Abstract
A completed pregnancy at a young age reduces a woman’s lifetime risk of breast cancer by up to 50%. A similar protective effect of an early pregnancy has been observed in rodent models using chemical carcinogens. However, the mechanisms responsible for this protective effect remain unclear. Stem cells have been proposed to be the cells of origin for breast cancer. We hypothesized that an early pregnancy reduces adult levels of either mammary stem cells or mammary multipotent progenitor cells. Unsorted mammary cells from adult mice that had undergone an early parity had the same mammosphere formation efficiency as cells from age-matched virgin mice. However, when we transplanted adult mammary cells in limiting dilutions into cleared fat pads of syngeneic mice, we found a significant reduction in the outgrowth potential of the cells from early parous mice as compared with age-matched virgin mice. The extent of fat pad filling in successful outgrowths did not change, suggesting that while mammary stem cells in parous mice retained their functional competence, the number of mammary stem cells was reduced. Our results provide the first direct evidence that an early pregnancy has an effect on mammary stem cells.
Keywords: Epithelial cells, Mammary glands, animal, Pregnancy, Stem cells, Stem cell transplantation
Introduction
Women completing their first pregnancy before age 20 have about half the risk of breast cancer compared to nulliparous women 1–6. This protective effect has also been observed in rodent models of carcinogenesis 7–9. The mechanisms underlying the protective effect of an early parity remain unclear, though several explanations have been proposed10–23.
Stem cells exist in the mammary gland 24–34, and have been proposed to be the cellular origin of cancer 33, 35–42. Although it has been speculated that changes in mammary stem cells following an early pregnancy could be responsible for reducing breast cancer risk 8, 43, 44, the effect of an early pregnancy on mammary stem cells has not been rigorously studied.
Methods
Mice and cells
Female FVB mice were allocated into age-matched pairs, with ages differing by no more than one week. One mouse of each pair was mated at five weeks of age and allowed to complete one pregnancy. Pups were weaned at 21 days, and parous mice were maintained a minimum of eight weeks after weaning to allow complete involution before being used for assays. Single cell suspensions were prepared as described 33.
Mammosphere formation & mammary transplantation
Mammosphere assays were performed essentially as reported 45. Single cell suspensions from each parous donor mouse, serially diluted in DMEM/F12 + 5% FBS, were injected immediately after preparation into the #4 cleared fat pads of recipient virgin mice (age: 3–15 weeks; cleared at 3 weeks) as reported 46. The contralateral fat pad received an equal number of mammary cells from an age-matched virgin donor. Eight weeks later, transplanted glands were stained in carmine alum.
Results
Pregnancy causes mammary gland alterations, including an expansion of the ductal tree and the development of alveoli, which regress during involution. We first ascertained that an early pregnancy did not alter the total number of cells, or the luminal epithelial or myoepithelial cell content in the adult mammary gland (5–15 months of age) (Figure 1A & 1B).
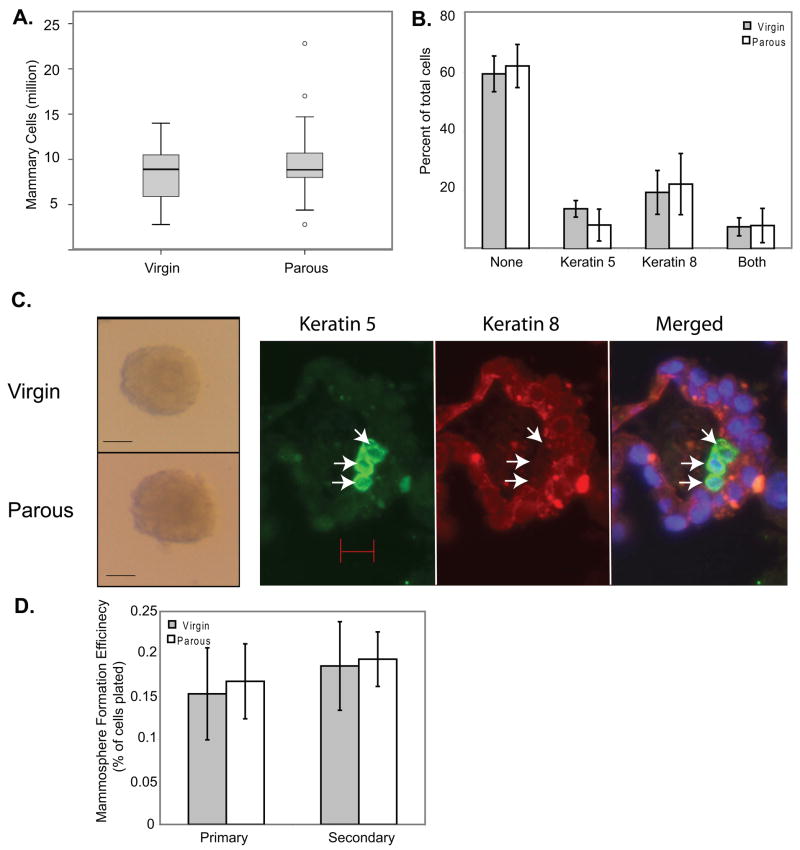
Figure 1. An early pregnancy does not induce a persistent change in total mammary cells or epithelial content, and does not cause a persistent change in mammosphere forming cells.
