Abstract
Nutrient requirements increase during periods of growth and development such as pregnancy and lactation. In response, many clinicians recommend dietary supplements during these important periods of the life cycle. Although there exist some recommendations concerning the need for a limited number of nutrients in supplemental form (eg, iron, folic acid, and iodine), there is a relative paucity of data concerning the use of dietary supplements during pregnancy and lactation. Limited data suggest, however, that usage is dependent on demographic, sociologic, and economic factors. Thus, it is possible that the nation's most at-risk populations may be those who are least likely to comply with these recommendations. As researchers continue to study what is meant by “optimal nutrition” during pregnancy and lactation, it is likely that additional recommendations concerning dietary supplements will emerge. For example, it is possible that increased consumption of some of the long-chain polyunsaturated fatty acids during pregnancy or lactation may impart a benefit to infant health. Understanding better the population dynamics related to supplement use during these periods will be critical in implementation of campaigns designed to encourage appropriate use—and discourage inappropriate use—of dietary supplements during these important phases of human reproduction. The purpose of this article is to briefly review what is known about the use of dietary supplements in North America and, more specifically, in pregnant and lactating women. In addition, information concerning barriers to supplement use is discussed as are current recommendations for dietary supplement consumption during these periods of the life cycle.
INTRODUCTION
Nutrient requirements during periods of the life span characterized by intensive physical growth and/or developmental maturation are generally greater than during other life stages. Consequently, consumption of appropriate foods that supply adequate amounts of nutrients to meet nutrient requirements may be especially difficult, and important, during growth and development. Such periods of the life span include pregnancy and lactation, during which time nutrient requirements increase to support fetal and then infant growth and development (1). For example, recommended intakes for 14 of the 21 essential micronutrients increase during pregnancy. These nutrients comprise 7 vitamins, 5 minerals, and choline (2). As such, it is important to increase one's intake of foods containing these nutrients to prevent risk of deficiencies. It is also important during these periods of the life span to not consume too much of each nutrient to reduce risk for levels of intake that may be harmful. Although meeting these increased nutrient requirements can and perhaps should be achieved by the consumption of appropriate amounts of foods in a balanced and varied diet, the use of dietary supplements may be beneficial in some situations (3).
It is important to recognize that the term dietary supplement refers to a specific group of products available to consumers. Specifically, the Dietary Supplement Health and Education Act of 1994 issued by the US Food and Drug Administration defined a dietary supplement as a product that collectively meets the following requirements (4):
A product (other than tobacco) intended to supplement the diet or that contains one or more of the following: vitamin, mineral, herb or other plant-derived substance (eg, ginseng or garlic), amino acid, concentrate, metabolite, constituent, or extract
A product intended for ingestion in pill, capsule, tablet, or liquid form
A product not represented for use as a conventional food or as the sole item of a meal or diet
The fundamental purpose of this article is to provide a brief review of the published literature concerning the use of dietary supplements in women in general and specifically during pregnancy and lactation. We also review current recommendations concerning dietary supplement use and what is known about demographic factors and barriers related to supplement use during these periods of the life span. This information is crucial for informing public health professions in their decision-making process related to whether recommendations for supplementation with other nutrients, such as those covered by this symposium (methyl donors, iodine, and long-chain polyunsaturated fatty acids), are warranted or desirable on both population and individual levels in North American women. A review of the safety and efficacy of dietary supplements during pregnancy and lactation is beyond the scope of this article. Instead, the reader is directed to other reviews of this topic (1, 5, 6).
