Abstract
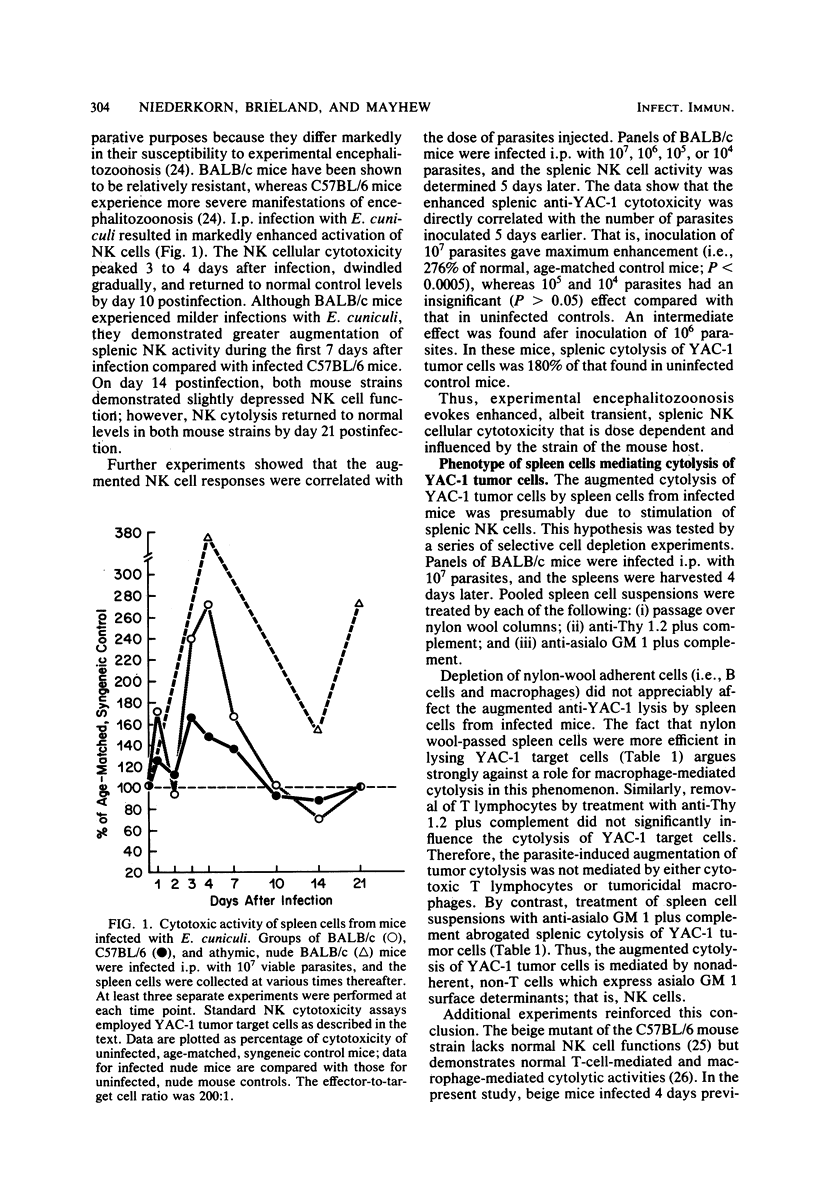
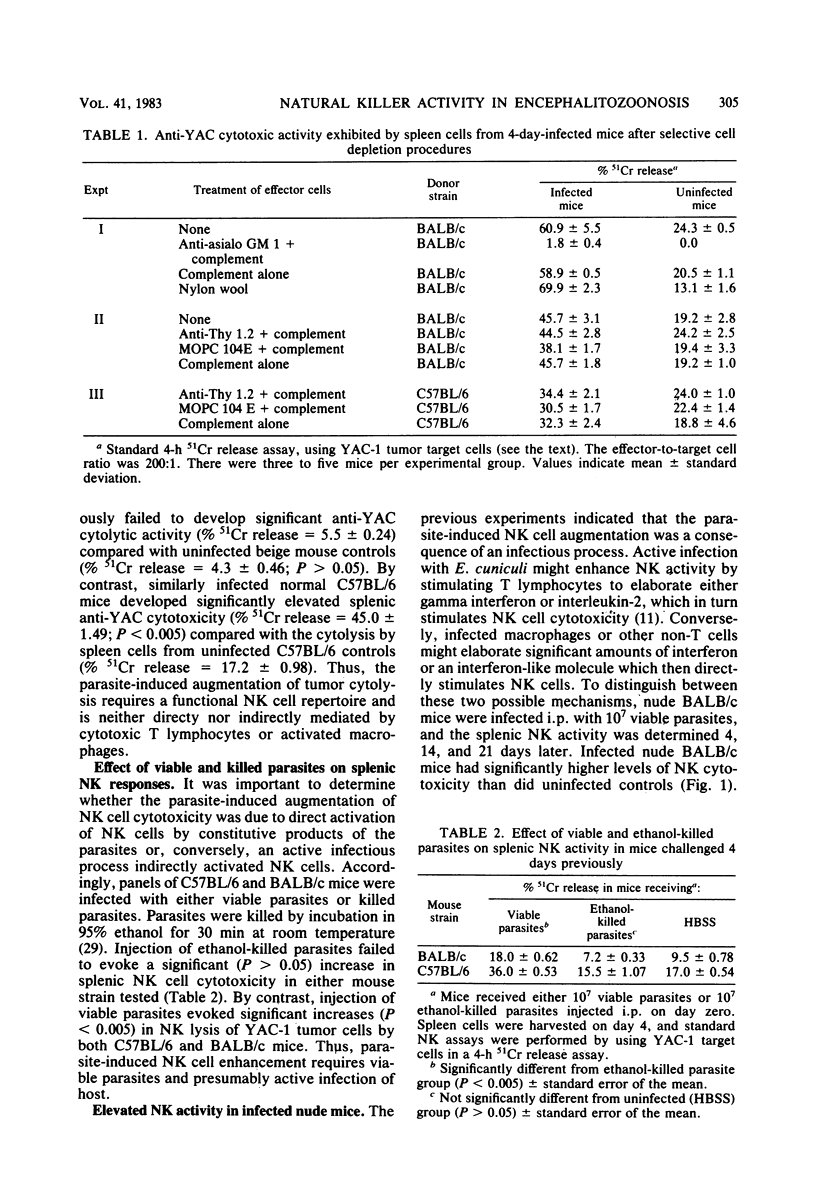
Spleen cells from mice infected with the protozoan parasite Encephalitozoon cuniculi demonstrated enhanced in vitro cytolysis of YAC-1 lymphoma cells. Selective cell depletion experiments showed that the dominant cell population mediating cytolysis of YAC-1 tumor cells expressed the characteristic phenotype of murine natural killer (NK) cells because (i) pretreatment of spleen cells with anti-asialo GM 1 antiserum plus complement abolished the cytotoxic activity; (ii) augmented cytolysis was found in athymic nude mice; (iii) pretreatment of spleen cells with anti-Thy 1.2 plus complement did not affect the level of cytolysis; and (iv) nylon wool removal of adherent cells did not reduce the augmented cytolysis. The augmented cytolysis peaked 7 days after infection, gradually diminished, and finally returned to control levels by 21 days postinfection. The parasite-induced augmentation of NK cell activity was dose-dependent: inoculation of 10(7) parasites gave maximum enhancement, whereas 10(5) or 10(4) parasites had an insignificant effect on spontaneous NK cell cytolysis. The augmented NK cell cytotoxicity was dependent upon viable parasites; inoculation of killed parasites failed to stimulate a significant increase in spontaneous cytolysis. An active infectious process was an important component of this process. The peak of NK activity in euthymic mice was closely correlated with the active stage of infection, and reduction of NK cell activity coincided with recovery from infection. By contrast, athymic nude mice were unable to control E. cuniculi infections yet maintained persistently elevated NK responses. The present data, along with previous reports, indicate that infection with E. cuniculi evokes transient modulation of host immune functions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arison R. N., Cassaro J. A., Pruss M. P. Studies on a murine ascites-producing agent and its effect on tumor development. Cancer Res. 1966 Sep;26(9):1915–1920. [PubMed] [Google Scholar]
