Abstract
Age-associated changes in glial reactivity may predispose individuals to exacerbated neuroinflammatory cytokine responses that are permissive to cognitive and behavioral complications. The purpose of this study was to determine if aging is associated with an exaggerated sickness response to central innate immune activation. Our results show that intracerebroventricular (i.c.v) administration of lipopolysaccharide (LPS) caused a heightened proinflammatory cytokine response (IL-1β, IL-6, and TNFα) in the cerebellum 2 h post i.c.v injection in aged mice compared to adults. This amplified inflammatory profile was consistent with a brain region-dependent increase in reactive glial markers (MHC class II, TLR2 and TLR4). Moreover, LPS caused a prolonged sickness behavior response in aged mice that was paralleled by a protracted expression of brain cytokines in the cerebellum and hippocampus. Finally, central LPS injection caused amplified and prolonged IL-6 levels at the periphery of aged mice. Collectively, these data establish that activation of the central innate immune system leads to exacerbated neuroinflammation and prolonged sickness behavior in aged as compared to adult mice.
Keywords: Brain, Lipopolysaccharide, Age, Glia, Cytokines, Mice, Behavior, Intracerebroventricular
1. Introduction
Microarray analysis of brain mRNA reveal that aging is associated with a gene expression profile indicative of oxidative stress, complement activation, and glia reactivity [5, 25, 35]. These reports support the premise that brain glial cells, including astrocytes and microglia, become more active or reactive during normal aging [22]. For example, microglia expression of major histocompatibility complex (MHC) class II, a marker of microglial cell activation, is increased in brains of aged but otherwise healthy humans, non-human primates, and rodents [20, 25, 41, 51, 52, 56, 64–66]. An age-associated change in glial phenotypes is also indicated by increased expression of scavenger receptors [25, 71], complement receptors [56] and glial fibrillary acidic protein (GFAP) [25, 35, 41]. It is noteworthy that the actual number of resident astrocytes and microglia is not increased in the brain with age [37, 43]. Therefore, glial reactivity appears to increase in existing astrocytes and microglia populations.
Understanding changes in brain glia during normal aging is important because microglia and astrocytes are part of the brain’s innate immune system and play an important role in receiving and propagating inflammatory signals in response to activation of the peripheral immune system. The bidirectional interaction between the brain and immune system is necessary for mounting the appropriate immunological, physiological, and behavioral responses to immune stimulation [32]. This response is mediated, in part, by proinflammatory cytokines including IL-1β, IL-6, and TNFα. These cytokines are produced within the brain by glia and act on neuronal substrates to elicit a sickness behavior syndrome that is normally adaptive and beneficial to the host [32, 33]. A dysregulated cytokine response, however, can lead to neurological complications [6, 9, 16] including cognitive impairment [2, 29, 46, 68], anorexia [17], and mood and depressive disorders [10, 47, 58].
Activation of the peripheral immune system exacerbates neurodegenerative diseases such as Alzheimer’s, Multiple Sclerosis, and prion disease by increasing cytokine production by activated microglia [12, 57]. Thus, a potential consequence of increased glial reactivity in the aged brain may be hypersensitivity to peripheral or central immune challenge. For instance, in a murine model of aging, peripheral stimulation of the innate immune system with LPS causes an exaggerated inflammatory cytokine response in the brain of old mice [25]. This heightened neuroinflammatory response in old mice is associated with a delayed recovery from the behavioral symptoms of sickness [25]. Moreover, elevated neuroinflammation in the aged rodent brain is permissive to longer lasting cognitive impairments [3]. This hypersensitivity is interpreted to be glial derived because primary mixed glia cultures and coronal brain sections established from the brain of aged rodents are hyper-responsive to LPS stimulation and produce more inflammatory cytokines (IL-1β and IL-6) compared to cultures established from adult brain [72, 73]. Aged rodents are also more sensitive to endotoxic shock induced by i.c.v. injection of high levels of either LPS [31] or TNFα/IFNγ̣ [14]. It is unknown, however, if transient stimulation of the central innate immune system leads to prolonged neuroinflammation and behavioral deficits in aged mice.
Our experiments were carried out to test the hypothesis that brain hypersensitivity to immune activation and prolonged sickness behavior in the aged was caused by an amplified cytokine response within the brain. In order to remove any potential confound of the peripheral immune system, the present set of experiments were carried out by challenging mice i.c.v. with LPS. Here we show that central LPS challenge caused a prolonged sickness behavior response in aged mice, which was paralleled by a protracted expression of brain proinflammatory cytokines in the cerebellum and hippocampus. Moreover, the magnitude and duration of the increase in plasma IL-6 following central injection of LPS was greater in aged mice compared to adults. Furthermore, we show that the expression of potential markers of glial reactivity including MHC class II, TLR2, and TLR4, were increased in the brain of aged mice. High expression of these markers in the cerebellum of aged mice was associated with a greater initial cytokine response to i.c.v LPS. These findings support our hypothesis that sensitization of the brain cytokine system in aged individuals promotes an exaggerated response to either peripheral or central innate immune stimulation and might underlie the increased prevalence of behavioral and cognitive complications in this population.
2. Methods
2.1 Animals
Male BALB/c mice from the National Institute on Aging specific pathogen free colony were used. The mean lifespan for BALB/c mice is approximately 26 months [42], so to investigate changes that occur from young adulthood to what is considered aged, young adult (3–4-month-old) and aged (20–22 month-old) males were used. Mice were housed in polypropylene cages and maintained at 21° C under a 12 h light: 12 h dark cycle with ad libitum access to water and rodent chow. Male juvenile conspecifics (4 to 5 weeks old) used in the social exploratory behavior paradigm were maintained under identical conditions. At the end of each study, mice were examined postmortem for gross signs of disease (e.g. splenomeglia or tumors). Data from mice determined to be unhealthy were excluded from analysis (< 5%). All procedures were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2 Intracerebroventricular cannulation
The i.c.v cannulation was performed as previously described with a few modifications [24]. In brief, mice were deeply anesthetized using ketamine and xylazine (100 mg and 10 mg/kg BW i.p., respectively) and the surgical site was shaved and sterilized. Mice were positioned in a sterotaxic instrument so that the plane formed by the frontal and parietal bones was parallel to the table top. An incision, 1.5 cm in length, was made on the cranium to reveal the bregma and a 26-guage stainless-steel guide cannula was placed in the lateral cerebral ventricle using the following stereotaxic coordinates: Lat 1.6 mm; and A–P 1 mm to the bregma; and Hor -2 mm from the dura mater. Two anchoring cranial screws were inserted adjacent to the cannula and the cannula was secured with cranioplastic cement. A dummy cannula was inserted in the guide cannula to prevent occlusion and infection. Mice were injected subcutaneously with Buprinex (111 µg /kg BW) following surgery and then again 12 h later. Mice were provided a minimum of 7 d to recover before any treatment was administered. Accurate cannula placement was confirmed by injecting trypan blue dye and examining the dye diffusion throughout the ventricles.