OVERVIEW OF SUPPLEMENT INTAKE IN THE GENERAL POPULATION
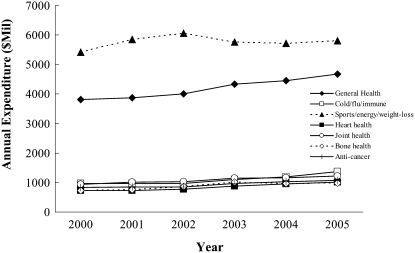
Data suggest that use of dietary supplements in the general US population is quite high and growing in most sectors. According to the Nutrition Business Journal's 2006 report, >$21 billion are spent annually on dietary supplements, with sales increasing consistently by ≈4% each year since 2000 (Figure 1) (7). Interestingly, although prescription drug expenditures ($252 billion) are much higher than those on dietary supplements, annual growth of the former has experienced a consistent decline, with annual growth rates decreasing from 18.7% to 5.4% between 2000 and 2005. In general, the majority of dollars spent on dietary supplements goes toward those taken to enhance sports performance, increase energy, and promote weight loss followed by those that support general health.
FIGURE 1.
Trends in annual fiscal expenditures on the top-selling, condition-specific supplement markets between 2000 and 2005 in the United States. Adapted from data from reference 7.
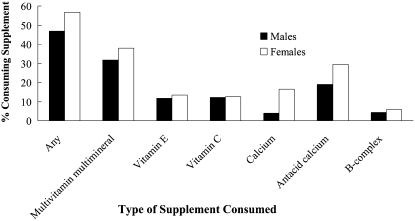
Data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES), which likely represents a more nationally representative survey of the US population than that sampled by the Nutrition Business Journal described above, also suggest high dietary supplement use among Americans (8). In this study conducted by Radimer et al (8), supplement use in US adults (n = 4880) ≥20 y was assessed by asking participants whether they had taken any vitamins, minerals, or other dietary supplements (including prescription supplements) in the past month. When possible (78% of the time), supplement containers were examined, and the name and manufacturer of the supplement were obtained from label information. Trained NHANES nutritionists then matched reported supplements to known products (9). This survey found that 52% of adults took a multivitamin and multimineral supplement, with vitamin C, vitamin E, B-complex, and calcium being taken by >5% of those surveyed, which supports the previous literature that suggested a high degree of supplement use among Americans (10–12). Factors related to supplement use included being female, older, more educated, non-Hispanic white, physically active, normal- or underweight, having formerly smoked, and reporting excellent or very good health. Supplement use by sex is shown in Figure 2. Of the women surveyed who took supplements, 44.6% took 1 supplement daily, 22.3% took 2, and 32.1% took ≥3.
FIGURE 2.
Percentage of US adults (>20 y of age) consuming dietary supplements and effect of sex as indicated by data from the National Health and Nutrition Examination Survey (NHANES), 1999–2000. Adapted from data from reference 8.
Although very little has been published about the characteristics of women who use dietary supplements, Yu et al (13) analyzed data from the 2000 National Health Interview Survey and reported that 56.9% of the women (n = 18,388) included in this study used supplements. Logistic regression suggested that female supplement users tended to be non-Hispanic white, married, older, more educated, not poor (≥200% of the poverty threshold), former smokers, alcohol users (≥1 drink/d in the past year), and regular exercisers. Those who were obese or overweight and women who had not been in contact with a health professional in the past 12 mo were less likely to use supplements. In summary, data, albeit limited in scope, suggest high use of dietary supplements among the US population and even higher use among women. Variables related to greater supplement use include race (non-Hispanic white used more supplements than others); being married, older, and more educated; and being a former smoker and a regular exerciser. Although correlation does not infer causality, it is likely that these factors, all of which tend to be related to positive health practices, drive supplement use and that individuals at greater risk of poor health are generally less likely to take supplements. Additional longitudinal, mixed-methods research will be required to understand the decision-making paradigms related to and determining supplement use in the US population as well as in subpopulations therein.
