Abstract
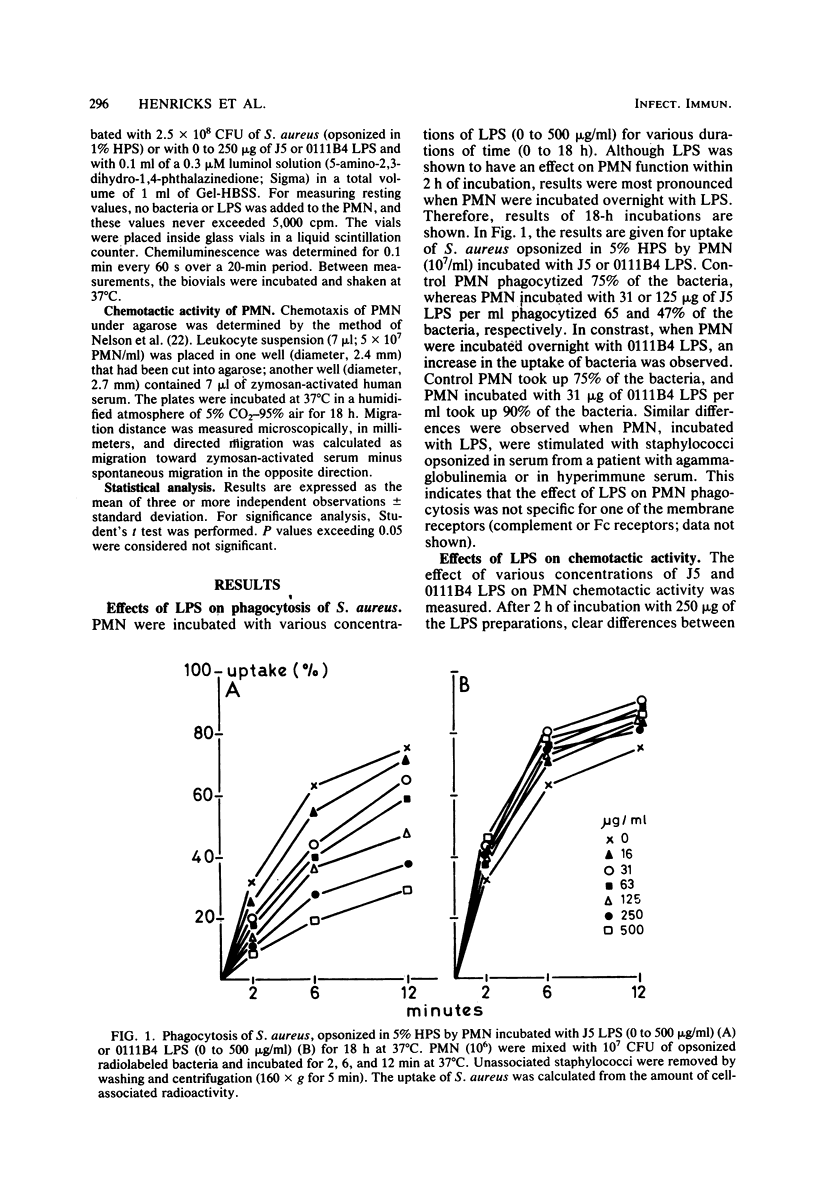
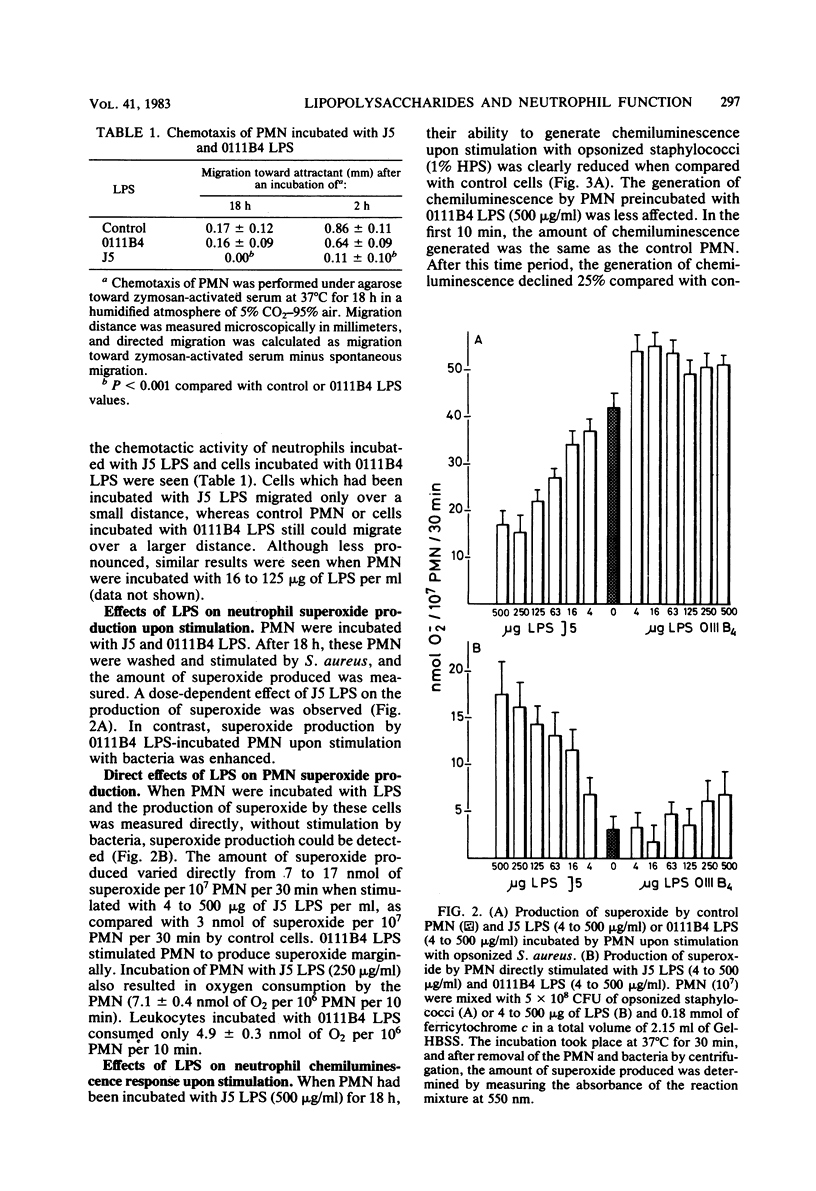
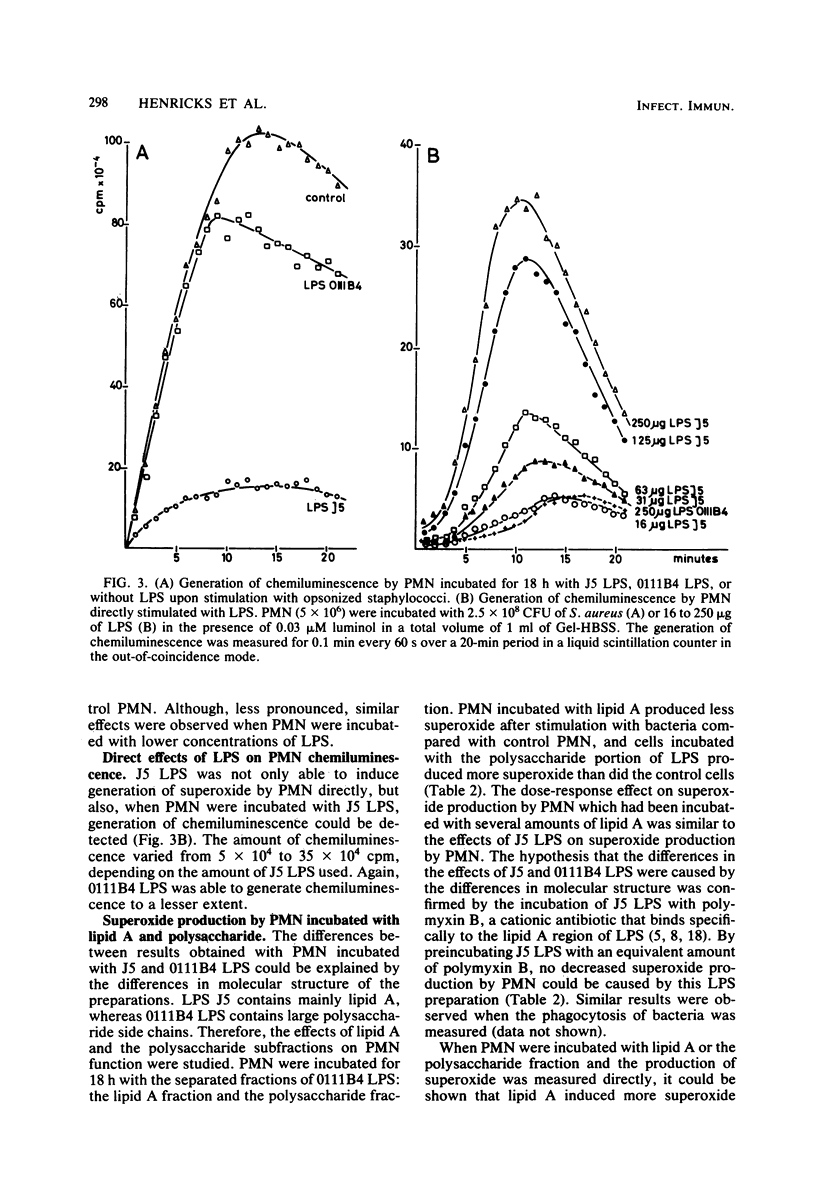
The effects of the lipopolysaccharide (LPS) of Escherichia coli J5 and 0111B4 on the function of human polymorphonuclear leukocytes (PMN) were tested. E. coli J5 is a UDP-galactose-4-epimerase-deficient mutant of E. coli 0111B4, and its LPS, therefore, contains mainly lipid A, as it lacks the polysaccharide side chains. PMN which had been incubated with J5 LPS showed decreased phagocytic, chemotactic, and metabolic activities as compared with control PMN. In contrast, incubation of PMN with 0111B4 LPS had no effect or even an enhancing effect on PMN function. When lipid A and the polysaccharide fraction were isolated from 0111B4 LPS, it was shown that lipid A had the same deleterious effect on PMN function as did J5 LPS and that the LPS fraction had no effect. When PMN were incubated with J5 LPS or lipid A, it could be shown that these structures were able to induce PMN to generate superoxide and chemiluminescence. 0111B4 LPS and the polysaccharide component were able to generate a metabolic burst by the PMN to a lesser extent. The induced defects in PMN function by J5 LPS could be prevented when polymyxin B or an oxygen-radical scavenger was present. We hypothesize that the lipid A portion of LPS is toxic for PMN due to the induction of toxic oxygen species by the PMN. These toxic oxygen species destroy the phagocytic, chemotactic, and metabolic activities of the PMN.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Bannatyne R. M., Harnett N. M., Lee K. Y., Biggar W. D. Inhibition of the biologic effects of endotoxin on neutrophils by polymyxin B sulfate. J Infect Dis. 1977 Oct;136(4):469–474. doi: 10.1093/infdis/136.4.469. [DOI] [PubMed] [Google Scholar]