2.3 Behavior Tests
Locomotor activity
Mice were maintained in their home cage, which provided a floor area of 26 × 20 cm, and locomotor activity was video recorded during 3-min tests. On the video records, cages were divided into 6 identical rectangles and a trained observer who was blind to experimental treatments determined the incidence of line crossing.
Social exploratory behavior
To assess the motivation to engage in social exploratory behavior, a novel juvenile conspecific was introduced into the test subject’s home cage for a 10-min period. Behavior was video taped and the cumulative amount of time the subject engaged in social investigation was determined from the video records by a trained observer who was blind to experimental treatments. Baseline social behavior was measured at time 0 for all experimental treatments. Social behavior was determined as the amount of time that the experimental subject spent investigating (e.g., anogenital sniffing, trailing) the juvenile [7]. Results are expressed as percent decrease in time engaged in social behavior compared to respective baseline measures.
2.4 Quantitative Real Time PCR
Total RNA was isolated from brain using the Tri Reagent protocol (Sigma, St. Louis, MO). RNA samples were subjected to a DNase I digestion procedure and then reverse transcribed to cDNA using a RT RETROscript kit (Ambion, Austin, TX). Quantitative real time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol as previously described [25]. In brief, cDNA was amplified by real time PCR where a target cDNA (IL-1β, IL-6, TNFα, or MHC class II) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems, CA). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference [36].
2.5 Plasma Cytokine Measurement
IL-6 was measured in the plasma as previously described [23]. In brief, mice were asphyxiated by CO2 inhalation and blood was collected by cardiac puncture into EDTA coated syringes. Samples were centrifuged (6000 × g for 15 min at 4°C) and plasma was collected and stored frozen (−80°C) until assaying. Plasma samples were assayed for IL-6 using a customized ELISA that we have described in detail [23]. Assays were sensitive to 8 pg/ml of IL-6, and inter-and intra-assay coefficients of variation were less than 10%.
2.6 Experimental protocols
For all studies, adult and aged mice were prepared with an indwelling i.c.v. cannula. After recovery mice were injected i.c.v. with sterile saline or Escherichia coli LPS (10 ng, 5ng/ul; serotype 0127:B8, Sigma, St. Louis, MO). The dosage of LPS used for i.c.v injection was used to elicit a mild and transient sickness response [30]. For i.c.v. injection into the lateral ventricle, the indwelling cannula was connected with sterile tubing to a Hamilton syringe and injections were administered in a 2 µl volume using a KS Scientific precision syringe pump. In the first set of studies, locomotor activity, social behavior, and food intake, were measured 0, 2, 4, 8 and 24 h after i.c.v injection of saline or LPS (n=8). In the second set of studies, mice were injected i.c.v. with either saline or LPS and sacrificed by CO2 asphyxiation either 2 or 8 h later. Brains were removed, dissected, and stored in a RNA preservative solution at −20° C. Total RNA was isolated from brain samples and assayed using real time PCR (n=7). Plasma was also collected and stored (−80° C) until assaying.
2.7 Statistical analysis
All data were analyzed using Statistical Analysis Systems (SAS) General Linear Model procedures. Data were subjected to two- (Age × LPS) or three-way (Age × LPS × Time) ANOVA to determine significant main effects as well as interactions between main factors. When appropriate, differences between treatment means were evaluated by an F-protected t-test using the LSD procedure of SAS. All data are expressed as treatment means ± standard error of mean (SEM).
3. Results
3.1 Central LPS injection induces a prolonged sickness response in aged mice
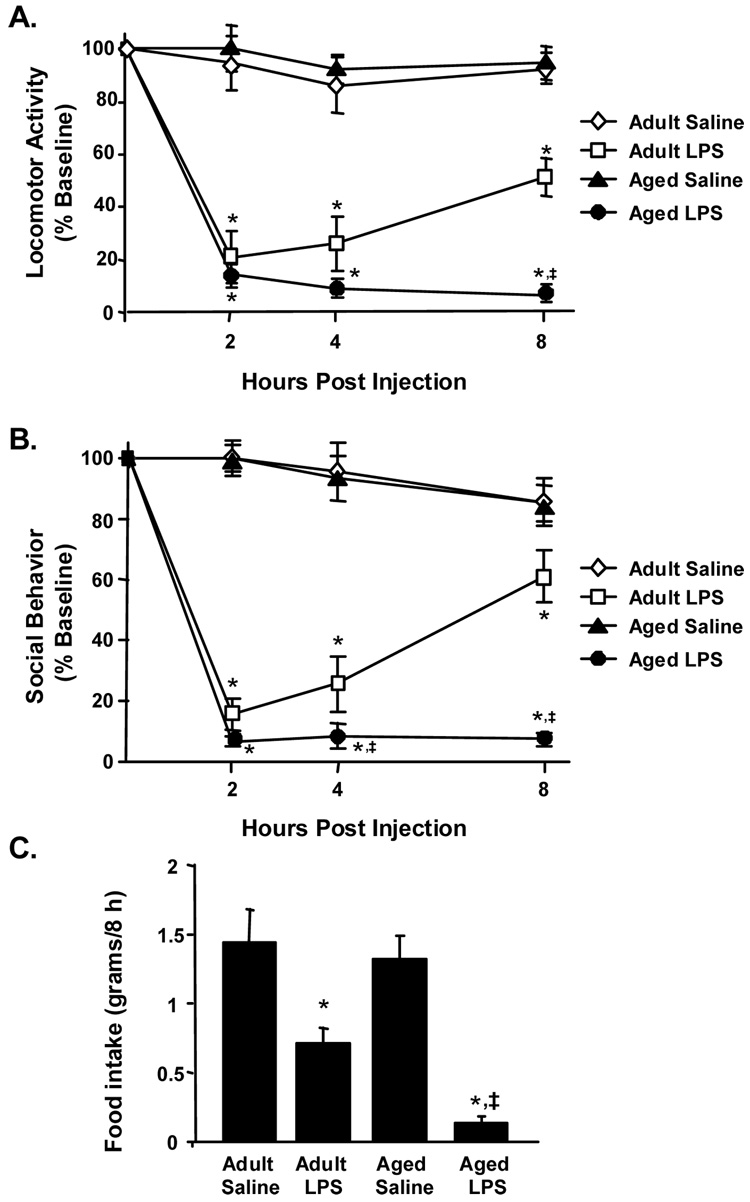
Since peripheral LPS injection caused an exaggerated sickness response in aged BALB/c mice [25], we sought to determine if sickness behavior was exacerbated in aged mice following central (i.c.v) LPS challenge (10 ng). To determine if central injection of LPS caused a more severe sickness behavior syndrome in aged mice, locomotor activity, motivation to engage in social behavior, and food intake were assessed in adult and aged mice injected i.c.v. with either saline or LPS. Behavior was measured just before i.c.v. injection and again 2, 4, and 8 h later (Fig. 1). ANOVA of locomotor activity revealed a significant main effect of LPS (F(1,31)= 246.7 P < 0.001) and an Age × LPS interaction (F(3,31)= 12.46, P < 0.001) (Fig. 1A). While adult mice showed improvement in locomotor activity 8 h after i.c.v. LPS injection, locomotor activity of aged mice was still markedly reduced (P < 0.001).