- Armstrong J. A., Ke Y. H., Breinig M. C., Ople L. Virus resistance in rabbit kidney cell cultures contaminated by a protozoan resembling Encephalitozoon cuniculi. Proc Soc Exp Biol Med. 1973 Apr;142(4):1205–1208. doi: 10.3181/00379727-142-37209. [DOI] [PubMed] [Google Scholar]
- Bismanis J. E. Detection of latent murine nosematosis and growth of Nosema cuniculi in cell cultures. Can J Microbiol. 1970 Apr;16(4):237–242. doi: 10.1139/m70-044. [DOI] [PubMed] [Google Scholar]
- Cox J. C. Altered immune responsiveness associated with Encephalitozoon cuniculi infection in rabbits. Infect Immun. 1977 Feb;15(2):392–395. doi: 10.1128/iai.15.2.392-395.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt R. E., Jackson S. J. Renal nosematosis in young rabbits. Pathol Vet. 1970;7(6):492–497. doi: 10.1177/030098587000700603. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Glimcher L., Shen F. W., Cantor H. Identification of a cell-surface antigen selectively expressed on the natural killer cell. J Exp Med. 1977 Jan 1;145(1):1–9. doi: 10.1084/jem.145.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O. A., Gidlund M., Kurnick J. T., Wigzell H. In vivo generation of mouse natural killer cells: role of the spleen and thymus. Scand J Immunol. 1978;8(3):207–213. doi: 10.1111/j.1365-3083.1978.tb00512.x. [DOI] [PubMed] [Google Scholar]
- Haller O., Kiessling R., Orn A., Wigzell H. Generation of natural killer cells: an autonomous function of the bone marrow. J Exp Med. 1977 May 1;145(5):1411–1416. doi: 10.1084/jem.145.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E., Cerrone M. C., Burton R. C. Increased natural killer cell activity in experimental American trypanosomiasis. J Immunol. 1981 Sep;127(3):1126–1130. [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Bartram S., Haskill J. S., Nunn M., Holden H. T., West W. H. Fc receptors on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1977 Jul;119(1):322–326. [PubMed] [Google Scholar]