OVERVIEW OF DIETARY SUPPLEMENT INTAKE IN PREGNANCY
Even less is known about dietary supplement use during pregnancy. This is somewhat surprising because health care providers generally recommend that pregnant women consume a standard prenatal multivitamin and multimineral supplement as insurance against inadequate micronutrient intake. Interestingly, however, evidence to support a benefit from this practice for most women in developed countries such as the United States is weak. Nonetheless, there are a handful of reports that suggest that multivitamin and multimineral use is related to a decreased risk of fetal malformations. For example, Botto et al (14) conducted a retrospective case-control study in which they compared the incidence of conotruncal heart defects in 2 groups of women living in the Atlanta, GA, area: those giving birth to infants with conotruncal defects (n = 158) and unaffected women (n = 3026). Multivitamin use was defined as “the reported regular use from 3 mo before conception through the third month of pregnancy.” Data from this study suggest that periconceptional multivitamin use was associated with a reduced risk of this kind of malformation. However, because this study was not an intervention trial, there was no standardization of the type of prenatal supplement taken by the women. Thus, it is quite possible that confounding factors may have influenced the findings.
Another study that used a more rigorous study design provides additional, but limited, evidence that periconceptional multivitamin supplementation may be beneficial (15, 16). This study was conducted as a randomized, double-blind, placebo-like controlled study with 4682 subjects. Data suggest that multivitamin supplementation can reduce the risk of urinary tract abnormalities, cardiovascular malformations, and neural tube defects. Interestingly, these authors also found an effect on fertility and multiple births, such that the women receiving the multivitamin supplement had a 5% shorter time in the achievement of subsequent pregnancy and experienced higher rates of monozygotic twinning (17, 18). There was no effect of multivitamin consumption on the rate of fetal deaths, low birth weight, or preterm birth in singletons. However, care should be taken when drawing conclusions from this study, because the placebo-like group actually received a supplement containing several trace elements. Other observational studies support the possible effects of prenatal multivitamin and multimineral use on the risk of preeclampsia, preterm birth, cleft palate, and other fetal malformations (19–22). However, more controlled, prospective studies are needed to confirm these findings.
DIETARY SUPPLEMENT USE DURING PREGNANCY
Although most pregnant women are advised to consume multivitamin and multimineral supplements, their use during pregnancy is not well documented. Perhaps the best data in this regard were those reported by Suitor and Gardner (23) almost 2 decades ago. In this epidemiologic study, the researchers documented the self-reported rates of supplement use in 344 low-income pregnant women. Eighty-six percent of subjects reported consumption (≥4 times/wk) of prenatal vitamins, which were routinely prescribed for all patients. Another 5% took these products 2–3 times/wk. Clearly, more research is needed to understand multivitamin and multimineral supplement use in contemporary pregnant women living in North America. Nonetheless, this study suggests that dietary supplement use is quite common in women, even in those at the lower spectrum of socioeconomic status.
Additional information concerning dietary supplement use during pregnancy can be garnered from national survey data (n = 10,046; ≥14 y of age) evaluated by Cogswell et al (24), who examined data from NHANES III (1988–1994) to assess iron supplement use among women in the United States. Included in their study population were 9000 nonpregnant and nonlactating women, 295 pregnant women, and 97 lactating women. Data were obtained from a series of questions about vitamins and minerals that the subject had taken within the past month, and participants who answered affirmatively were asked to show the supplement containers to the investigators. The authors found that there was a significant (P < 0.003) relation between reproductive status and iron supplement usage. Whereas only 9%–23% of the nonpregnant, nonlactating women consumed iron supplements, 72% and 60% of the pregnant and lactating, respectively, did. In addition, several sociocultural factors were related to supplement usage, including ethnicity and income. In general, non-Hispanic white women were more likely (P < 0.04) than non-Hispanic black or Mexican American women to take supplements, and women with incomes <130% of the poverty:income ratio tended to be less likely than those at >130% of the poverty:income ratio to take iron during pregnancy. Of interest is the finding that the mean amount of iron consumed from supplements among pregnant women who consumed supplements with iron was 78.4 ± 6.6 mg/d, which is a value much greater than the Institute of Medicine's upper limit level of 45 mg/d (25).