(A) Single cell suspensions were made from the pooled #2–4 mammary glands of each mouse, and the total number of viable cells recovered from each mouse was determined by FACS and hemocytometry. n=19 (virgin) or 20 (parous). (B) Single cell suspensions made from parous and age-matched virgin mouse mammary glands were spotted on slides and probed by immunofluorescence for luminal (keratin 8) and myoepithelial (keratin 5) markers. n=3. (C) Mammary cells from early parous or age-matched virgin mice were plated at 10,000 cells/well under ultra-low adherent culture conditions. Sample mammospheres that formed after 10 days in culture are shown. Scale bar = 50 μm. Primary mammospheres were fixed, sectioned, and stained for myoepithelial (keratin 5, left, arrows) and luminal (keratin 8, center) cell markers. All mammospheres contained keratin 8+ cells; approximately one in three also contained keratin 5+ cells. Scale bar = 10 μm. (D) Primary mammospheres larger than 50 μm were quantitated at 10 days, dissociated to single cells (by digestion in 0.05% trypsin EDTA followed by pipetting), and replated at 5000 cells/well to measure secondary mammosphere formation. The number of mammospheres formed as a percentage of cells plated is shown. n=9 (primary mammospheres) or 5 (secondary mammospheres).
In non-adherent cultures, a small subset of mammary cells form mammospheres containing both epithelial and basal cell types, implying a multipotent progenitor origin 45. Cells from both parous and age-matched virgin adult mice (8–15 months of age) formed mammospheres that were similar in appearance and cell composition (Figure 1C) to each other and to mammospheres derived from human mammary cells 45. Cells from parous mice had the same capacity to form both primary and secondary mammospheres as cells from virgin mice (Figure 1D). Therefore, an early pregnancy does not induce lasting changes in the multipotent progenitor cell population detectable by the mammosphere assay.
The classical assay for mammary stem cells is serial dilution transplantation into epithelium-cleared fat pads to evaluate ductal tree regeneration potential. We used this assay to compare mammary cells isolated from parous and age-matched virgin mice. Donors ranged in age from 5 months (n=3 for both virgin and parous mice) to 10–15 months of age (n=8). The overall take rate from virgin donors (Figure 2A) is in general accordance with other studies using similar methods of cell preparation 33, 46. The take rate from parous donors was significantly less than that from virgin mice (Figure 2A & 2B, p=0.017; main effect of parity across all dilutions). Even when only transplants from older (10–15 month) donor mice are considered, a significant parity-induced decrease in take rate was observed (p=0.03). Therefore, we conclude that an early age pregnancy has an adverse effect on the mammary stem cell population.
