Abstract
Germ cell tumor development in humans has been proposed to be part of testicular dysgenesis syndrome (TDS), which manifests as undescended testes, sterility, hypospadias, and, in extreme cases, as germ cell tumors. Males of the Ter mouse strain show interesting parallels to TDS because they either lack germ cells and are sterile or develop testicular germ cell tumors. We found that these defects in Ter mice are due to mutational inactivation of the Dead-end (Dnd1) gene. Here we report that chromo-some X modulates germ cell tumor development in Ter mice. We tested whether the X or the Y chromosome influences tumor incidence. We used chromosome substitution strains to generate two new mouse strains: 129-Ter/Ter that carry either a C57BL/6J (B6)-derived chromosome (Chr) X or Y. We found that Ter/Ter males with B6-Chr X, but not B6-Chr Y, showed a significant shift in propensity from testicular tumor development to sterile testes pheno-type. Thus, our studies provide unambiguous evidence that genetic factors from Chr X modulate the incidence of germ cell tumors in mice with inactivated Dnd1.
Introduction
Testicular germ cell tumors in humans and mice originate from germ cells during fetal development (Rajpert-De Meyts et al. 1998; Skakkebaek et al. 1987; Stevens 1967). Although these tumors occur in males of all ages, from infants to men over 50 years old, they are the most common cancers in young males (better known as testicular cancer) between the ages of 15 to 35 years (Oosterhuis and Looijenga 2005). Moreover, testicular germ cell tumor development in humans has been proposed to be part of a broader testicular dysgenesis syndrome (TDS), which manifests as undescended testes, sterility, hypospadias, and, in extreme cases, as germ cell tumors (Skakkebæk et al. 2001). There is strong evidence for genetic predisposition to germ cell tumor development (Tollerud et al. 1985; United Kingdom Testicular Cancer Study Group 1994) and studies suggest that multiple susceptibility loci with weak effects contribute to the disease (Crockford et al. 2006).
The Ter mouse strain has interesting parallels to human germ cell tumor development and TDS because males of this strain either lack germ cells and have small, sterile testes or they develop testicular germ cell tumors. Germ cell tumors of the Ter/Ter mice were originally reported on the 129 strain background (129-Ter/Ter males) (Noguchi and Noguchi 1985; Stevens 1973). These tumors in mice are similar to pediatric germ cell tumors (or testicular type I germ cell tumors) (Horwich et al. 2006; Oosterhuis and Looijenga 2005; Rescorla 1999) in humans.
Males homozygous for Ter on a 129 mouse strain background (129-Ter/Ter) develop either of two pheno-types: the majority develops testicular germ cell tumors (TGCT) whereas others have small testes lacking germ cells. The defect in Ter mice is inactivation of the Dnd1 gene. Inactivation of Dnd1 causes progressive loss of primordial germ cells (PGC) during embryogenesis thus resulting in sterility (Weidinger et al. 2003; Youngren et al. 2005). In addition, for reasons not understood, some PGCs escape death to undergo cancerous transformation to eventually develop into testicular tumors.
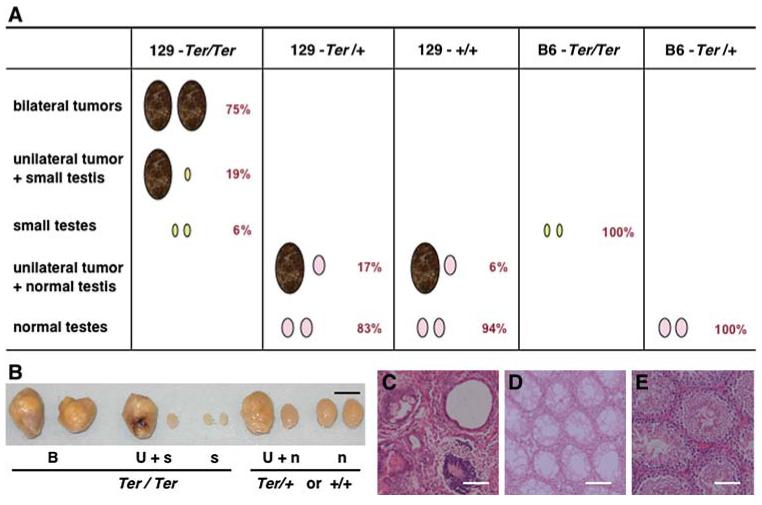