Fig. 1. Aging prolonged LPS-induced deficits in locomotor activity, social behavior, and food intake.
Adult and aged mice were injected i.c.v. with either saline or LPS and (A) locomotor activity, (B) social behavior, and (C) food intake were measured 0, 2, 4, and 8 hours post injection. Bars represent the mean ± SEM (n=8). Means with * are different (P < 0.05) from baseline controls and means with ‡ are significantly different from Adult LPS.
The effect of i.c.v. LPS injection on social behavior is shown in Figure 1B. ANOVA of social behavior revealed a significant main effect of Age (F(1,31)= 11.5, P < 0.001), LPS (F(1,31)= 347, P < 0.001), and an Age × LPS interaction (F(3,31)= 12.46, P < 0.001). Whereas adult mice began to recover between 4 to 8 h post LPS injection, social behavior in aged mice was still considerably reduced at 4 h (P < 0.04) and 8 h (P < 0.001). It is important to note that both age groups returned to baseline social investigation by 24 h after i.c.v. injection of LPS (data not shown). Finally, the delayed recovery from sickness behavior was substantiated by a greater reduction in food intake (P < 0.05) in aged mice receiving LPS compared with adults given LPS (Fig.1C). Collectively these data indicate that central LPS injection induces a prolonged sickness response in aged mice compared to adults.
3.2 Central LPS injection induces an elevated proinflammatory cytokine response in the cerebellum of aged mice
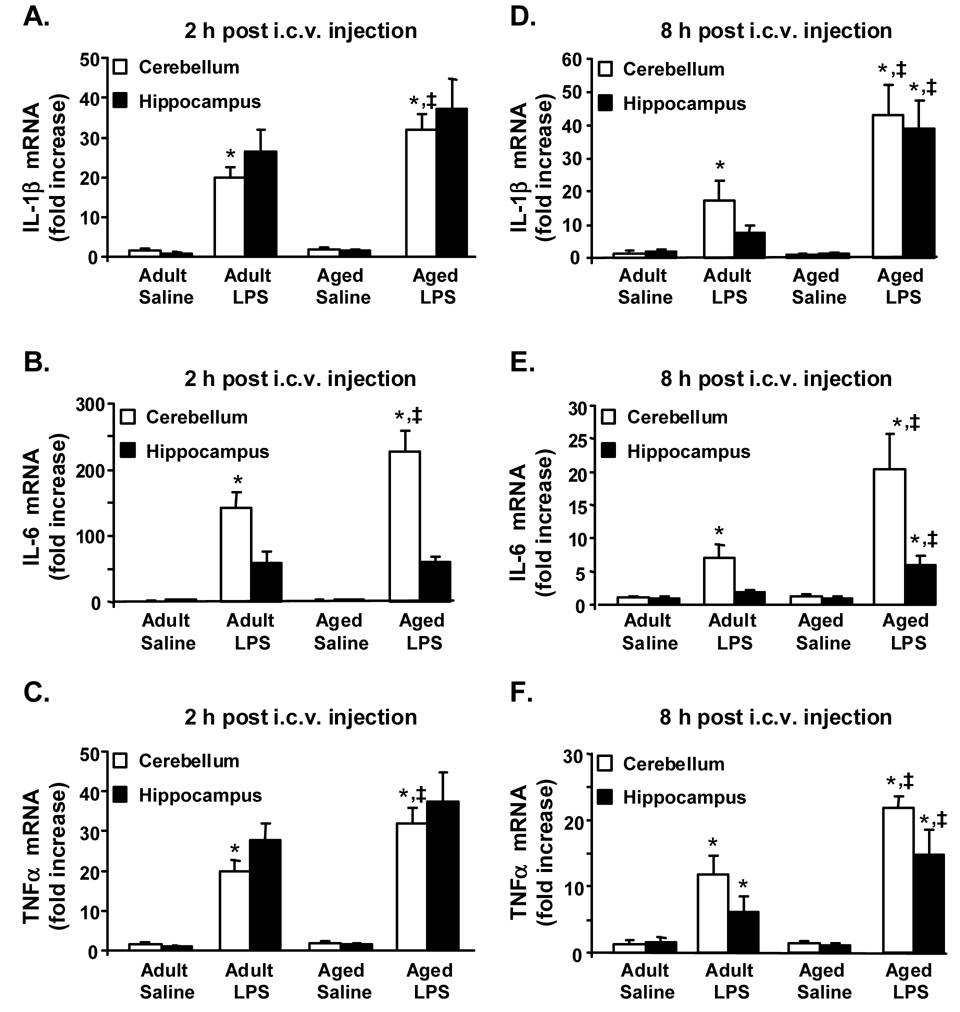
Since central LPS injection caused an exaggerated sickness response in aged mice, we next sought to determine if there was an amplified and prolonged proinflammatory cytokine response in the aged brain following central LPS challenge. The proinflammatory cytokines, IL-1β, IL-6, and TNFα, were selected for these experiments because they are critical CNS mediators of behavior symptoms of sickness [32]. In this experiment, mice were injected i.c.v. with either saline or LPS and IL-1β, IL-6, and TNFα steady state mRNA levels were measured in discrete brain regions collected either 2 or 8 h later (Fig. 2). While multiple discrete brain regions were anticipated to show differential responses to immune challenge (i.e., hypothalamus, amygdala, striatum), the cerebellum and hippocampus were selected because these regions are predicted to be involved in the cognitive, motor, and behavioral deficits associated with prolonged or excessive neuroinflammation [22].
