Abstract
Objective:
Determine whether sleep extension (a) improves alertness and performance during subsequent sleep restriction and (b) impacts the rate at which alertness and performance are restored by post-restriction recovery sleep.
Design:
Participants were randomly assigned to an Extended (10 h time in bed [TIB]) or Habitual TIB [mean (SD) hours = 7.09 (0.7)] sleep group for one week, followed by 1 Baseline (10 hours or habitual TIB), 7 Sleep Restriction (3 h TIB), and 5 Recovery Sleep nights (8 h TIB). Performance and alertness tests were administered hourly between 08:00–18:00 during all in-laboratory phases of the study.
Setting:
Residential sleep/performance testing facility.
Participants:
Twenty-four healthy adults (ages 18–39) participated in the study.
Interventions:
Extended vs. habitual sleep durations prior to sleep restriction.
Results:
Psychomotor vigilance task (PVT) lapses were more frequent and modified maintenance of wakefulness (MWT) sleep latency was shorter in the Habitual group than in the Extended group across the sleep restriction phase. During the Recovery phase, PVT speed rebounded faster (and PVT lapsing recovered significantly after the first night of recovery sleep) in the Extended group. No group differences in subjective sleepiness were evident during any phase of the study.
Conclusion:
The extent to which sleep restriction impairs objectively measured alertness and performance, and the rate at which these impairments are subsequently reversed by recovery sleep, varies as a function of the amount of nightly sleep obtained prior to the sleep restriction period. This suggests that the physiological mechanism(s) underlying chronic sleep debt undergo long-term (days/weeks) accommodative/adaptive changes.
Citation:
Rupp TL; Wesensten NJ; Bliese PD; Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. SLEEP 2009;32(3):311–321.
Keywords: Sleep restriction, partial sleep deprivation, cognitive performance, sleep extension, recovery sleep, discontinuous growth modeling
AMERICAN ADULTS REPORT SLEEPING AN AVERAGE OF 6.8 HOURS ON WEEKNIGHTS1—CONSIDERABLY LESS THAN THE 8 H OF SLEEP THOUGHT TO BE necessary to restore and sustain optimal daytime alertness. However, despite its near-ubiquitousness, chronic sleep restriction has not been scientifically studied to the same extent as acute, total sleep deprivation. In part, this has most likely been due to (a) the relative logistical difficulties associated with studying chronic sleep restriction in a controlled manner, and (b) an implicit, parsimonious assumption that the effects of chronic sleep restriction are qualitatively identical to those of acute total sleep deprivation, differing only in terms of the rate at which the deficits accrue.
In previous studies of chronic sleep restriction (≥ 7 consecutive nights) it has been shown that performance and alertness are degraded in a dose-dependent manner.2,3 Also apparent in both studies were (a) substantial individual differences in performance during (resilience to) sleep restriction, and (b) a failure for some aspects of performance to be restored to baseline levels after 3 nights of recovery sleep (with 8 h time in bed per night). Extrapolating from total sleep deprivation studies,4–6 this amount of recovery sleep may not have been sufficient for full recovery, but still was anticipated to produce greater levels of performance improvement than what was observed. Instead, this finding suggested the intriguing possibility that the neurobiological mechanism(s) underlying performance and alertness vary as a function of (and perhaps adapt to) habitual, nightly sleep duration, and that such changes have a relatively long time constant—requiring multiple (e.g., 7) days of continuously elevated sleep pressure (i.e., a longer duration than would typically be imposed in a formal total sleep deprivation study or be manifested in nature as a result of exposure to stressors). Consistent with the possibility of a slow-to-adapt physiological mechanism that mediates alertness and performance, cross-study comparisons of results from some other of our studies7,8 were also consistent with the possibility that recovery rate following multiple days of sleep restriction varies as a function of prior, habitual sleep duration: during which full recovery occurred in volunteers after 2 nights of 8 h time in bed (TIB) when sleep restriction was preceded by 1 week of self-reported TIB of 10 h/night.
Therefore, the present study was conducted to systematically determine the effects of prior sleep history on rates of performance and alertness degradation during chronic (7 nights) sleep restriction and during the subsequent recovery period. Specifically, it was predicted that “banking extra sleep” by extending nightly TIB would confer protective benefits during subsequent sleep restriction and facilitate recovery from that sleep restriction.
METHODS
This study was approved by the Walter Reed Army Institute of Research Human Use Review Committee and the United States Army Medical Research and Materiel Command Human Subjects Research Review Board and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Participants
Civilian and active duty military men and women 18 to 39 years of age were recruited via flyers posted at local colleges, universities, and military installations. After providing informed consent, participants completed questionnaires to determine eligibility based on physical state, psychological state, sleep habits, and chronotype. Participants also underwent a physical examination including a 12-lead electrocardiogram (ECG) and evaluation of blood and urine samples to determine general health, including pregnancy and drug use. In order to reduce intersubject variability in nighttime sleep, participants were excluded if they reported any of the following for the preceding month: (1) habitual daytime napping (> 1 nap per week), (2) an average of > 7 h sleep per night (the national average) Sunday through Thursday, (3) average nighttime lights-out times earlier than 21:00 Sunday through Thursday, (4) average morning wake-up times later than 09:00 Monday through Friday, or (5) time zone travel across > 3 time zones within the last 3 months. Additional study exclusionary criteria included: cardiovascular disease; hypertension or high blood pressure; resting pulse > 95 bpm; past or present neurologic, psychiatric, or sleep disorder; present or past use of over-the-counter substances with purported psychoactive properties (e.g., Ginko, St. John's Wort); asthma or other reactive airways diseases; prior history of cancer; allergies; regular nicotine use (or addiction) within the last 3 years; current heavy alcohol use; current use of other illicit drugs (to include but not limited to benzodiazepines, amphetamines, cocaine, and marijuana); known liver disease or liver abnormalities as determined by a laboratory test; self-reported history of caffeine use > 400 mg (8 caffeinated sodas or 3–4 cups of coffee) per day on average; score ≥ 41 on either side of the State Trait Anxiety Inventory,9 score ≥ 13 on the Beck Depression Inventory,10,11 score < 31 or > 69 on the Horne Ostberg Morningness-Eveningness Questionnaire12 (indicative of extreme morning or evening preference); and pregnancy.