We sought to identify genetic loci that modulate the testicular phenotypes of Ter mice. In other words, what determines development of tumors rather than sterile testes? For our studies we made use of the fact that the testes phenotypes of Ter mice on 129 and other strain backgrounds have been very well characterized (Noguchi and Noguchi 1985). Mice homozygous for Ter (also referred to as Ter/Ter, Dnd1Ter/Ter, or Dnd1-/-) can be distinguished from heterozygous Ter mice or wild-type mice based on their testicular phenotypes (Noguchi and Noguchi 1985) (as illustrated in Fig. 1). 129-Ter/Ter mice either develop testicular tumors or have small, sterile testes. Ninety-four percent of 129-Ter/Ter males have at least one tumor and 75% of the tumors affect both testes (bilateral). The rest (6%) have bilateral small testes lacking germ cells and for these reasons Ter/Ter males are infertile (Fig. 1). In contrast, most heterozygote males (129-Ter/+) are normal but some develop testicular tumors (17% incidence). The tumors in 129-Ter/+ males usually affect only one testis (unilateral) and the contralateral testis is normal (Fig. 1B and E). The 129-Ter/+ males with unilateral tumor and contra-lateral normal testes are fertile and able to sire progeny. About 6% of 129-+/+ males also develop testicular germ cell tumors (Matin et al. 1999; Noguchi and Noguchi 1985). Tumors of the 129-+/+ males are unilateral and the contralateral testis is normal, as in 129-Ter/+ males. Thus, these testicular phenotypes distinguish 129-Ter/Ter from the 129-Ter/+ and 129-+/+ males. The phenotypes can also be verified by genotyping (Youngren et al. 2005).
Fig. 1.
A Illustration of testicular phenotypes that distinguish Ter/Ter males from Ter/+ and +/+ males. Ter/Ter males on 129 strain background have bilateral tumors (75% of Ter/Ter males), unilateral tumors accompanied with small testis (19% of Ter/Ter males), and bilateral small testes (6% of Ter/Ter males) (Noguchi and Noguchi 1985). Seventeen percent of Ter/+ and 6% of 129-+/+ males develop unilateral testicular tumors with contralateral normal testes. The majority have normal testes. When Ter/Ter is congenic on the C57BL/6J strain (mice designated as B6.129-Ter/Ter or B6-Ter/Ter), 100% of B6-Ter/Ter mice develop bilateral small testes. In contrast, all B6-Ter/+ males have normal testes. B Testicular phenotypes of the Ter strain. Examples of bilateral tumors (B), unilateral tumor with contralateral small testis (U+s), bilateral small testes (s) from 129-Ter/Ter adult males compared to unilateral tumor with contralateral normal testis (U+n), or normal (n) testes from 129-Ter/+ and 129-+/+ mice. Black scale bar = 0.5 cm. C Histology of testicular tumor from male, adult 129-Ter/Ter showing differentiated cell types. D Histology of small testes with no germ cells from male, adult 129-Ter/Ter. E Histology of normal testes from male, adult 129-Ter/+ mice. White scale bar = 100 μm
In addition, tumor development due to Ter occurs in a 129 strain-specific manner because when Ter is transferred onto the C57BL/6J background to create a B6.129-Ter congenic strain (or referred to as B6-Ter congenic strain), tumor incidence decreases to zero and the predominant phenotype is sterile, small gonads (Sakurai et al. 1994, 1995; Youngren et al. 2005) (illustrated in Fig. 1). The B6-Ter strain carries a 5-Mb region from 129-Ter that flanks the Ter locus and has been made congenic on the C57BL/6J strain background (Youngren et al. 2005) (Fig. 2A).
Fig. 2.