Fig. 2. Proinflammatory cytokine mRNA expression was prolonged in hippocampus and cerebellum of aged mice after central injection of LPS.
Adult and aged mice were injected i.c.v. with either saline or LPS and IL-1β (A, D), IL-6 (B, E), and TNFα (C, F) mRNA levels were measured in the cerebellum (white bars) and hippocampus (black bars) collected either 2 (A, B, C) or 8 h later (D, E, F). Bars represent the mean ± SEM (n=7). For each brain region, means with * are significantly different (P < 0.05) from saline controls and means with ‡ are significantly different from Adult LPS.
As expected, i.c.v injection of LPS increased IL-1β, IL-6, and TNFα mRNA expression in the cerebellum and hippocampus at both time points (P < 0.001, for each). In the cerebellum, the expression levels of all three proinflammatory cytokines were appreciably higher at 2 h after central LPS injection in aged mice compared to adults (Fig. 2 A–C). ANOVA of cerebellum cytokine mRNA expression revealed a significant Age × LPS interaction for IL-1β (F(3, 27)= 6.1, P < 0.05), IL-6 (F(3, 27)= 4.2, P < 0.05) and TNFα (F(3, 27)= 18.86, P < 0.01). The steady state levels of cerebellum IL-1β, IL-6, and TNFα mRNA were approximately 1.6 fold higher (P< 0.001, P < 0.01, and P< 0.001) in aged compared to adult mice injected with LPS. In the hippocampus, however, there was not a significant Age × LPS interaction for inflammatory cytokine expression. Thus, in hippocampus at 2 h, cytokine mRNA levels were increased due to LPS, but this cytokine increase was independent of age. Taken together these results indicate that central LPS injection causes an elevated proinflammatory cytokine response in aged mice that is brain region dependent.
Figure 2 D–F shows that 8 h after central LPS administration, IL-1β, IL-6, and TNFα were increased in the LPS treated mice compared to those receiving saline (P < 0.001, for each). Analysis of cerebellum cytokine mRNA levels revealed a significant Age × LPS interaction for IL-1β (F(3, 27)= 5.1, P < 0.04), IL-6 (F(3, 27)= 8.3, P <0.01) and TNFα(F(3, 27)= 4.21, P < 0.05). The steady state level of cerebellum mRNA for IL-1β, IL-6, and TNFα, respectively, were 3-fold (P < 0.003), 6 fold (P< 0.001) and 2 fold (P < 0.004) higher in aged mice compared to adult mice injected i.c.v. with LPS. Similar to results from the cerebellum at 8 h, IL-1β, IL-6, and TNFα mRNA levels were higher in the hippocampus of mice injected i.c.v. with LPS compared to those receiving saline (P < 0.001, for each). ANOVA of hippocampal cytokine mRNA levels revealed a significant Age × LPS interaction for IL-1β̣ (F(3, 27)= 13.10, P < 0.002), IL-6 (F(3, 27)= 7.21 , P < 0.02) and TNFα F(3, 27)= 4.52, P < 0.05). The steady state level of hippocampal mRNA for IL-1β, IL-6, and TNFα, respectively, were 5.4-fold (P < 0.001), 2.8 fold (P < 0.001), and 2.4 fold (P < 0.01) higher in aged mice compared to adults administered LPS. Notably, cortex mRNA was also analyzed 2 and 8 h after i.c.v injection. Similar to the proinflammatory profile of the hippocampus after LPS there was not an Age × LPS interaction in the cortex at 2 h, but there was at 8 h (data not shown). Collectively, these data indicate that the propagation of a central inflammatory signal is exacerbated within the brain of aged mice and indicate that there is a relationship between a prolonged neuroinflammatory cytokine response and protracted sickness behavior in the aged.
3.3 Elevated and prolonged IL-6 levels in the plasma of aged mice after central injection of LPS
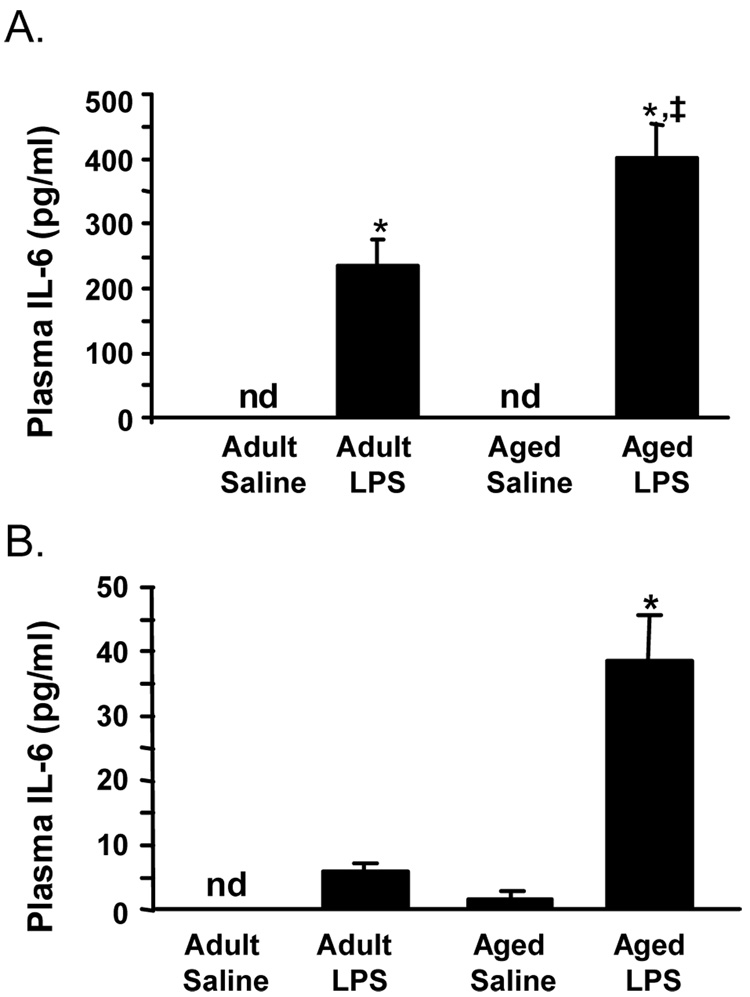
To determine if central LPS injection caused an elevated inflammatory response at the periphery of aged mice, plasma levels of proinflammatory cytokines were determined 2 and 8 h after i.c.v LPS injection (Fig 3). ANOVA of plasma IL-6 cytokine revealed a significant Age × LPS interaction at 2 h (F(3, 31)= 8.1, P < 0.01) and at 8 h (F(3, 27)= 14.33, P < 0.001). At 2 and 8 h after i.c.v. LPS injection, plasma IL-6 levels were 2- and 6.6-fold higher, respectively, in aged mice compared to adults (P< 0.01 at each time). All three proinflammatory cytokines (IL-1β, TNFα, and IL-6) were assayed in the plasma but only IL-6 was consistently detected post i.c.v injection of LPS. This is consistent with our previous work showing i.c.v injection of LPS in rats induced an increase in circulating IL-6 [18]. Taken together, these findings indicate that the exaggerated proinflammatory cytokine response to LPS in the aged brain was paralleled with increased IL-6 in the plasma.