To our knowledge, there has been only a single randomized study to investigate factors related to supplement adherence during pregnancy. In this trial, Jasti et al (26) randomly assigned low-income pregnant women (n = 244) to consume either a placebo or an iron-containing supplement. This population was characterized by ethnic diversity (31% white and 62% black), low education (77% with a high school or less education), and nonmarital status (80% unmarried). Adherence to supplement consumption was assessed by documenting pill counts. As the study of Cogswell et al (24) described previously, Jasti et al (26) reported that supplement consumption was highly dependent on ethnicity. However, ethnicity interacted with several factors, such as 1) gravidity, 2) supplement use before pregnancy, 3) smoking, and 4) pregnancy-related nausea, in predicting supplement adherence. For example, white but not black women were more likely to take the supplements in their first pregnancy as compared with later pregnancies, whereas black but not white women were more likely to take their supplements if they had taken supplements during previous pregnancies. Clearly, further research investigating the influence of cultural factors is necessary to understand adherence to supplement use among ethnic groups.
In summary, limited data suggest a high degree of supplement use during pregnancy. However, supplement use appears to vary among ethnic and socioeconomic groups, which indicates that disparities might exist among subgroups within the general North American population. Additional research should focus on understanding better these possible differences and their potential consequences.
RECOMMENDATIONS FOR DIETARY SUPPLEMENT USE DURING PREGNANCY
The American Dietetic Association and the Institute of Medicine recommend that all pregnant women who smoke or abuse alcohol or drugs take multivitamin and multimineral supplements as should those with iron deficiency anemia or poor-quality diets (2, 27). This recommendation also applies to vegans and women carrying ≥2 fetuses. In addition, because of the convincing evidence that periconceptional folic acid supplementation can decrease neural tube defects in some women, many health organizations recommend routine folic acid supplementation during this period. For example, the US Centers for Disease Control and Prevention (CDC) recommends that all women of childbearing age who are capable of becoming pregnant should consume 0.4 mg/d folic acid. This recommendation has been adopted by several clinical practice associations, such as the American Academy of Pediatrics and the National Healthy Mothers, Healthy Babies Coalition (28, 29). Similarly, the Institute of Medicine recommends that “to reduce the risk of neural tube defects, women able to become pregnant should take 0.4 mg of folic acid daily from fortified foods, supplements, or both, in addition to consuming food folate from a varied diet” (30). Data from the 2005 March of Dimes Gallup survey indicated that only 33% of US women of childbearing age reported taking supplemental folic acid daily (31).
Furthermore, because of the recognized benefits of additional iron during pregnancy, the World Health Organization recommends daily iron supplementation (60 mg/d) for all pregnant women for 6 mo or, if 6 mo of treatment cannot be achieved during the pregnancy, either continuation of supplementation during the postpartum period or an increased dosage of 120 mg/d iron during pregnancy (32, 33). Other recommendations include that of the CDC, which advises that oral, low-dose (30 mg/d) supplements of iron be provided to all pregnant women at the first prenatal visit (34). To our knowledge, the only other essential nutrient for which there is a recommendation for supplementation during pregnancy is iodine: the American Thyroid Association recommends that all pregnant women living in the United States or Canada consume 150 μg/d of supplemental iodine (35).
OVERVIEW OF SUPPLEMENT USE IN LACTATION
Like pregnancy, lactation represents a period of the life cycle characterized by increased nutrient requirements that may or may not be easily achieved by diet alone. Thus, many women turn to dietary supplements to supply some of these nutrients. However, use of dietary supplements during lactation is even less well documented than that during pregnancy. In a recent report, Stultz et al (36) studied the extent of medication (including multivitamins and select micronutrients) during this period of the life cycle in a prospective cohort of 46 breastfeeding women (≥18 y of age) from birth until cessation of breastfeeding or 1 y postpartum. Their data suggest that most (73%) women took a multivitamin preparation, with fewer reporting use of calcium (11%), folic acid (7%), or iron (4%). Interestingly, their data suggest that, in this relatively small nonrepresentative sample, significantly more (P < 0.05) women reported taking dietary supplements during pregnancy than during lactation. Whether this is true in the general US population as well is not known. Nonetheless, these data suggest a relatively high usage of multivitamin preparations during lactation with relatively low consumption of other supplemental micronutrients such as calcium, folic acid, and iron.