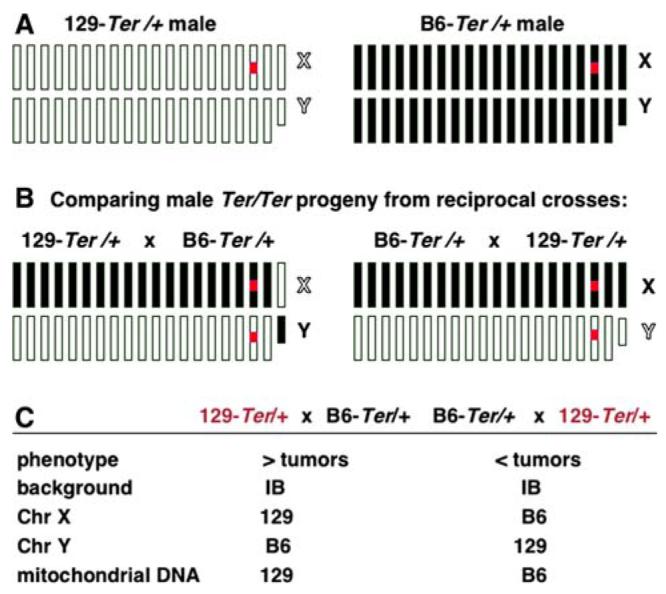
Parental effects on tumor development. A Diagram illustrating autosomal and sex chromosomes of 129-Ter/+ and B6-Ter/+ males. White boxes represent 129 chromosomes and black boxes represent C57BL/6J chromosomes. The X chromosome (marked X) is the 20th box and Y (marked Y) is represented by the smaller box. The red box represents the Ter (Dnd1Ter) mutation on Chr 18. B (left) Schematic diagram of chromosomes of male Ter/Ter progeny expected from reciprocal intercrosses of 129-Ter/+ female and B6-Ter/+ male. The progeny from both sets of crosses will be heterozygous for every autosome as represented by white and black boxes. The X chromosome will be derived from the 129 mother and the Y chromosome will be from the B6 father. (right) Chromosomes of progeny expected from the intercross B6-Ter/+female × 129-Ter/+ male. The X chromosome will be derived from B6 mother and the Y chromosome will be from the 129 father. C Summary of characteristics of progeny from the two crosses. IB refers to heterozygous for 129 and B6. Progeny from 129-Ter/+ female × B6-Ter/+ male cross have a higher incidence of tumors compared to those from the reciprocal cross whose parents are B6-Ter/+ female × 129-Ter/+ male
Materials and methods
Mouse strains
129-Ter (129T1/Sv-+pTyrc-ch Ter/+@Na) and B6.129-Ter (also referred to as B6-Ter) have been described (Youngren et al. 2005). 129S1/SvImJ-Chr YC57BL/6J/NaJ (also referred to as 129.B6-Chr Y or 129-X1YB, Jackson Laboratory stock No. 005548) and 129S1/SvImJ-Chr XC57BL/6J/NaJ (also referred to as 129.B6-Chr X, 129-XBY1 male, 129-XBXB female) were made as described below. C57BL/6J (also referred to as B6) was Jackson Laboratory stock No. 000664. Mice were maintained on a 12/12-h light/dark cycle, and fed NIH-31 diet ad libitum.
Generation of 129.B6-Chr X and 129. B6-Chr Y chromosome substitution strains (or consomic strains)
The 129.B6-Chr X strain was made by intercrossing C57BL/6J and 129. The progeny were backcrossed to 129 strains for the next nine generations. At each generation, tail DNA from progeny was genotyped for SSLP (simple sequence length polymorphism) markers DXMit136, DXMit208, DXMit64, and DXMit186, which are polymorphic between the 129 and C57BL/6J strains. These markers map at 2.8 cM, 16.5 cM, 45.0 cM, and 69 cM along mouse Chr X. Progeny that carried B6 alleles for all the Chr X markers, as determined by genotyping, were selected and backcrossed to 129. At the tenth generation, less than 0.2% of the genome, except for the selected X chromosome, is expected to be of B6 origin. For at least one cross, the female used was 129 such that the mitochondrial DNA in this strain is 129-derived. Male and female mice of the tenth generation were intercrossed. Selected females that were homozygous for B6-derived Chr X, as determined by genotyping, were crossed to males with B6-derived Chr X and the line has been subsequently maintained by brother-sister mating so that males and females of this strain carry B6-derived Chr X.
The 129.B6-Chr Y strain was made by an initial intercross of 129 female and C57BL/6J male. Male progeny from the next and subsequent generations were backcrossed to 129 strain females for another nine generations. As 129 strain females were was used in the crosses, mitochondrial DNA in this strain is also 129-derived.
Creation of 129-Ter/Ter,XBY1 and 129-Ter/Ter,X1YB
A three-generation cross was used to derive 129-Ter/Ter,XBY1 males. Intercrosses were set up to prevent recombination of the B6-derived X during the crosses. For cross I, 129.B6-X (CSS, 129-XBXB) females were inter-crossed to male 129-Ter/+ (or 129-Ter/+,X1Y1). The male progeny from this cross were selected by genotyping for Ter/+. These males would carry B6-derived X (129-Ter/ +,XBY1). For the second cross (II), 129-Ter/+,XBY1 males were intercrossed with 129.B6-X (129-XBXB) females. The female progeny from this cross were selected by geno-typing for Ter/+. These females would carry two B6-derived X (129-Ter/+,XBXB). In the final cross (III), 129-Ter/+,XBXB females from II were crossed to 129-Ter/ +,XBY1 males. Approximately 25% of the resulting progeny are expected to be Ter/Ter and carry B6-derived Chr X (129-Ter/Ter,XBY1). These males should display one of three testicular phenotypes shown in Fig. 1.