Fig. 3. IL-6 levels were amplified and protracted in the plasma of aged mice in response to central LPS injection.
Adult and aged mice were injected i.c.v. with either saline or LPS and IL-6 was measured in plasma collected (A) 2 and (B) 8 h later. Bars represent the mean ± SEM (n=7). Means with * are significantly different (P < 0.05) from saline controls and means with ‡ are significantly different from Adult LPS.
3.2 Reactive microglial markers are expressed in the brain of aged mice and are enhanced by central LPS injection
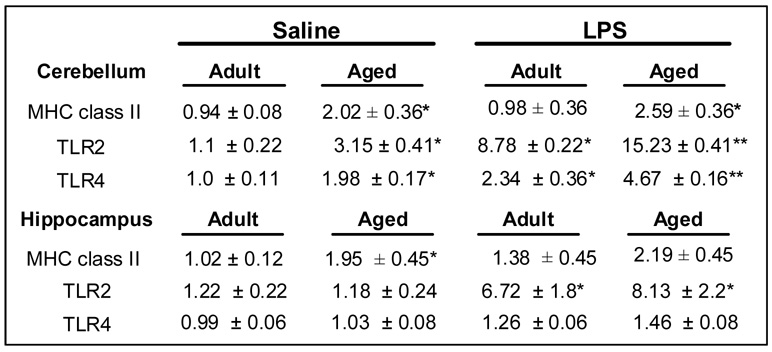
To begin to understand why there were brain region-dependent differences in the cytokine expression profile of aged mice post central LPS challenge, a number of glial markers, MHC class II, Toll-Like Receptor (TLR)-2 and -4, were examined in the brain of adult and aged mice. In this experiment, mice were injected i.c.v. with either saline or LPS and MHC class II, Toll-Like Receptor (TLR)-2 and -4 mRNA levels were measured in discrete brain regions collected 8 h later (Fig. 4).
Fig. 4. TLR2 and TLR4 mRNA expression was increased in the cerebellum of aged mice after central LPS injection.
Adult and aged mice were injected i.c.v. with either saline or LPS and mRNA levels of MHC class II, TLR2, and TLR4 were determined. The table represents the fold increase mean ± SEM (n=7). Means with * are significantly different (P < 0.05) from saline controls and means with ‡ are significantly different from Adult LPS.
Analysis of brain MHC class II mRNA levels revealed a significant main effect of Age (cerebellum, F(1, 27)= 9.17, P < 0.01 and hippocampus, (F(1, 27)= 8.45, P < 0.01). In both brain regions, MHC class II mRNA expression was increased approximately 2-fold with age (P < 0.01, for each). Elevated MHC class II mRNA expression is relevant because MHC class II is regulated primarily at the level of transcription [19]. Moreover, increased MHC class II expression is consistent with previous reports showing an increase in reactive microglia in the brain of healthy aged rodents [20, 25]. Despite this age difference in MHC class II, central injection of LPS did not significantly alter MHC class II mRNA levels at 8 h.
Because TLR2 is a marker of brain inflammation [50] and TLR4 can mediate LPS-induced inflammatory signal transduction in microglia/macrophages [34, 53], TLR2 and TLR4 mRNA levels were determined. Analysis of the cerebellum revealed a significant main effect of Age (TLR2, F(1, 27)= 10.98, P < 0.01 and TLR4, F(1, 27)= 26.8, P < 0.001), LPS (TLR2, F(1, 23)= 40.97, P < 0.001 and TLR4, F(1, 27)= 40.43, P < 0.001) and an Age × LPS interaction (tendency for TLR2, F(1, 27)= 3.14, P = 0.09, and TLR4, F(3, 27)= 4.84, P < 0.001). The levels of TLR2 and TLR4 mRNA in the cerebellum of aged mice were increased 3- and 2-fold, respectively, compared to adult mice. These data also indicate that LPS injection induced the highest overall increase in the steady state transcription for both TLR2 and TLR4 in the cerebellum of aged mice. These findings are consistent with the notion that there is an age-associated exacerbated cytokine response to central LPS injection.
In the hippocampus the steady state mRNA levels of TLR2 and TLR4 were not increased due to age. Each was increased due to LPS (TLR2, F(1, 27)= 21.74, P < 0.001 and TLR4, F(1, 27)= 18.18, P < 0.001) but these increases were irrespective of age. These data indicate that a brain regional difference TLR4 expression could explain why central LPS challenge caused a heightened age-dependent proinflammatory cytokine response at 2 h in the cerebellum but not in the hippocampus (Fig. 2 A–C). Taken together, these TLR findings indicate that central LPS challenge induces a high level of TLR reactivity in the cerebellum of aged mice.