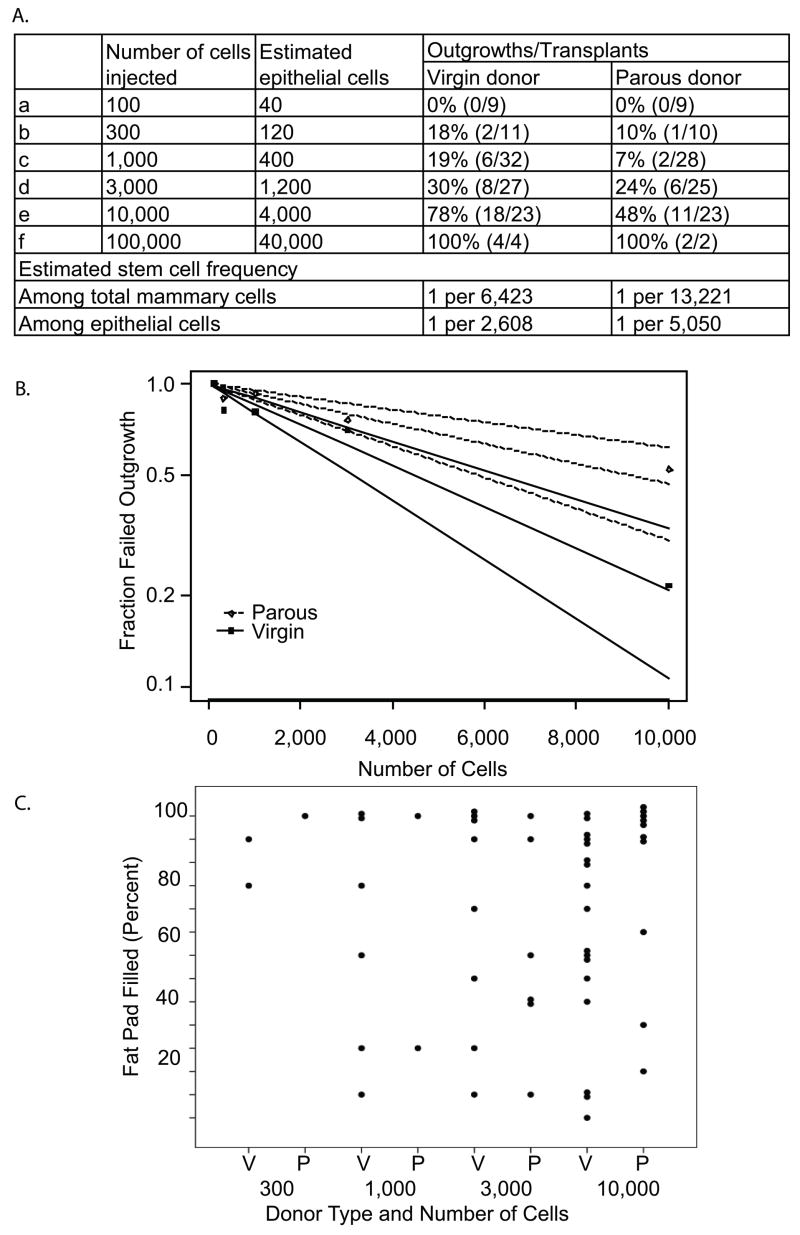
Figure 2. An early pregnancy reduces the number of mammary stem cells.
(A) The indicated number of mammary cells isolated from parous (n=11) or age-matched virgin mice (n=11) was injected into the cleared #4 fat pads of recipient mice. The number of successful outgrowths (>5% fat pad-filling) after 8 weeks and total number of transplants performed are shown. These data were analyzed by using Generalized Estimating Equations in a Generalized Linear Model with a logit link function (PROC GENMOD, SAS V9.1, Cary, NC)53. This ANOVA-like analysis, which tests for overall effects of parity while accounting for dilution and the paired nature of the outgrowth data, found that an early parity significantly reduced the take rate compared to virgin (p=0.017). Limiting dilution analysis was conducted to estimate the frequency of mammary stem cells per total mammary cells by fitting the single-hit Poisson model (SHPM) to the limiting-dilution data from (A) using a complementary log-log generalized linear model54. The fit of the model was checked using the method proposed by Bonnefoix et al 55. (B) Regression lines of the estimated outgrowth frequency and 95% confidence intervals are graphed. The Wald confidence intervals were calculated via delta method for the frequency of regenerative stem cells. Limiting-dilution statistical analyses were performed using the limdil function in the Statmod package 56 in the software R 57. (C) The extent of the cleared fat pad filled by outgrowths in (A) is shown.
The extent of the fat pad filled by parous donor cells was comparable to that of virgin donor cells, even at higher dilution points (Figure 2C), suggesting that our observed decrease in take rate is due to a parity-induced loss of stem cells. By a single-hit Poisson model, the frequency of regenerative stem cells in virgin mice was 1 per 6,423 cells with a 95% confidence interval of [4,493, 9,187], similar to the estimation reported by other groups using comparable transplantation methods 33, 46, 47. In parous mice, the frequency of regenerative stem cells was 1 per 13,221 cells [8,442, 20,708] (Figure 2A). The confidence intervals overlap only slightly, and by a Wald test the frequency difference was significant (p=0.01). Therefore, we conclude that an early age pregnancy decreases the mammary stem cell population by ~50% (Figure 2A).