A two-generation cross was used to derive 129-Ter/Ter,X1YB males. For cross I, 129-Ter/+ females (or 129-Ter/+,X1X1) were intercrossed to male 129.B6-Y (CSS, 129-XIYB). The male progeny from this cross were selected by genotyping for Ter/+. These males would carry B6-derived Y (129-Ter/+,X1YB). For the second cross (II), 129-Ter/+ females were intercrossed with 129-Ter/+,X1YB males. Approximately 25% of the resulting progeny are expected to be Ter/Ter and carry B6-derived Chr Y (129-Ter/Ter,X1YB) and display one of three testicular pheno-types of Ter/Ter as shown in Fig. 1.
Genotyping
Genotyping for Ter was performed using primers 4.13a-F and R followed by Dde1 digestion and electrophoresis as described (Youngren et al. 2005).
Tumor characterization
Adult males (4-6 weeks old) were sacrificed and their testes were examined for tumors. Most tumors can be detected visually at that age. For some cases, it was unclear by visual inspection if tumors were present in the testes. For these cases, histologic sections of the testes were evaluated for presence of tumors. The tissues were preserved in 10% phosphate-buffered formalin for at least 48 h before sectioning (5 μm) and staining with hematoxylin and eosin.
Results
The incidences of tumors or sterile testes in Ter mice on the 129 and B6 strain backgrounds have been well characterized (Noguchi and Noguchi 1985) and, therefore, we used the phenotypes of Ter (as described above and in Fig. 1) to identify genetic loci that modulate the testicular pheno-types of Ter mice. The clue about the involvement of the sex chromosomes in modulating tumor development came first from our observation of parental effects on progeny from reciprocal crosses using the 129-Ter and the B6-Ter strains.
Reciprocal crosses were set up using heterozygous mice from the two strains (female × male): (cross 1) 129-Ter/ + × B6-Ter/+ and (cross 2) B6-Ter/+ × 129-Ter/+ (Fig. 2). More than 100 male progeny from the two crosses were examined for tumors or small testes (Table 1). Seventeen male progeny from the 129-Ter/+ × B6-Ter/+ cross developed testicular tumors whereas 54 had small gonads (Table 1). In contrast, 29 male progeny from the B6-Ter/ + × 129-Ter/+ cross had bilateral small gonads but only 1 developed a testicular tumor. The testicular phenotypes (incidence of tumors or small testes) of male progeny from the two reciprocal crosses were significantly different (Fisher’s exact test) with p = 0.0115.
Table 1.
Parental effect on tumor development in progeny from reciprocal crosses of 129-Ter/+ and B6-Ter/+ mice
| Genotype Phenotype |
Ter/Ter or Ter/+ No. of males with unilateral or bilateral tumors |
Ter/Ter No. of males with small testesa |
Total No. of males | |
|---|---|---|---|---|
| Cross 1 | 129-Ter/+ × B6-Ter/+ | 17 (12%) | 54 (38%) | 143 |
| Cross 2 | B6-Ter/+ × 129-Ter/+ | 1 (1%) | 29 (28%) | 102 |
The incidence of tumors and small testes of the 143 male progeny from cross 1 was significantly different compared to that of the 102 male progeny from cross 2, with p = 0.0115 (Fisher’s exact test, 2-sided p value)
Percentages indicate fraction of males with the specified phenotype compared to the total number of males
Bilateral small testes
The male progeny from both cross 1 and cross 2 are identical in the constitution of their autosomes in that they are heterozygous for 129 and B6 chromosomes (illustrated in Fig. 2B and C). Male progeny with tumors or small gonads carry Ter/Ter alleles on Chr 18 (a small percent of males with tumors may also be Ter/+). However, progeny from crosses 1 and 2 differ regarding their Chr X, Chr Y, and mitochondrial genome (Fig. 2C). Male progeny derived from the 129-Ter/+ × B6-Ter/+ cross (cross 1) carry 129-derived Chr X, B6-derived Chr Y, and 129-derived mitochondrial genome. In contrast, male progeny from the B6-Ter/+ × 129-Ter/+ cross (cross 2), carry B6-derived Chr X, 129-derived Chr Y, and B6-derived mitochondrial genome.
