Abstract
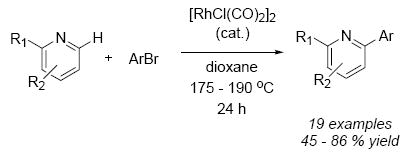
A Rh(I)-catalyzed direct arylation of pyridine and quinoline heterocycles has been developed. The method provides rapid entry into an important class of bis(hetero)aryl products employing inexpensive and readily available starting materials.
The pyridine and quinoline nuclei are privileged scaffolds that occupy a central role in many medicinally relevant compounds.1 Consequently, methods for their expeditious functionalization are of immediate interest. However, despite the immense importance of transition-metal catalyzed cross-coupling for the functionalization of aromatic scaffolds, general solutions for coupling 2-pyridyl organometallics with aryl halides have only recently been presented.2 Direct arylation at the ortho position of pyridine would constitute an even more efficient approach because it eliminates the need for the stoichiometric preparation and isolation of 2-pyridyl organometallics.3,4 Progress towards this goal has been achieved by activation of the pyridine nucleus for arylation via conversion to the corresponding pyridine N-oxide5 or N-iminopyridinium ylide.6 However, this approach necessitates two additional steps: activation of the pyridine or quinoline starting material, and then unmasking the arylated product. The use of pyridines directly would clearly represent the ideal situation both in terms of cost and simplicity. We now wish to document our efforts in this vein, culminating in an operationally simple Rh(I)-catalyzed direct arylation of pyridines and quinolines.
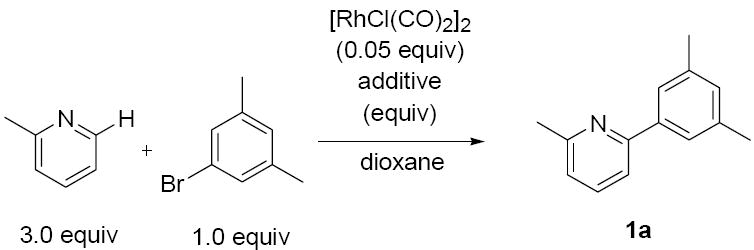
We recently developed an electron-rich Rh(I) system for catalytic alkylation at the ortho position of pyridines and quinolines with alkenes.7,8 Therefore, we initially focused our attention on the use of similarly electron-rich Rh(I) catalysts for the proposed direct arylation. After screening an array of electron-rich phosphine ligands and Rh(I) salts, only marginal yields (<20%) of the desired product were obtained. Much more efficient was an electron-poor Rh(I) system with [RhCl(CO)2]2 as precatalyst (Table 1).9,10 For the direct arylation of picoline with 3,5-dimethyl-bromobenzene, addition of P(OiPr)3 afforded a promising 40% yield of the cross coupled product 1a (entry 1). The exclusion of phosphite additive proved even more effective, with the yield of 1a improving to 61% (entry 2). Further enhancement in yield was not observed upon the inclusion of other additives such as MgO (entry 3), various organic bases (entries 4, 5) and inorganic bases (entry 6), or a protic acid source (entry 7). Absolute concentration proved very important, with the best results being obtained at relatively high concentrations of the aryl bromide (compare entries 8 and 9). A marginal improvement was observed upon running the reaction with 6 equivalents of 2-methyl pyridine (entry 10).11,12 The reaction temperature could also be increased to 175 or 190 °C while maintaining reaction yield, to enable the reaction time to be reduced to 24 h (entries 11 and 12).
Table 1.
Direct Arylation of 2-Methyl Pyridine
 | ||||
|---|---|---|---|---|
| entry | additive (equiv) | conc. (M)a | temp (°C) | % yieldb,c |
| 1 | P(OiPr)3 (0.30) | 0.8 | 165 | 40 |
| 2 | None | 0.8 | 165 | 61 |
| 3 | MgO (3.0) | 0.8 | 165 | 33 |
| 4 | NiPr2iBu (3.0) | 0.8 | 165 | 0 |
| 5 | 2,6-lutidine (3.0) | 0.8 | 165 | 47 |
| 6 | K2CO3 (3.0) | 0.8 | 165 | 0 |
| 7 | 2,6-lut HCl(0.25) | 0.8 | 165 | 55 |
| 8 | none | 0.15 | 165 | 34 |
| 9 | none | 1.1 | 165 | 61 |
| 10 | none | 0.8 | 165 | 63d |
| 11 | none | 0.8 | 175 | 60d,e |
| 12 | none | 0.8 | 190 | 62d,e |
Refers to the absolute concentration in aryl bromide;
determined by GC analysis with hexamethylbenzene as an internal standard;
the reaction time was 48 h unless otherwise indicated;
6.0 equiv of 2-methyl pyridine was employed;
the reaction time was 24 h.