Discussion
We report here a long-term decrease in the number of mammary repopulating units in the mammary glands following an early pregnancy. This decrease is most likely due to a reduction in the number of mammary epithelial stem cells. Since mammary stem cells are likely a major cellular target of breast cancer, our observation of reduced mammary stem cells may help explain why early pregnancy reduces the risk of breast cancer.
The cancer-protective effect of an early pregnancy persists throughout a woman’s lifetime. However, there is a heightened risk of breast cancer in the years immediately following pregnancy 6, 48–50. For this reason, we allowed parous mice a minimum of eight weeks between the onset of involution and analysis, so that transitory changes in this potential window of increased risk would not complicate our analysis and that our test would reveal persistent changes in mammary stem cells. Of note, most other reports on parity-induced changes in the mammary gland examined the gland only a few weeks after the initiation of involution 14, 15, 51.
Stromal, immune, and systemic changes have also been reported in animals following pregnancy 15, 17; these changes may have an impact on mammary stem cells and their susceptibility to tumorigenesis 9, 17, 52. Our study does not address the impact of hormonal and immune changes on mammary stem cells. We do not know if parity-induced changes in stromal cells incidentally transplanted with the mammary epithelial cells might have contributed to our observed decrease in the take rate.
Mammary stem cells have been reported to be enriched in the subset of mammary cells that are lin−/CD24+/CD49fhi, lin−/CD24+/CD29hi, or CD24low 30, 33, 34. However, the poor extent of stem cell enrichment (up to 1 stem cell in 64 lin−/CD24+/CD29hi cells 33) makes it unlikely that changes in the number of stem cells can be detected by examining these stem cell-enriched populations as a whole. Indeed, we did not observe a significant difference between parous and virgin mice in the lin−/CD24+/CD29hi population, nor in the lin−/CD24+/CD29low progenitor population (Supplementary Figure 1). This finding is in agreement with a recent report of no difference between virgin and primiparous mice in the percentage of CD24+/CD49f+ cells, although the age of pregnancy was not described 51. Flow cytometry using more specific stem cell markers may unmask differences in stem cell numbers between these two groups of mice.
In conclusion, we present strong evidence that an early pregnancy reduces the number or function of mammary stem cells. Further progress in understanding an early pregnancy’s protection from breast cancer requires determining the cellular targets of oncogenic transformation. It is crucial to test whether virgin mice with depleted numbers of mammary stem cells have reduced tumorigenesis upon carcinogen exposure. If so, targeting mammary stem cells becomes a new approach towards clinical interventions to replicate in nulliparous women the protective effect of an early childbirth.
Supplementary Material
Mammary cells from adult mice (six months of age) were analyzed by flow cytometry to identify the lineage−/CD24+/CD29hi (stem cell-enriched) population and the lineage−/CD24+/CD29lo (progenitor enriched) population. Representative plots are shown (A). The percentage of cells in the stem cell-enriched (B) and progenitor cell-enriched (C) populations are plotted. N=11 (parous), 16 (virgin) mice.
Acknowledgments
We thank Dr. Daniel Medina for invaluable discussions of the research design and data interpretation and critical reading of the manuscript, Dr. Jeffrey Rosen, Dr. Adrian Lee, and Ricardo Moraes for discussions and assistance with data analysis, Dr. Gary Chamness for helpful comments on the manuscript, Francis Kittrell for demonstrating the mammary transplantation technique, Cassandra Horne and Drs. Fariba Behbod and Mei Zhang for assistance and suggestions with flow cytometry, and Dr. Heather LaMarca for instruction on mammosphere formation. This study was supported in part by National Institutes of Health R01 CA113869 (to YL) and National Cancer Institute Grants P50 CA058183 (to YL; principal investigator: Dr. C. Kent Osborne, Baylor College of Medicine) and P30 CA125123 (to HL; principal investigator: Dr. C. Kent Osborne, Baylor College of Medicine). SKS was supported by a training grant from the National Institutes of Health, T32 CA 090221.
Footnotes
The authors declare no conflicts of interest
Author contribution: Stefan Siwko: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Jie Dong: collection and/or assembly of data; Michael Lewis: data analysis and interpretation; Hao Liu: data analysis and interpretation; Susan Hilsenbeck: data analysis and interpretation; Yi Li: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support.