To test if the strain source of Chr X or Y modulates testicular tumor development as opposed to small gonads, we generated two new mouse strains. We generated mice of 129 background, carrying the Ter defect, and that carry either Chr X or Chr Y from the B6 strain. These mice were made by crossing the 129-Ter/+ strain with the chromo-some substitution strains (CSS) 129.B6-Chr X and 129.B6-Chr Y (Fig. 3 and Supplementary Fig. 1). 129.B6-Chr X (for clarity, they are also referred to as 129-XBY1 males and 129-XBXB females) carries the intact Chr X from the B6 strain. The 129.B6-Chr Y strain (also referred to as 129-X1YB males) carries the intact Chr Y from the B6 strain on 129 background (XB and X1 denote Chr X from B6 and 129 strains, respectively; YB and Y1 denote Chr Y from B6 and 129 strains, respectively). The derivation and description of the two CSS is described in the Methods section and Supplementary Fig. 1. The gonads of the males of the CSS, 129.B6-Chr X and 129.B6-Chr Y strains, are normal. The testicular tumor incidences of 129.B6-Chr X and 129.B6-Chr Y strains were 0 and 4.5% (unilateral tumors with normal contralateral testes), respectively.
Fig. 3.
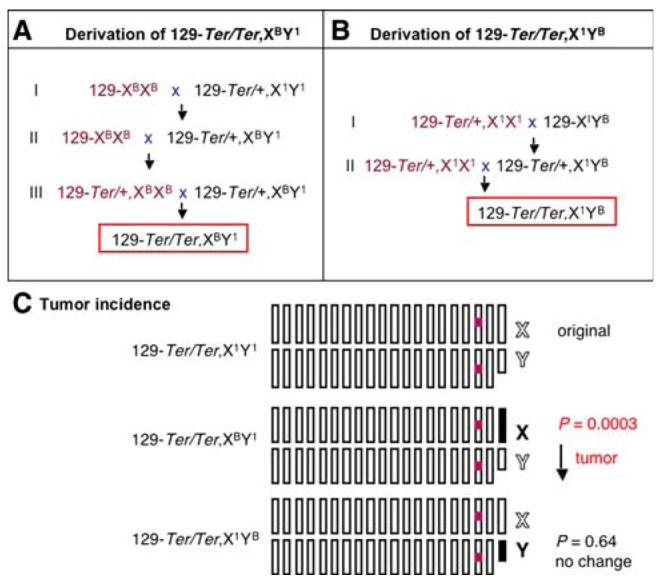
Derivation of 129-Ter/Ter males with B6-derived Chr X or Chr Y. A A three-generation cross was used to derive 129-Ter/Ter males with B6-derived Chr X. Intercrosses were set up to prevent recombination of the B6-derived Chr X during the crosses. For cross I, 129.B6-X (129-XBXB) females were intercrossed to male 129-Ter/+ (designated as 129-Ter/+,X1Y1). Male progeny were selected by genotyping for Ter/+. These males would carry B6-derived Chr X (129-Ter/+,XBY1). For the second cross (II), 129-Ter/+,XBY1 males were intercrossed with 129.B6-X (129-XBXB) females. Female progeny were selected for Ter/+. These females would carry two B6-derived Chr X (129-Ter/+,XBXB). In the final cross (III), 129-Ter/ +,XBXB females from II were crossed to 129-Ter/+,XBY1 males. Approximately 25% of the resulting progeny are expected to be Ter/Ter and carry B6-derived Chr X (129-Ter/Ter,XBY1) and display one of three testicular phenotypes shown in Fig. 1. B A two-generation cross was used to derive 129-Ter/Ter males with B6-derived Chr Y. For cross I, 129-Ter/+ females (designated as 129-Ter/+,X1X1) were intercrossed to male 129.B6-Y (129-XIYB). Male progeny were selected by genotyping for Ter/+. These males would carry B6-derived Chr Y (129-Ter/+,X1YB). For the second cross (II), 129-Ter/+ females were intercrossed with 129-Ter/+,X1YB males. Approximately 25% of the resulting progeny are expected to be Ter/Ter and carry B6-derived Chr Y (129-Ter/Ter,X1YB) and display one of three testicular phenotypes of Ter/Ter as shown in Fig. 1. C Summary of the incidence of testicular tumors in males of the new 129-Ter/Ter,XBY1 and 129-Ter/Ter,X1YB strains compared to the original 129-Ter/Ter strain (Noguchi and Noguchi 1985). The chromosomes of the original 129-Ter/Ter males (referred to as 129-Ter/Ter,X1Y1) and the new strains 129-Ter/Ter,XBY1 and 129-Ter/Ter,X1YB are shown. 129 chromosomes are represented by white boxes and B6 by black boxes. The X chromosome (marked X) is the 20th box and the Y (marked Y) is represented by the smaller box. The red box represents the Ter (Dnd1Ter) mutation
Crosses of 129-Ter/+ with the two CSS were used to derive males homozygous for Ter and with either XB or YB (see the Methods section and Fig. 3 for details of the cross). We then compared the effect of substituting the 129 for B6-derived Chr X or Y on the testicular phenotypes of Ter.