4. Discussion
We recently reported that peripheral stimulation of the innate immune system with LPS caused an exaggerated neuroinflammatory response and prolonged sickness behavior in aged BALB/c mice [25]. The aim of this study was to determine if direct CNS challenge with LPS also caused exaggerated brain cytokine and sickness responses in aged mice. These experiments were important to exclude any potential confound of age-dependent cytokine amplification at the periphery. We observed here that i.c.v. administered LPS caused a heightened proinflammatory cytokine response in the cerebellum, but not the hippocampus 2 h post injection in aged mice compared to adults. This amplified inflammatory cytokine response was consistent with a brain region-dependent increase in TLR4. Moreover, the inflammatory response induced by centrally injected LPS led to protracted behavioral symptoms of sickness in aged mice, which was paralleled by prolonged brain inflammatory cytokine mRNA expression in the cerebellum and hippocampus. Finally, our results indicate that an amplified neuroinflammatory response to central challenge was communicated to the periphery, potentially as an IL-6 mediated signal. Taken together, these data can be interpreted to suggest that exacerbated neuroinflammation and prolonged sickness behavior in aged mice is a consequence of an amplified cytokine response within the brain.
There is already evidence that brain glia become more reactive with age, and as a consequence, peripheral immune activation causes an exaggerated brain cytokine response [25]. These results have been interpreted to suggest that the amplification of the cytokine signal occurs within the brain for several reasons. First, primary mixed glia cultures established from the brain of aged rats show increased expression of MHC class II indicating that these cells are more active [62]. In fact, related studies have shown that glial cultures or brain slices derived from aged brains are hyper-responsive to LPS stimulation and produce more inflammatory cytokines 7 (IL-1β and IL-6) compared to cultures established from adult brains [72, 73]. Second, peripheral levels of IL-1β do not mirror the exaggerated neuroinflammatory cytokine response following peripheral innate immune challenge [2, 25]. This may reflect a general tendency for the innate immune cells at the periphery to be less responsive in older animals and humans [11]. Third, peripheral LPS injection caused markedly elevated neuronal activity in several circumventricular organs (CVO) and in the brainstem nucleus of the solitary tract of aged mice 4 h post injection [21]. Because peripheral immune signals can be relayed to the CNS via CVO organs and vagus nerve stimulation of the brainstem [26, 27, 39], higher neuronal activity in these areas indicates that the immune signal from the periphery is amplified within the aged brain. However, these findings do not eliminate the potential for the peripheral innate immune system to contribute to the exacerbated neuroinflammation in the aged. One possibility is that peripheral immune challenge compromises the blood brain barrier allowing infiltration of peripheral immune cells into the brain [4]. However, compromise of the BBB is not a prerequisite for immune to brain cytokine signaling [59, 60]. The results of current study are significant because they indicate that direct central immune activation causes an exaggerated cytokine response within the aged brain and support the premise that a dysregulated response within the brain of aged mice accounts for at least part of the amplified and prolonged cytokine profile induced by systemic immune activation.
Since aged BALB/c mice have an increased neuroinflammatory profile [25, 61], we anticipated that the initial proinflammatory cytokine response to central LPS injection would be exaggerated. While MHC class II levels were increased due to age in all brain regions examined, these increases were not reliable predictors of an amplified response to i.c.v injection of LPS. LPS-induced inflammatory cytokine (IL-1β, IL-6 and TNFα) mRNA levels were only significantly different in the cerebellum of aged mice compared to adult mice 2 h post i.c.v. injection (Fig. 2 A–C). Since both TLR2 and TLR4 can mediate LPS signaling in microglia [34, 53] the specificity of the amplified cytokine response to LPS in the cerebellum of aged mice may be explained by age-dependent increase in TLR2 and TLR4 steady state mRNA levels in this region (Fig. 4). In fact, LPS caused a marked increase in the steady state transcription for both TLR2 and TLR4 in the cerebellum of aged mice compared to adults. Taken together, these data can be interpreted to suggest that there was a pronounced TLR reactivity to central LPS challenge in the cerebellum of aged mice.
While the glia of the aged brain may not necessarily have an increased sensitivity to direct TLR4 stimulation, they may have an increased activity in response to inflammatory cytokine exposure [14, 48]. Propagation of the LPS-induced inflammatory cytokine signal was prolonged in both the cerebellum and hippocampus of aged mice. At 8 h post i.c.v. injection of LPS, IL-1β, IL-6, and TNFα mRNA levels were significantly higher in the cerebellum and hippocampus of aged mice compared to adult cohorts (Fig. 2 D–F). It is also important to note that innate immune activators such as LPS do not typically gain access into the brain, so age-associated increases in brain TLR expression would have little impact on the response to a peripheral infection. However, the significant point is that independent of the stimulus (i.e. peripheral or central) inflammatory cytokines are protracted in the aged brain. One explanation is that reactive glia in the aged brain become activated to a greater extent following cytokine exposure and respond by producing larger amounts of these inflammatory mediators than the glia of adult mice. This notion is supported by a study where i.c.v. injection of TNFα and IFNγ is caused a greater reactivity of microglia in the aged brain [14]. It is also plausible that there is an age-dependent reduction in anti-inflammatory mediators or growth factors, such as IL-10 [38, 73] or insulin-like growth factor-1 (IGF-1) [8, 13], which would impair the ability to resolve a brain inflammatory response. Whatever the mechanism, it is clear that aged mice experience unresolved brain inflammation for a longer duration following central immune activation (Fig. 2).
The critical finding of this study was that the prolonged cytokine exposure in the aged brain was associated with a protracted sickness behavior syndrome. Since IL-1β, IL-6 and TNFα are involved in either inducing or maintaining sickness behavior [32], it is not surprising that the prolonged LPS-induced sickness in aged mice was mirrored by exaggerated brain cytokine levels 8 h after LPS i.c.v. injection (Fig. 2 D–F). While adult mice show significant improvement in recovery from LPS-induced sickness at 8 h, aged mice did not (Fig. 1). It is important to note that 2 h after LPS, sickness behavior was similar in each age group. This is likely to reflect that the cumulative brain cytokine response (i.e. cortex and hippocampus) was similar in both age groups. Prolonged sickness was not a function of increased mortality because aged mice recovered to baseline social investigation and cytokine mRNA expression by 24 h after central LPS injection (data not shown). This is a relevant point because a previous study showed that a high dosage of LPS (10 µg) injected i.c.v. caused a greater mortality in aged mice that was associated with higher whole brain and circulating levels of TNFα [31]. Excessive or septic levels of LPS are likely to compromise the integrity of the blood brain barrier [4] and allow for infiltration of peripheral immune cells into the brain [74]. In the current study, however, we used a low dose of LPS (10 ng) to induce a mild and transient sickness response that was not associated with mortality or detectable levels of circulating TNFα or IL-1β. A prolonged cytokine-mediated sickness response is consistent with our previous findings in aged mice using peripheral LPS injection [25] and central HIV gp-120 injection [1].