- Bradley S. G. Cellular and molecular mechanisms of action of bacterial endotoxins. Annu Rev Microbiol. 1979;33:67–94. doi: 10.1146/annurev.mi.33.100179.000435. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Corrigan J. J., Jr, Sieber O. E., Jr, Ratajczak H., Bennett B. B. Modification of human neutrophil response to endotoxin with polymyxin B sulfate. J Infect Dis. 1974 Oct;130(4):384–387. doi: 10.1093/infdis/130.4.384. [DOI] [PubMed] [Google Scholar]
- Davis W. B., Barsoum I. S., Ramwell P. W., Yeager H., Jr Human alveolar macrophages: effects of endotoxin in vitro. Infect Immun. 1980 Dec;30(3):753–758. doi: 10.1128/iai.30.3.753-758.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks P. A., van Erne-van der Tol M. E., Verhoef J. Partial removal of sialic acid enhances phagocytosis and the generation of superoxide and chemiluminescence by polymorphonuclear leukocytes. J Immunol. 1982 Aug;129(2):745–750. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mills E. L., Rholl K. S., Quie P. G. X-linked inheritance in females with chronic granulomatous disease. J Clin Invest. 1980 Aug;66(2):332–340. doi: 10.1172/JCI109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Verbrugh H. A., Verhoef J. Suppression of phagocytosis and chemotaxis by cell wall components of Staphylococcus aureus. J Immunol. 