The testes of all 129-Ter/Ter male progeny with Chr XB or YB were examined for testicular tumors and small gonads (Table 2). At this stage, the laterality of the tumors (bilateral tumors versus unilateral tumors) and whether tumors were accompanied with small, sterile, contralateral testes were noted (Table 2). Our results showed that 40% of 129-Ter/Ter,XBY1 males had bilateral tumors, 32% had unilateral tumors accompanied with small, contralateral testes, and 28% had bilateral small testes. In contrast, 72% of the 129-Ter/Ter,X1YB males had bilateral testicular tumors, 25% had unilateral tumors plus small contralateral testes, and only 3% had bilateral, small testes. Thus, the incidence of tumors and small testes (testicular profiles) of 129-Ter/Ter,XBY1 males was significantly different from that of 129-Ter/Ter,X1YB males (p = 0.0057). The overall incidence of testicular tumors had decreased by 25%. The incidence of bilateral testicular tumors had decreased by 30% in 129-Ter/Ter,XBY1 compared to 129-Ter/Ter,X1YB males. There was a corresponding 25% increase in incidence of bilateral small, sterile testes in 129-Ter/Ter,XBY1 males. Thus, the decrease in testicular tumor incidence in Ter mice due to substitution of B6-Chr X was modest (decease by 25-30%) but nevertheless significant (p = 0.0057).
Table 2.
Comparing the incidence of tumors and small testes from the new strains 129-Ter/Ter,X1YB and 129-Ter/Ter,XBY1 to that of the original 129-Ter/Ter strain
| Genotype Phenotype |
Ter/Ter No. of males with bilateral tumors |
Ter/Ter No. of males with unilateral tumors + small testes |
Ter/Ter No. of males with small testesa |
Total No. of Ter/Ter males |
Total No. of males |
|---|---|---|---|---|---|
| 129-Ter/Ter,X1YB | 26 (72%) | 9 (25%) | 1 (3%) | 36 | 128 |
| 129-Ter/Ter,XBY1 | 10 (40%) | 8 (32%) | 7 (28%) | 25 | 88 |
| 129-Ter/Ter,X1Y1 | 133 (75%) | 34 (19%) | 11 (6%) | 178b |
The incidence of tumors and small testes in males of the 129-Ter/Ter,X1YBstrain and the 129-Ter/Ter,XBY1strain was significantly different, p = 0.0057 (Fisher’s exact test)
The incidence of tumors and small testes in males of the 129-Ter/Ter,X1YBstrain and the original 129-Ter/Ter strain (129-Ter/Ter,X1Y1) was not different, p = 0.64 (Fisher’s exact test)
The incidence of tumors and small testes in males of the 129-Ter/Ter,XBY1strain and the original 129-Ter/Ter strain (129-Ter/Ter,X1Y1) was significantly different, p = 0.0003 (Fisher’s exact test)
Percentages indicate fraction of Ter/Ter males with the specified phenotype compared to the total number of Ter/Ter males
Bilateral small testes
Numbers for 129.Ter/Ter are from Noguchi and Noguchi (1985)
We compared the testicular profiles of the males of the new strains 129-Ter/Ter,XBY1 and 129-Ter/Ter,X1YB to the testicular profile reported for the original 129-Ter/Ter (or 129-Ter/Ter,X1Y1) strain (Noguchi and Noguchi 1985). The incidence of tumors and small gonads of the 129-Ter/Ter,X1YB males was not significantly different from that of the original 129-Ter/Ter,X1Y1 males (Noguchi and Noguchi 1985) (p = 0.64) (Table 2). This indicates that Chr Y from either 129 or B6 has no significant effect on incidence of tumor development due to Ter. In contrast, the incidence of tumors and small gonads of the 129-Ter/Ter,XBY1 males was significantly different from that of the original strain 129-Ter/Ter,X1Y1 males (Noguchi and Noguchi 1985) (p = 0.0003) (Table 2). We thus conclude that the presence of B6-derived Chr X in Ter (Dnd1-/-) mice results in decreased incidence of testicular tumors but increased incidence in sterility (summarized in Fig. 3C).
Discussion
Inactivation of Dnd1 (in Ter mice) causes primordial germ cell loss and results in a fully penetrant phenotype that manifests as either small gonads or testicular tumors. Our results here show that at least one locus on Chr X influences the development of testicular tumors in males with inactivated Dnd1. Genes from this locus likely affect germ cell survival or transformation or both.