Data from Cogswell et al (24) in which a more heterogeneous, representative sample was studied using data from NHANES III support the contention that overall dietary supplement usage is lower during lactation than during pregnancy. In addition, as previously discussed, these investigators found that ethnicity and income predicted iron supplementation during lactation, with 77% of non-Hispanic white women reporting supplement use compared with 41% of non-Hispanic black or Mexican American women. Women who reported being at >130% of the poverty level were more than twice (P < 0.001) as likely as poorer women to take iron supplements during this time period.
To our knowledge, there are no consistently made recommendations concerning dietary supplement use during lactation, although the American Thyroid Association recommends that breastfeeding women receive 150-μg iodine supplements daily. However, in its most recent report addressing nutrition during lactation, the Institute of Medicine recommended that lactating women be encouraged to obtain their nutrients from a well-balanced, varied diet rather than from vitamin and mineral supplements (37). For women whose eating patterns lead to a very low intake of one or more nutrients, individualized dietary counseling is preferred and dietary supplementation may be necessary. For example, vegans might be advised to take vitamin B-12 supplements daily, and those who avoid all dairy products might be advised to take calcium and vitamin D supplements.
SUMMARY AND RESEARCH NEEDS
In summary, although dietary supplementation is commonly recommended during pregnancy and lactation, there are only a handful of reports that document the extent of this practice in North America. These suggest that well more than one-half of pregnant or lactating women take some form of dietary supplement, that usage is likely greater during pregnancy than during lactation, and that usage is dependent on a web of social, ethnic, and demographic variables. For example, high-income white women are more likely than their low-income Mexican American counterparts to consume supplements during the period of reproduction. In addition, almost nothing is currently known about usage in our most at-risk subpopulations. Of particular note, in one of the few publications documenting use of iron supplements by pregnant and lactating women in the United States, data suggest that supplementary iron intakes well above the upper-limit-level values recommended by the Institute of Medicine. Clearly, these findings need to be corroborated or refuted by additional large-scale human studies.
Collection of these types of data is critical not only to document current dietary supplement usage but also to help implement future campaigns concerning dietary supplement use in pregnancy or lactation should they be needed to ensure adequate micronutrient intakes. This is likely most pressing for the issues of folic acid and iodine supplements, both of which are currently recommended for use during pregnancy and lactation. In addition, it is important for both scientific and public health purposes to understand to what extent supplements contribute to overall nutrient intakes during pregnancy and lactation. For example, do dietary supplements contribute additional nutrients to diets already deemed adequate or do they fulfill basic nutrient needs? Of course, it should go without saying that rigorous, controlled human trials continue to be needed to assess the efficacy and safety of dietary supplements recommended during pregnancy and lactation to both mother and infant in years to come. Ongoing longitudinal, national nutrition-monitoring programs are needed for collection of such critically important data. (Other articles in this supplement to the Journal include references 38–42.)
Acknowledgments
Each author took equal responsibility for researching and drafting this manuscript. Neither of the authors had a known conflict of interest related to this publication.
REFERENCES
- 1.Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr 2003;133(suppl):1997S–2002S [DOI] [PubMed] [Google Scholar]
- 2.Allen LH. Pregnancy and lactation. Bowman BA, Russell RM, eds Present knowledge in nutrition. 9th ed Vol 2 Washington, DC: International Life Sciences Institute, 2006529–43 [Google Scholar]
- 3.Subcommittee on Dietary Intake and Nutrient Supplements During Pregnancy. Nutrition during pregnancy. Washington, DC: National Academy Press, 1990 [Google Scholar]
- 4.Center for Food Safety and Applied Nutrition. Overview of dietary supplements. Washington, DC: US Food and Drug Administration, 2001. Available from: http://www.cfsan.fda.gov/∼dms/ds-oview.html#what (cited 23 April 2007) [Google Scholar]
- 5.Picciano MF, McGuire MK. Efficacy and safety of dietary supplements during pregnancy. Lammi-Keefe CJ, Couch SC, Philipson E, eds Nutrition and health: handbook of nutrition in pregnancy. Totowa, NJ: Humana Press, 2008:191–214 [Google Scholar]
- 6.O'Connor DL, Houghton LA, Sherwood KL. Nutrition issues during lactation. Lammi-Keefe CJ, Couch SC, Philipson E, eds Nutrition and health: handbook of nutrition in pregnancy. Totowa, NJ: Humana Press, 2008:257–82 [Google Scholar]
- 7.Nutrition Business Journal. 2006. Supplement business report 2006. San Diego, CA: New Hope Natural Media, Penton Media Inc.