1981 Jul;127(1):84–88. [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Proctor R. A. Endotoxin in vitro interactions with human neutrophils: depression of chemiluminescence, oxygen consumption, superoxide production, and killing. Infect Immun. 1979 Sep;25(3):912–921. doi: 10.1128/iai.25.3.912-921.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., Mills E. L., Holmes B. Molecular events during phagocytosis by human neutrophils. Prog Hematol. 1977;10:193–210. [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Peters R., Peterson P. K., Verhoef J. Phagocytosis and killing of staphylococci by human polymorphonuclear and mononuclear leucocytes. J Clin Pathol. 1978 Jun;31(6):539–545. doi: 10.1136/jcp.31.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Van Dijk W. C., Peters R., Van Der Tol M. E., Peterson P. K., Verhoef J. Staphylococcus aureus opsonization mediated via the classical and alternative complement pathways. A kinetic study using MgEGTA chelated serum and human sera deficient in IgG and complement factors C1s and C2. Immunology. 1979 Mar;36(3):391–397. [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Human polymorphonuclear leucocyte receptors for staphylococcal opsonins. Immunology. 1977 Aug;33(2):231–239. [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Weening R. S., Roos D., Loos J. A. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974 Apr;83(4):570–577. [PubMed] [Google Scholar]
- Westphal O. Bacterial endotoxins. The second Carl Prausnitz Memorial Lecture. Int Arch Allergy Appl Immunol. 1975;49(1-2):1–43. [PubMed] [Google Scholar]