References
- 1.MacMahon B, Cole P, Lin TM, et al. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43:209–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson BE, Powell D, Rosario I, et al. An epidemiologic study of breast cancer. J Natl Cancer Inst. 1974;53:609–614. doi: 10.1093/jnci/53.3.609. [DOI] [PubMed] [Google Scholar]
- 3.Trichopoulos D, Hsieh CC, MacMahon B, et al. Age at any birth and breast cancer risk. Int J Cancer. 1983;31:701–704. doi: 10.1002/ijc.2910310604. [DOI] [PubMed] [Google Scholar]
- 4.White E. Projected changes in breast cancer incidence due to the trend toward delayed childbearing. Am J Public Health. 1987;77:495–497. doi: 10.2105/ajph.77.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols HB, Trentham-Dietz A, Love RR, et al. Differences in breast cancer risk factors by tumor marker subtypes among premenopausal Vietnamese and Chinese women. Cancer Epidemiol Biomarkers Prev. 2005;14:41–47. [PubMed] [Google Scholar]
- 6.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 7.Sivaraman L, Medina D. Hormone-induced protection against breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:77–92. doi: 10.1023/a:1015774524076. [DOI] [PubMed] [Google Scholar]
- 8.Britt K, Ashworth A, Smalley M. Pregnancy and the risk of breast cancer. Endocr Relat Cancer. 2007;14:907–933. doi: 10.1677/ERC-07-0137. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar L, Kittrell FS, Guzman RC, et al. Hormone-induced protection of mammary tumorigenesis in genetically engineered mouse models. Breast Cancer Res. 2007;9:R12. doi: 10.1186/bcr1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2:5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 11.Russo J, Russo IH. Susceptibility of the mammary gland to carcinogenesis. II. Pregnancy interruption as a risk factor in tumor incidence. Am J Pathol. 1980;100:497–512. [PMC free article] [PubMed] [Google Scholar]
- 12.Sivaraman L, Conneely OM, Medina D, et al. p53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12379–12384. doi: 10.1073/pnas.221459098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginger MR, Shore AN, Contreras A, et al. A noncoding RNA is a potential marker of cell fate during mammary gland development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5781–5786. doi: 10.1073/pnas.0600745103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginger MR, Gonzalez-Rimbau MF, Gay JP, et al. Persistent changes in gene expression induced by estrogen and progesterone in the rat mammary gland. Mol Endocrinol. 2001;15:1993–2009. doi: 10.1210/mend.15.11.0724. [DOI] [PubMed] [Google Scholar]
- 15.D’Cruz CM, Moody SE, Master SR, et al. Persistent parity-induced changes in growth factors, TGF-beta3, and differentiation in the rodent mammary gland. Mol Endocrinol. 2002;16:2034–2051. doi: 10.1210/me.2002-0073. [DOI] [PubMed] [Google Scholar]
- 16.Blakely CM, Stoddard AJ, Belka GK, et al. Hormone-induced protection against mammary tumorigenesis is conserved in multiple rat strains and identifies a core gene expression signature induced by pregnancy. Cancer Res. 2006;66:6421–6431. doi: 10.1158/0008-5472.CAN-05-4235. [DOI] [PubMed] [Google Scholar]
- 17.Thordarson G, Jin E, Guzman RC, et al. Refractoriness to mammary tumorigenesis in parous rats: is it caused by persistent changes in the hormonal environment or permanent biochemical alterations in the mammary epithelia? Carcinogenesis. 1995;16:2847–2853. doi: 10.1093/carcin/16.11.2847. [DOI] [PubMed] [Google Scholar]
- 18.Kwa HG, Cleton F, Bulbrook RD, et al. Plasma prolactin levels and breast cancer: relation to parity, weight and height, and age at first birth. Int J Cancer. 1981;28:31–34. doi: 10.1002/ijc.2910280106. [DOI] [PubMed] [Google Scholar]
- 19.Musey VC, Collins DC, Musey PI, et al. Long-term effect of a first pregnancy on the secretion of prolactin. The New England journal of medicine. 1987;316:229–234. doi: 10.1056/NEJM198701293160501. [DOI] [PubMed] [Google Scholar]
- 20.Eliassen AH, Tworoger SS, Hankinson SE. Reproductive factors and family history of breast cancer in relation to plasma prolactin levels in premenopausal and postmenopausal women. Int J Cancer. 2007;120:1536–1541. doi: 10.1002/ijc.22482. [DOI] [PubMed] [Google Scholar]
- 21.Balogh GA, Heulings R, Mailo DA, et al. Genomic signature induced by pregnancy in the human breast. International Journal of Oncology. 2006;28:399–410. [PubMed] [Google Scholar]
- 22.Medina D. Breast cancer: the protective effect of pregnancy. Clin Cancer Res. 2004;10:380S–384S. doi: 10.1158/1078-0432.ccr-031211. [DOI] [PubMed] [Google Scholar]
