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117:359–367. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 32.Soejima K, Rollins BJ. A functional IFN-gamma-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J Immunol. 2001;167:6576–6582. doi: 10.4049/jimmunol.167.11.6576. [DOI] [PubMed] [Google Scholar]
- 33.Scotton C, Milliken D, Wilson J, Raju S, Balkwill F. Analysis of CC chemokine and chemokine receptor expression in solid ovarian tumours. Br J Cancer. 2001;85:891–897. doi: 10.1054/bjoc.2001.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res. 2004;64:4693–4698. doi: 10.1158/0008-5472.CAN-03-3437. [DOI] [PubMed] [Google Scholar]
- 35.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58:1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 36.Inoue K, Slaton JW, Kim SJ, Perrotte P, Eve BY, Bar-Eli M, Radinsky R, Dinney CP. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 2000;60:2290–2299. [PubMed] [Google Scholar]
- 37.Reiland J, Furcht LT, McCarthy JB. CXC-chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate. 1999;41:78–88. doi: 10.1002/(sici)1097-0045(19991001)41:2<78::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 38.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 39.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O'Brien T, Kerin MJ. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 40.Bendre MS, Margulies AG, Walser B, Akel NS, Bhattacharrya S, Skinner RA, Swain F, Ramani V, Mohammad KS, Wessner LL, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-κB ligand pathway. Cancer Res. 2005;65:11001–11009. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]