- 8.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol 2004;160:339–49 [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics National Health and Nutrition Examination Survey. Available from: http://www.cdc.gov/nchs/about/major/nhanes/NHANES99_00.htm; questionnaire files heading (cited 3 May 2008)
- 10.Slesinski MJ, Subar AF, Kahle LL. Trends in use of vitamin and mineral supplements in the United States: the 1987 and 1992 National Health Interview Surveys. J Am Diet Assoc 1995;95:921–3 [DOI] [PubMed] [Google Scholar]
- 11.Balluz LS, Kieszak SM, Philen RM, Mulinare J. Vitamin and mineral supplement use in the United States: results from the third National Health and Nutrition Examination Survey. Arch Fam Med 2000;9:258–62 [DOI] [PubMed] [Google Scholar]
- 12.Ervin RB, Wright JD, Kennedy-Stephenson J. Use of dietary supplements in the United States, 1988–94. Vital Health Stat 111999;244:i–iii, 1–14 [PubMed] [Google Scholar]
- 13.Yu SM, Kogan MD, Huang Z. Vitamin-mineral supplement use among US women, 2000. J Am Med Womens Assoc 2003;58:157–64 [PubMed] [Google Scholar]
- 14.Botto LD, Khoury MJ, Mulinare J, Erickson JD. Periconceptional multivitamin use and the occurrence of conotruncal heart defects: results from a population-based, case-control study. Pediatrics 1996;98:911–7 [PubMed] [Google Scholar]
- 15.Czeizel AE. Reduction of urinary tract and cardiovascular defects by periconceptional multivitamin supplementation. Am J Med Genet 1996;62:179–83 [DOI] [PubMed] [Google Scholar]
- 16.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5 [DOI] [PubMed] [Google Scholar]
- 17.Czeizel AE, Metneki J, Dudas I. The effect of preconceptional multivitamin supplementation on fertility. Int J Vitam Nutr Res 1996;66:55–8 [PubMed] [Google Scholar]
- 18.Czeizel AE, Metneki J, Dudas I. The higher rates of multiple births after periconceptional multivitamin supplementation: an analysis of causes. Acta Genet Med Gemellol (Roma) 1994;43:175–84 [DOI] [PubMed] [Google Scholar]
- 19.Bodnar LM, Tang G, Ness RB, Harger G, Roberts JM. Periconceptional multivitamin use reduces the risk of preeclampsia. Am J Epidemiol 2006;164:470–7 [DOI] [PubMed] [Google Scholar]
- 20.Lammer EJ, Shaw GM, Iovannisci DM, Finnell RH. Periconceptional multivitamin intake during early pregnancy, genetic variation of acetyl-N- transferase 1 (NAT1), and risk for orofacial clefts. Birth Defects Res A Clin Mol Teratol 2004;70:846–52 [DOI] [PubMed] [Google Scholar]
- 21.Correa A, Botto L, Liu Y, Mulinare J, Erickson JD. Do multivitamin supplements attenuate the risk for diabetes-associated birth defects?. Pediatrics 2003;111:1146–51 [PubMed] [Google Scholar]
- 22.Vahratian A, Siega-Riz AM, Savitz DA, Thorp JM., Jr Multivitamin use and the risk of preterm birth. Am J Epidemiol 2004;160:886–92 [DOI] [PubMed] [Google Scholar]
- 23.Suitor CW, Gardner JD. Supplement use among a culturally diverse group of low-income pregnant women. J Am Diet Assoc 1990;90:268–71 [PubMed] [Google Scholar]
- 24.Cogswell ME, Kettel-Khan L, Ramakrishnan U. Iron supplement use among women in the United States: science, policy and practice. J Nutr 2003;133:1974S–7S [DOI] [PubMed] [Google Scholar]