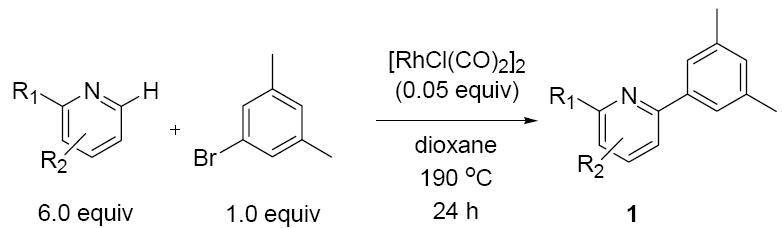
With a set of optimized conditions in hand, we next set out to investigate the generality of the catalytic direct arylation with a variety of substituted pyridine derivatives (Table 2). Good arylation yields were observed for pyridine derivatives with straight chain (entries 1 and 2), β-branched (entry 3), and α-branched (entry 4) substituents at the C-2 position. Tetrahydroquinoline also provided the arylated product in good yield (entry 5) as did 2,4-dimethylpyridine (entry 6). In contrast, when a substituent at the C-2 position is not present, arylation does not occur (entry 7). This result parallels our previous studies on Rh-catalyzed pyridine alkylation.7,13 The steric interactions provided by a substituent at this position may reduce the energy difference between the N-bound complex and the C-H activation intermediate. Consistent with the requirement for C-2 substitution, quinoline is also a highly effective substrate for direct arylation (entry 8). As illustrated in entry 9, the compatibility of the reaction conditions to chloro substitution should enable efficient further elaboration of the arylation product by standard cross coupling methods. However, no arylation was observed for 2- and 2-choropyridine.
Table 2.
Investigation of Scope in Heterocycle
 | |||
|---|---|---|---|
| entry | heterocycle | product | % yielda |
| 1 | 1a | 53 | |
| 2 | 1b | 51 | |
| 3 | 1c | 78 | |
| 4 | 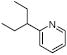 |
1d | 70 |
| 5 | 1e | 76 | |
| 6 | 1f | 67 | |
| 7 | 1g | 0 | |
| 8 | 1h | 86b | |
| 9 | 1i | 65b | |
Isolated yield of analytically pure product; 0.4 mmol scale in ArBr; 0.8 M absolute concentration in ArBr;
reaction run at 175 °C and 0.3 M absolute concentration in ArBr.
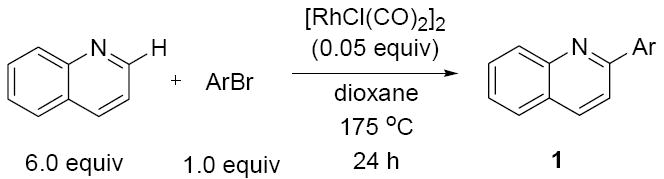
The substrate scope in aryl bromide was also evaluated with quinoline as the coupling partner (Table 3). Both electron-rich and electron-poor aryl bromides are accommodated with equal efficiency (compare for example entries 4 and 11). A variety of useful functional groups are tolerated, including aryl and alkyl ethers (entries 3, 4), chloride (entry 8), fluoride (entry 9), and ketone functionality (entry 10). While cross coupling proceeds smoothly with meta substitution on the aryl bromide ring (see for example entry 1), attempted cross coupling with 2-methyl-bromobenzene failed to afford product, with only unreacted starting material recovered.
Table 3.
Investigation of Scope in Aryl Bromide
 | |||
|---|---|---|---|
| entry | ArBr | product | % yielda |
| 1 | 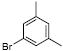 |
1h | 86 |
| 2 | 1j | 74 | |
| 3 | 1k | 62 | |
| 4 | 1l | 70 | |
| 5 | 1m | 73 | |
| 6 | 1n | 77 | |
| 7 | 1o | 69 | |
| 8 | 1p | 68 | |
| 9 | 1q | 69 | |
| 10 | 1r | 70 | |
| 11 | 1s | 65 | |
| 12 | 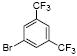 |
1t | 45 |
Isolated yield of analytically pure product; 0.4 mmol scale in ArBr; 0.3 M absolute concentration in ArBr.