One explanation is that B6-derived Chr X encodes tumor suppressors to inhibit testicular tumor development due to Ter. Alternatively, the B6-derived Chr X may be neutral for tumor predisposition but the 129-derived Chr X may carry novel tumor susceptibility gene(s). Further studies will be required to clarify the nature of the germ cell tumor susceptibility locus that maps to Chr X. The effect of Chr X is not fully penetrant because although overall tumor incidence in 129-Ter/Ter,XBY1 males is reduced by 25%, it is not abolished. Therefore, other unidentified genetic loci, likely residing on autosomal chromosomes, are also required for germ cell transformation that ultimately results in the extremely high, 94% incidence of testicular tumors in 129-Ter/Ter males.
Inactivation of the Dnd1 gene in the Ter strain primarily causes loss of germ cells. Our data are the first report of involvement of Chr X in modulating tumor incidence due to Ter. As mentioned earlier, the etiology of germ cell tumor development in Ter mice shares similarities with tumor development in humans. Sterility is a risk factor associated with germ cell tumor susceptibility (Jacobsen et al. 2000; Skakkebaek et al. 2003) in humans and germ cell tumor development is one aspect of testicular dysgenesis syndrome. In humans, the data suggest that testicular germ cell tumor predisposition in humans is likely due to a combination of multiple susceptibility loci with weak effects, and weak linkage has been identified to human Chr Xq27–28 (Crockford et al. 2006; Holzik et al. 2006; Rapley et al. 2000). Genetic and population heterogeneity in humans likely complicates the evidence for linkage to Xq27. In addition, Chr X abnormalities frequently have been reported in testicular cancer cells with gain in Xq found in 50% of testicular cancers (Henegarui et al. 2004; Kraggerud et al. 2002) and with frequent amplification at Xq28 (Henegarui et al. 2004; Skotheim et al. 2001). Testicular germ cell tumor susceptibility genes from human Xq27—Xq28 have not been identified. Thus, although TGCT susceptibility loci on human Chr X have been strongly implicated, it has not yet been proven. Here, we provide clear evidence for the role of Chr X in modulating tumor incidence of 129-Ter mice. Our experiments therefore provide the first experimental evidence for maternal contribution toward development of testicular germ cell tumors in males.
Our study also illustrates the advantage and power of using chromosome substitution strains to map modifiers or disease susceptibility loci (Matin et al. 1999; Nadeau et al. 2000; Singer et al. 2004). Even in mice, detection of susceptibility loci with weak effects has proven to be difficult by linkage analysis of segregating crosses. However, by utilizing the CSS 129.B6-Chr X or 129.B6-Chr Y, we ensured that the genetic background is uniformly homo-zygous for 129 autosomes. By eliminating genetic heterogeneity and by minimizing the effect of segregating background genes, we have been able to detect, using a reasonably small sample size of mice, a modest but statistically significant effect of Chr X on tumor susceptibility. In summary, our results provide clear evidence for the role of mouse Chr X in modulating the incidence of testicular germ cell tumors in Ter mice and support future efforts to identify germ cell tumor susceptibility genes from mouse and human Chr X.
Supplementary Material
Acknowledgments
The authors thank Lianchun Xiao for help with statistical calculations and Glenda Seawood for assistance with mouse husbandry. They also thank J. H. Nadeau for critical reading of the manuscript. This work was supported by National Cancer Institute grants CA93754 and CA75056 and David Carmine Cancer Research Fund.
Footnotes
Electronic Supplementary Material The online version of this article (doi: 10.1007/s00335-007-9075-8) contains supplementary material, which is available to authorized users.