- 23.Guzman RC, Yang J, Rajkumar L, et al. Hormonal prevention of breast cancer: mimicking the protective effect of pregnancy. Proc Natl Acad Sci U S A. 1999;96:2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeOme KB, Faulkin LJ, Jr, Bern HA, et al. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- 25.Daniel CW, De Ome KB, Young JT, et al. The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc Natl Acad Sci U S A. 1968;61:53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel CW, Deome KB. Growth of Mouse Mammary Glands in Vivo after Monolayer Culture. Science. 1965;149:634–636. doi: 10.1126/science.149.3684.634. [DOI] [PubMed] [Google Scholar]
- 27.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 28.Welm BE, Tepera SB, Venezia T, et al. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 29.Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2004 doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- 30.Sleeman KE, Kendrick H, Ashworth A, et al. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sleeman KE, Kendrick H, Robertson D, et al. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 34.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 35.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004;51:1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Horwich A, Swerdlow AJ. Second primary breast cancer after Hodgkin’s disease. Br J Cancer. 2004;90:294–298. doi: 10.1038/sj.bjc.6601499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Land CE, McGregor DH. Breast cancer incidence among atomic bomb survivors: implications for radiobiologic risk at low doses. J Natl Cancer Inst. 1979;62:17–21. [PubMed] [Google Scholar]
- 38.Fialkow PJ, Jacobson RJ, Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. American Journal of Medicine. 1977;63:125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- 39.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Welm B, Podsypanina K, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu BY, McDermott SP, Khwaja SS, et al. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci U S A. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Tognon CE, Godinho FJ, et al. ETV6-NTRK3 fusion oncogene initiates breast cancer from committed mammary progenitors via activation of AP1 complex. Cancer Cell. 2007;12:542–558. doi: 10.1016/j.ccr.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo J, Moral R, Balogh GA, et al. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005;7:131–142. doi: 10.1186/bcr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trichopoulos D, Lagiou P, Adami HO. Towards an integrated model for breast cancer etiology: the crucial role of the number of mammary tissue-specific stem cells. Breast Cancer Res. 2005;7:13–17. doi: 10.1186/bcr966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39:21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- 47.Welm BE, Dijkgraaf GJ, Bledau AS, et al. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell. 2008;2:90–102. doi: 10.1016/j.stem.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janerich DT, Hoff MB. Evidence for a crossover in breast cancer risk factors. Am J Epidemiol. 1982;116:737–742. doi: 10.1093/oxfordjournals.aje.a113462. [DOI] [PubMed] [Google Scholar]
- 49.Lambe M, Hsieh C, Trichopoulos D, et al. Transient increase in the risk of breast cancer after giving birth. The New England journal of medicine. 1994;331:5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 50.Albrektsen G, Heuch I, Hansen S, et al. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92:167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Abrams TJ, Guzman RC, Swanson SM, et al. Changes in the parous rat mammary gland environment are involved in parity-associated protection against mammary carcinogenesis. Anticancer Res. 1998;18:4115–4121. [PubMed] [Google Scholar]
- 53.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford: Clarendon Press; 1994. [Google Scholar]
- 54.Bonnefoix T, Bonnefoix P, Verdiel P, et al. Fitting limiting dilution experiments with generalized linear models results in a test of the single-hit Poisson assumption. J Immunol Methods. 1996;194:113–119. doi: 10.1016/0022-1759(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 55.Bonnefoix T, Bonnefoix P, Callanan M, et al. Graphical representation of a generalized linear model-based statistical test estimating the fit of the single-hit Poisson model to limiting dilution assays. J Immunol. 2001;167:5725–5730. doi: 10.4049/jimmunol.167.10.5725. [DOI] [PubMed] [Google Scholar]
- 56.Smyth G. Statmod: Statistical Modeling. R package 1.3.1. 2007. [Google Scholar]
- 57.Team RDC. R: A language and environment for statistical computing. 2007
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mammary cells from adult mice (six months of age) were analyzed by flow cytometry to identify the lineage−/CD24+/CD29hi (stem cell-enriched) population and the lineage−/CD24+/CD29lo (progenitor enriched) population. Representative plots are shown (A). The percentage of cells in the stem cell-enriched (B) and progenitor cell-enriched (C) populations are plotted. N=11 (parous), 16 (virgin) mice.