Studies exploring reaction scope were conducted using 5 mol% of the Rh(I) catalyst. However, the reaction can be performed with comparable efficiency employing only 1 mol% of the catalyst. In this case, the reaction was run neat, resulting in complete consumption of the aryl bromide after 24 h and a 68% isolated yield of the arylated product 1e (eq 1).
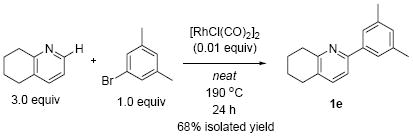 |
(1) |
In summary, we have developed a Rh(I)-catalyzed strategy for the direct arylation of pyridines and quinolines. The heterocycle is used without the need for prefunctionalization, and all reaction components are inexpensive and readily available. The strategy represents an expeditious route to an important class of bis(hetero)aryls and should be of broad utility.14
Supplementary Material
Experimental procedures and analytical data for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by NIH Grant GM069559 to J.A.E. and the DOE, Office of Basic Energy Sciences, Chemical Sciences Division, U.S. Department of Energy, under Contract DE-AC03-76SF00098 to R.G.B. A.M.B. was supported by a NRSA postdoctoral fellowship (GM082080).
References
- 1.For an analysis of heterocycles used in the preparation of drug candidates, see: Carey JS, Laffan D, Thomson C, Williams MT. Org Biomol Chem. 2006;4:2337. doi: 10.1039/b602413k.
- 2.Billingsley KL, Buchwald SL. Angew Chem Int Ed. 2008;47:4695. doi: 10.1002/anie.200801465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For a recent comprehensive review on direct arylation, see: Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174. doi: 10.1021/cr0509760.
- 4.Excess Zn and catalytic Pd/C has been used to prepare 2-phenylpyridine in 52% yield from pyridine and phenyl chloride: Mukhopadhyay S, Rothenberg G, Gitis D, Baidossi M, Ponde DE, Sasson Y. Perkin Trans 2. 2000;9:1809.
- 5.(a) Campeau L-C, Rousseaux S, Fagnou K. J Am Chem Soc. 2005;127:18020. doi: 10.1021/ja056800x. [DOI] [PubMed] [Google Scholar]; (b) Leclerc J-P, Fagnou K. Angew Chem Int Ed. 2006;45:7781. doi: 10.1002/anie.200602773. [DOI] [PubMed] [Google Scholar]; (c) Cho SH, Hwang SJ, Chang S. J Am Chem Soc. 2008;130:9254. doi: 10.1021/ja8026295. [DOI] [PubMed] [Google Scholar]
- 6.Larivee A, Mousseau JJ, Charette AB. J Am Chem Soc. 2008;130:52. doi: 10.1021/ja710073n. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JC, Bergman RG, Ellman JA. J Am Chem Soc. 2007;129:5332. doi: 10.1021/ja070388z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For Ni and Lewis acid catalyzed C-2 alkenylation of pyridines with alkynes, see: Nakao Y, Kanyiva KS, Hiyama T. J Am Chem Soc. 2008;130:2448. doi: 10.1021/ja710766j.
- 9.The direct arylation of azoles proceeds most efficiently with Rh(I) salts containing alkene rather than CO ligands and requires the addition of either an electron rich trialkylphosphine or a phosphepine, see: Lewis JC, Berman AM, Bergman RG, Ellman JA. J Am Chem Soc. 2008;130:2493. doi: 10.1021/ja0748985.
- 10.For use of [RhCl(CO)2]2 in a different type of C-H bond functionalization reaction, see: Zhao X, Yu Z. J Am Chem Soc. 2008;130:8136. doi: 10.1021/ja803154h.
- 11.The role of excess heterocycle may be to both stabilize the Rh catalyst and act as an acid scavenger.
- 12.Mass balance experiments indicate <10% unproductive loss of the heterocycle after complete consumption of the aryl bromide. For all of the aryl bromides investigated, neither hydrodehalogenation nor dimerization was observed.
- 13.For relevant studies on the impact of pyridyl C-2 substitution on C-H bond activation, see: iridium complexes: Alvarez E, Conejero S, Paneque M, Petronilho A, Poveda ML, del Rio D, Serrano O, Carmona E. J Am Chem Soc. 2006;128:13060. doi: 10.1021/ja0646592.. osmium complexes: Buil ML, Esteruelas MA, Garcés K, Oliván M, Oñate E. J Am Chem Soc. 2007;129:10998. doi: 10.1021/ja073673r.
- 14.For several mechanistic possibilities, please see the Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and analytical data for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.