References
- Crockford GP, Linger R, Hockley S, Dudakia D, Johnson L, et al. Genome-wide linkage screen for testicular germ cell tumour susceptibility loci. Hum Mol Genet. 2006;15:443–451. doi: 10.1093/hmg/ddi459. [DOI] [PubMed] [Google Scholar]
- Henegarui O, Heerema NA, Thurston V, Jung S-H, Pera M, et al. Characterization of gains, losses, and regional amplification in testicular germ cell tumor cell lines by comparative genomic hybridization. Cancer Genet Cytogenet. 2004;148:14–20. doi: 10.1016/s0165-4608(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Holzik MFL, Hoekstra HJ, Sijmons RH, Sonneveld DJA, van der Steege G, et al. Re-analysis of the Xq27-Xq28 region suggests a weak association of an X-linked gene with sporadic testicular germ cell tumor wihout cryptorchidism. Eur J Cancer. 2006;42:1869–1874. doi: 10.1016/j.ejca.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367:754–765. doi: 10.1016/S0140-6736(06)68305-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000;321:789–792. doi: 10.1136/bmj.321.7264.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraggerud SM, Skotheim RI, Szymanska J, Ekns M, Fosså SD, et al. Genome profiles of familial/bilateral and sporadic testicular germ cell tumors. Genes Chromosomes Cancer. 2002;34:168–174. doi: 10.1002/gcc.10058. [DOI] [PubMed] [Google Scholar]
- Matin A, Collin GB, Asada Y, Varnum D, Nadeau JH. Susceptibility to testicular germ-cell tumours in a 129.MOLFChr 19 chromosome substitution strain. Nat Genet. 1999;23:237–240. doi: 10.1038/13874. [DOI] [PubMed] [Google Scholar]
- Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24:221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Noguchi M. A recessive mutation (ter) causing germ cell deficiency and a high incidence of congenital testicular teratomas in 129/Sv-ter mice. J Natl Cancer Inst. 1985;75:385–392. [PubMed] [Google Scholar]
- Oosterhuis JW, Looijenga LHJ. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210–222. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Jørgensen N, Brøndum-Nielsen K, Müller J, Skakkebaek N. Developmental arrest of germ cells in the pathogenesis of germ cell neoplasia. APMIS. 1998;106:198–204. doi: 10.1111/j.1699-0463.1998.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Rapley EA, Crockford GP, Teare D, Biggs P, Seal S, et al. Localization to Xq27 of a susceptibility gene for testicular germ-cell tumors. Nat Genet. 2000;24:197–200. doi: 10.1038/72877. [DOI] [PubMed] [Google Scholar]
- Rescorla FJ. Pediatric germ cell tumors. Semin Surg Oncol. 1999;16:144–158. doi: 10.1002/(sici)1098-2388(199903)16:2<144::aid-ssu6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Katoh H, Moriwaki K, Noguchi T, Noguchi M. The ter primordial germ cell deficiency mutation maps near Grl-1 on mouse chromosome 18. Mamm Genome. 1994;5:333–336. doi: 10.1007/BF00356550. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Iguchi T, Moriwaki K, Noguchi M. The ter mutation first causes primordial germ cell deficiency in ter/ter mouse embryos at 8 days of gestation. Dev Growth Differ. 1995;37:293–302. doi: 10.1046/j.1440-169X.1995.t01-2-00007.x. [DOI] [PubMed] [Google Scholar]
- Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, et al. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Berthelsen JG, Giwercman A, Muller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987;10:19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Skakkebæk NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects: Opinion. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Holm M, Hoei-Hansen C, Jorgensen N, Rajpert-De Meyts E. Association between testicular dysgenesis syndrome (TDS) and testicular neoplasia: evidence from 20 adult patients with signs of maldevelopment of the testis. APMIS. 2003;111:1–11. doi: 10.1034/j.1600-0463.2003.11101031.x. [DOI] [PubMed] [Google Scholar]
- Skotheim RI, Kraggerud SM, Fossa SD, Stenwig AE, Gedde-Dahl T, et al. Familial/bilateral and sporadic testicular germ cell tumors show frequent genetic changes at loci with suggestive linkage evidence. Neoplasia. 2001;3:196–203. doi: 10.1038/sj.neo.7900153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LC. Origin of testicular teratomas from primordial germ cells in mice. J Natl Cancer Inst. 1967;38:549–552. [PubMed] [Google Scholar]
- Stevens LC. A new inbred subline of mice (129-terSv) with a high incidence of spontaneous congenital testicular teratomas. J Natl Cancer Inst. 1973;50:235–242. doi: 10.1093/jnci/50.1.235. [DOI] [PubMed] [Google Scholar]
- Tollerud DJ, Blattner WA, Fraser MC, Brown LM, Pottern L, et al. Familial testicular cancer and urogenital developmental anomalies. Cancer. 1985;55:1849–1854. doi: 10.1002/1097-0142(19850415)55:8<1849::aid-cncr2820550834>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- United Kingdom Testicular Cancer Study Group U Aetiology of testicular cancer: association with congenital abnormalities, age at puberty, infertility, and exercise. BMJ. 1994;308:1393–1399. [PMC free article] [PubMed] [Google Scholar]
- Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, et al. Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol. 2003;13:1429–1434. doi: 10.1016/s0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- Youngren KK, Coveney D, Peng X, Bhattacharya C, Schmidt LS, et al. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature. 2005;435:360–364. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.