Another interesting finding was that IL-6 was amplified (2 h) and prolonged (8 h) in the plasma of aged mice after i.c.v. LPS injection (Fig. 3). These results show that an elevated brain response to central immune challenge can be communicated to the periphery. It is unknown why no appreciable levels of either IL-1β or TNFα were detected in the plasma of either age group after i.c.v. LPS challenge (data not shown). The absence of detectable levels of IL-1β in the plasma following central LPS injection is consistent with a previous rodent study [59]. Our results may indicate a specific IL-6 response since an indirect leakage of cytokines from the brain would result in increased levels in all three cytokines. In fact, in our previous studies in rats we found i.c.v injection of either IL-1β or LPS induced a plasma increase in IL-6 [17, 18]. The specificity of increase in IL-6 may indicate a higher hypothalamus-pituitary-adrenal (HPA) axis [45] or sympathetic nervous system mediated [18] response to central innate immune challenge in the aged brain. This is of particular interest because higher levels of circulating IL-6 were correlated with cognitive and depressive complications in the elderly [55, 70]. Taken together, these findings indicate that an amplified neuroinflammatory response to central challenge can be communicated to the periphery, potentially as an IL-6-mediated signal.
Understanding a prolonged neuroinflammatory response in the aged brain is important because it can lead to severe cognitive and behavioral complications [22]. For instance, peripheral infections and inflammatory conditions are permissive to acute cognitive impairment, dementia, and mood disorders in the elderly [44, 55, 67]. Brain region specificity is important because regions such as the hippocampus that express IL-1β and IL-6 cytokine receptors [54, 63] appear to be more sensitive to excessive or prolonged cytokine exposure. For example, peripheral E. coli infection in aged rats promoted higher IL-1β production in the hippocampus and was associated with impaired memory consolidation [2]. Moreover, peripheral LPS injection caused an elevated inflammatory response in the hippocampus of aged mice compared to adults that was associated with impaired spatial memory and depressive-like behavior (unpublished findings). Other studies have found that excessive exposure to inflammatory cytokines was detrimental to neuronal plasticity in the hippocampus, with impairments in neurogenesis [15, 40], long-term potentiation [28, 69], and neurite branching [49].
In conclusion, the present study demonstrates that direct activation of the central innate immune system in aged mice resulted in exacerbated neuroinflammation and prolonged sickness behavior. Furthermore, these findings support the idea that the sensitization of the inflammatory cytokine system is occurring within the brain with age. These findings are important because a heightened neuroinflammatory response in the aged may also lead to other neurobehavioral impairments. Therefore pharmacological strategies aimed at specifically decreasing exacerbated glial reactivity associated with infection might be important for improving recovery from sickness and reducing neurobehavioral deficits in the elderly.
Acknowledgements
This research was supported by the American Federation for Aging Research (AFAR) grant-60007465 to J.P.G and the NIH grants (MH071349 & MH079829) to R.D. and (AG16710 & MH069148) to R.W.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors of this manuscript declare that there are no actual or potential conflicts of interest. The authors affirm that there are no financial, personal or other relationships with other people or organizations that have inappropriately influenced or biased their research.
References
- 1.Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28(1):5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23(9):3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3(4):169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 7.Bluthe RM, Dantzer R, Kelley KW. Interleukin-1 mediates behavioural but not metabolic effects of tumor necrosis factor alpha in mice. Eur J Pharmacol. 1991;209(3):281–283. doi: 10.1016/0014-2999(91)90184-r. [DOI] [PubMed] [Google Scholar]
- 8.Bluthe RM, Kelley KW, Dantzer R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav Immun. 2006;20(1):57–63. doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90(21):10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller H. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54(9):906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 11.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31(2):578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dantzer R, Gheusi G, Johnson RW, Kelley KW. Central administration of insulin-like growth factor-1 inhibits lipopolysaccharide-induced sickness behavior in mice. Neuroreport. 1999;10(2):289–292. doi: 10.1097/00001756-199902050-00015. [DOI] [PubMed] [Google Scholar]
- 14.Deng XH, Bertini G, Xu YZ, Yan Z, Bentivoglio M. Cytokine-induced activation of glial cells in the mouse brain is enhanced at an advanced age. Neuroscience. 2006;141(2):645–661. doi: 10.1016/j.neuroscience.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fattori E, Lazzaro D, Musiani P, Modesti A, Alonzi T, Ciliberto G. IL-6 expression in neurons of transgenic mice causes reactive astrocytosis and increase in ramified microglial cells but no neuronal damage. Eur J Neurosci. 1995;7(12):2441–2449. doi: 10.1111/j.1460-9568.1995.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 17.Finck BN, Johnson RW. Anorexia, weight loss and increased plasma interleukin-6 caused by chronic intracerebroventricular infusion of interleukin-1beta in the rat. Brain Res. 1997;761(2):333–337. doi: 10.1016/s0006-8993(97)00451-4. [DOI] [PubMed] [Google Scholar]
- 18.Finck BN, Dantzer R, Kelley KW, Woods JA, Johnson RW. Central lipopolysaccharide elevates plasma IL-6 concentration by an alpha-adrenoreceptor-mediated mechanism. Am J Physiol. 1997;272(6 Pt 2):R1880–R1887. doi: 10.1152/ajpregu.1997.272.6.R1880. [DOI] [PubMed] [Google Scholar]
- 19.Fontes JD, Kanazawa S, Nekrep N, Peterlin BM. The class II transactivator CIITA is a transcriptional integrator. Microbes Infect. 1999;1(11):863–869. doi: 10.1016/s1286-4579(99)00232-4. [DOI] [PubMed] [Google Scholar]
- 20.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27(5):717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Gaykema RP, Balachandran MK, Godbout JP, Johnson RW, Goehler LE. Enhanced neuronal activation in central autonomic network nuclei in aged mice following acute peripheral immune challenge. Auton Neurosci. 2006 doi: 10.1016/j.autneu.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24(3):521–538. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Godbout JP, Berg BM, Kelley KW, Johnson RW. alpha-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J Neuroimmunol. 2004;149(1–2):101–109. doi: 10.1016/j.jneuroim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Godbout JP, Berg BM, Krzyszton C, Johnson RW. alpha-Tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005 doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19(10):1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 26.Goehler LE, Erisir A, Gaykema RP. Neural-immune interface in the rat area postrema. Neuroscience. 2006;140(4):1415–1434. doi: 10.1016/j.neuroscience.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 27.Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res. 1998;804(2):306–310. doi: 10.1016/s0006-8993(98)00685-4. [DOI] [PubMed] [Google Scholar]