- Herberman R. B. Natural killer cells and their possible relevance to transplantation biology. Transplantation. 1982 Jul;34(1):1–7. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kamiyama T., Hagiwara T. Augmented followed by suppressed levels of natural cell-mediated cytotoxicity in mice infected with Toxoplasma gondii. Infect Immun. 1982 May;36(2):628–636. doi: 10.1128/iai.36.2.628-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. An analysis of the murine NK cell as to structure, function and biological relevance. Immunol Rev. 1979;44:165–208. doi: 10.1111/j.1600-065x.1979.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Koller L. D. Spontaneous Nosema cuniculi infection in laboratory rabbits. J Am Vet Med Assoc. 1969 Oct 1;155(7):1108–1114. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. The role of activated macrophages in specific and nonspecific cytostasis of tumor cells. J Immunol. 1974 Aug;113(2):507–516. [PubMed] [Google Scholar]
- Minato N., Reid L., Bloom B. R. On the heterogeneity of murine natural killer cells. J Exp Med. 1981 Sep 1;154(3):750–762. doi: 10.1084/jem.154.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn J. Y., Shadduck J. A., Schmidt E. C. Susceptibility of selected inbred strains of mice to Encephalitozoon cuniculi. J Infect Dis. 1981 Sep;144(3):249–253. doi: 10.1093/infdis/144.3.249. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979 Nov;123(5):2174–2181. [PubMed] [Google Scholar]
- Roder J. C. The beige mutation in the mouse. I. A stem cell predetermined impairment in natural killer cell function. J Immunol. 1979 Nov;123(5):2168–2173. [PubMed] [Google Scholar]
- Shadduck J. A. Nosema cuiculi: in vitro isolation. Science. 1969 Oct 24;166(3904):516–517. doi: 10.1126/science.166.3904.516. [DOI] [PubMed] [Google Scholar]
- Shadduck J. A., Pakes S. P. Encephalitozoonosis (nosematosis) and toxoplasmosis. Am J Pathol. 1971 Sep;64(3):657–672. [PMC free article] [PubMed] [Google Scholar]
- Shadduck J. A., Polley M. B. Some factors influencing the in vitro infectivity and replication of Encephalitozoon cuniculi. J Protozool. 1978 Nov;25(4):491–496. doi: 10.1111/j.1550-7408.1978.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Young W. W., Jr, Hakomori S. I., Durdik J. M., Henney C. S. Identification of ganglio-N-tetraosylceramide as a new cell surface marker for murine natural killer (NK) cells. J Immunol. 1980 Jan;124(1):199–201. [PubMed] [Google Scholar]