- 25.National Academy of Science's Institute of Medicine Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press, 2001 [PubMed] [Google Scholar]
- 26.Jasti S, Siega-Riz AM, Cogswell ME, Hartzema AG, Bentley ME. Pill count adherence to prenatal multivitamin/mineral supplement use among low-income women. J Nutr 2005;135:1093–101 [DOI] [PubMed] [Google Scholar]
- 27.Kaiser LL, Allen L. American Dietetic Association. Position of the American Dietetic Association: nutrition and lifestyle for a healthy pregnancy outcome. J Am Diet Assoc 2002;102:1479–90 [DOI] [PubMed] [Google Scholar]
- 28.American Academy of Pediatrics, Committee on Genetics Folic acid for the prevention of neural tube defects. Pediatrics 1999;104:325–7 [DOI] [PubMed] [Google Scholar]
- 29.National Healthy Mothers Healthy Babies Coalition. Folic acid position statement. Available from: http://www.hmhb.org/ps_folicacid.html (cited 23 April 2008)
- 30.Institute of Medicine Dietary Reference Intakes: the essential guide to nutrient requirements. Otten JJ, Hellwig JP, Meyers LD, eds Washington, DC: The National Academies Press, 2006 [Google Scholar]
- 31.Centers for Disease Control and Prevention Use of dietary supplements containing folic acid among women of childbearing age: United States, 2005. MMWR Morb Mortal Wkly Rep 2005;54:955–8 [PubMed] [Google Scholar]
- 32.World Health Organization Iron and folate supplementation. Available from: http://www.who.int/making_pregnancy_safer/publications/standards1.8N.pdf (cited 23 April 2008)
- 33.Stolzfus RJ, Dreyfuss ML. Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Washington, DC: International Life Sciences Institute Press, 1998. Available from: http://inacg.ilsi.org/file/b2_vuhuq8ak.pdf (cited 23 April 2008) [Google Scholar]
- 34.Centers for Disease Control and Prevention Recommendations to prevent and control iron deficiency in the United States: 1998. MMWR Recomm Rep 1998;47:1–29 [PubMed] [Google Scholar]
- 35.Becker DV, Braverman LE, Delange F, et al. Iodine supplementation for pregnancy and lactation—United States and Canada: recommendations of the American Thyroid Association. Thyroid 2006;16:949–51 [DOI] [PubMed] [Google Scholar]
- 36.Stultz EE, Stokes JL, Shaffer ML, Paul IM, Berlin CM. Extent of medication use in breastfeeding women. Breastfeed Med 2007;2:145–51 [DOI] [PubMed] [Google Scholar]
- 37.Institute of Medicine (US), Subcommittee on Nutrition during Lactation. Nutrition during lactation. Washington, DC: National Academy Press, 1991 [Google Scholar]
- 38.Greer FR. Introduction. Am J Clin Nutr 2009;89(suppl):661S– 2S [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann MB. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr 2009;89(suppl):668S–72S [DOI] [PubMed] [Google Scholar]
- 40.Zeisel SH. Importance of methyl donors during reproduction. Am J Clin Nutr 2009;89(suppl):673S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson SE. Docosahexaenoic acid supplementation in pregnancy and lactation. Am J Clin Nutr 2009;89(suppl):678S–84S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeisel SH. Is maternal diet supplementation beneficial? Optimal development of infant depends on mother's diet. Am J Clin Nutr 2009;89(suppl):685S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]