- 28.Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99(4):1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- 29.Heyser CJ, Masliah E, Samimi A. IL Campbell, and LH Gold, Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94(4):1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson RW, Gheusi G, Segreti S, Dantzer R, Kelley KW. C3H/HeJ mice are refractory to lipopolysaccharide in the brain. Brain Res. 1997;752(1–2):219–226. doi: 10.1016/s0006-8993(96)01454-0. [DOI] [PubMed] [Google Scholar]
- 31.Kalehua AN, Taub DD, Baskar PV, Hengemihle J, Munoz J, Trambadia M, Speer DL, De Simoni MG, Ingram DK. Aged mice exhibit greater mortality concomitant to increased brain and plasma TNF-alpha levels following intracerebroventricular injection of lipopolysaccharide. Gerontology. 2000;46(3):115–128. doi: 10.1159/000022146. [DOI] [PubMed] [Google Scholar]
- 32.Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(1 Suppl):112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 33.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25(3):154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 34.Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. Faseb J. 2001;15(1):155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- 35.Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25(3):294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19(5):497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 38.Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10--a role for IL-1 beta? J Neurochem. 2004;88(3):635–646. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- 39.Marvel FA, Chen CC, Badr N, Gaykema RP, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav Immun. 2004;18(2):123–134. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 41.Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89(3):687–699. doi: 10.1016/s0306-4522(98)00334-0. [DOI] [PubMed] [Google Scholar]
- 42.Morley AA, Trainor KJ. Lack of an effect of vitamin E on lifespan of mice. Biogerontology. 2001;2(2):109–112. doi: 10.1023/a:1011589218219. [DOI] [PubMed] [Google Scholar]
- 43.Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956(1):30–35. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- 44.Mulsant BH, Ganguli M. Epidemiology and diagnosis of depression in late life. J Clin Psychiatry. 1999;60 Suppl 20:9–15. [PubMed] [Google Scholar]
- 45.Muramami N, Fukata J, Tsukada T, Kobayashi H, Ebisui O, Segawa H, Muro S, Imura H, Nakao K. Bacterial lipopolysaccharide-induced expression of interleukin-6 messenger ribonucleic acid in the rat hypothalamus, pituitary, adrenal gland, and spleen. Endocrinology. 1993;133(6):2574–2578. doi: 10.1210/endo.133.6.8243280. [DOI] [PubMed] [Google Scholar]
- 46.Murray CA, Clements MP, Lynch MA. Interleukin-1 induces lipid peroxidation and membrane changes in rat hippocampus: An age-related study. Gerontology. 1999;45(3):136–142. doi: 10.1159/000022076. [DOI] [PubMed] [Google Scholar]
- 47.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344(13):961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 48.Nelson KP, Marks NL, Heyen JR, Johnson RW. Behavior of adult and aged mice before and after central injection of interleukin-1beta. Physiol Behav. 1999;66(4):673–679. doi: 10.1016/s0031-9384(98)00339-4. [DOI] [PubMed] [Google Scholar]
- 49.Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde YA. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J Neurosci. 2002;22(3):854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 51.Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107(3):415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- 52.Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5(10):1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- 53.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173(6):3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 54.Parnet P, Amindari S, Wu C, Brunke-Reese D, Goujon E, Weyhenmeyer JA, Dantzer R, Kelley KW. Expression of type I and type II interleukin-1 receptors in mouse brain. Brain Res Mol Brain Res. 1994;27(1):63–70. doi: 10.1016/0169-328x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 55.Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54(5):566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 56.Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7(1):60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- 57.Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4(2):103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 58.Pollmacher T, Haack M, Schuld A, Reichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines--do they affect human brain functions? Brain Behav Immun. 2002;16(5):525–532. doi: 10.1016/s0889-1591(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 59.Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J Neuroimmunol. 1994;49(1–2):125–134. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 60.Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83(1):281–293. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 61.Richwine AF, Godbout JP, Berg BM, Chen J, Escobar J, Millard DK, Johnson RW. Improved psychomotor performance in aged mice fed diet high in antioxidants is associated with reduced ex vivo brain interleukin-6 production. Brain Behav Immun. 2005;19(6):512–520. doi: 10.1016/j.bbi.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging. 1998;19(1):97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- 63.Schobitz B, de Kloet ER, Sutanto W, Holsboer F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur J Neurosci. 1993;5(11):1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 64.Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19(1):47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- 65.Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol Aging. 1999;20(4):395–405. doi: 10.1016/s0197-4580(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 66.Streit WJ, Sparks DL. Activation of microglia in the brains of humans with heart disease and hypercholesterolemic rabbits. J Mol Med. 1997;75(2):130–138. doi: 10.1007/s001090050097. [DOI] [PubMed] [Google Scholar]
- 67.Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O'Brien JT. Increase in interleukin-1beta in late-life depression. Am J Psychiatry. 2005;162(1):175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- 68.Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22(2):486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vereker E, O'Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20(18):6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 71.Wong AM, Patel NV, Patel NK, Wei M, Morgan TE, de Beer MC, de Villiers WJ, Finch CE. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neurosci Lett. 2005;390(2):76–80. doi: 10.1016/j.neulet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 72.Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol. 2003;182(1):135–141. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 73.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9(4):183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 74.Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177(11):8103–8110. doi: 10.4049/jimmunol.177.11.8103. [DOI] [PubMed] [Google Scholar]