After eligibility to participate was ascertained, volunteers were randomly assigned to either the “Extended” or “Habitual” sleep group (described below) (n = 12 per group). Seven males and 5 females (mean age [SD] = 24.0 [6.1]) were assigned to the Extended group; 4 males and 8 females (mean age = 26.0 [7.1]) were assigned to the Habitual group. Mean (SD) values for other screening criteria by Group are summarized in Table 1. Power estimates calculated using Psychomotor Vigilance Task mean speed at baseline and sleep restriction Day 7 from a prior, similar sleep restriction study2 revealed that a sample size of 12 subjects per group would be sufficient to yield power of 0.90.
Table 1.
Group Demographics/ Screening Information (Mean [SD])
| Group | Weekday/Weekend Bedtime | Weekday/Weekend Rise time | Weekday TIB (min) | Weekend TIB (min) | MEQ | STAI state/trait | Beck |
|---|---|---|---|---|---|---|---|
| Extended | 23:25 (1:16)/24:32 (1:38) | 6:31 (1:42)/8:32 (2:03) | 421 (72) | 480 (66) | 50 (9) | 26 (5)/28 (5) | 1.3 (2) |
| Habitual | 24:13 (1:30)/01:37 (1:24) | 6:13 (1:09)/8:42 (2:00) | 360 (80) | 426 (110) | 54 (8) | 22 (3)/25 (3) | 1.2 (2) |
Abbreviations: TIB = time in bed; MEQ = Morningness/Eveningness Questionnaire; STAI = State Trait Anxiety Inventory; Beck = Depression Inventory
Testing Facilities
During testing and sleep periods, each subject was housed individually in a sound attenuated 8′ × 10′ room that included a bed and computer workstation. Ambient temperature was approximately 23°C, and lighting was approximately 500 lux (with lights off during sleep periods). Background white noise was 65 dB at all times. When not engaged in testing or sleep, participants remained in a common living area to play games, eat, read, or watch television and movies. Participants were monitored continuously by at least one laboratory technician. All volunteers were instructed by the principal investigator at the beginning of the study that discussion of how they were feeling (i.e., “sleepy”) or of testing or study procedures was not allowed and would be grounds for dismissal from the study.
Procedure
The study design consisted of 3 consecutive within-subjects' phases: (1) At home, (2) In-laboratory overnights, and (3) Full-time in-laboratory. Smoking was prohibited during all study phases, and caffeine use was prohibited beginning 48 h prior to beginning the Full-time in-laboratory phase (confirmed with daily log and urinalysis). Table 2 outlines the phases, described in more detail below.
14-Day At-Home Phase. For 14 days prior to the in-laboratory phases, participants wore a wrist actigraph continuously, recorded their daily sleep times, and called into a time-stamped answering machine before and after nocturnal sleep periods. They were instructed to maintain their habitual sleep/wake schedule (usual nightly TIB); otherwise, volunteers were allowed to maintain their usual lifestyles.
7-Day In-Laboratory Overnight phase (O1–O7). Immediately following the first phase, participants were randomly assigned to either a sleep “Extension” group (nightly TIB = 10 h) or a “Habitual” sleep group (usual nightly TIB). “Habitual” sleep schedule was determined from actigraphy, sleep logs, and telephone call-ins the prior 2 weeks (specific details of schedule determination provided under “Measures”). Day-of week entry into this in-lab segment was standardized to Sunday evening. During this phase, participants slept in the laboratory each night; both groups maintained a fixed wake time of 07:00 for this and all subsequent phases. Volunteers maintained a wake time of 07:00 during the overnight phase so that they would be accustomed to a wake time of 07:00 during in-laboratory testing. Actigraphy was used to provide an estimation of sleep parameters and ensure adherence to the TIB requirements of each group (PSG was not used during this phase to minimize the time and burden on volunteers during this phase). Participants left the laboratory during the day and maintained their usual daytime activities.
- Full-Time In-Laboratory Phase. Following the seventh night of Extension or Habitual sleep described above, participants returned to the laboratory for the in-laboratory phase consisting of baseline, sleep restriction, and recovery sub-phases (day-of week entry into this in-lab segment was standardized to Sunday). Upon arrival at 16:00, they were briefed on study procedures, vital signs were taken, and a urine sample collected for drug analyses in all participants and pregnancy screening in women. Polysomnographic (PSG) recording electrodes (electrooculogram [EOG], electromyogram [EMG], O1, O2, C3, and C4 electroencephalogram [EEG] sites) were applied, and participants continued to wear wrist actigraphs. Participants also were given instructions and practice on performance and alertness tasks described below. Upon awakening at 07:00, vital signs were measured (and monitored during waking), and participants were allowed to eat a meal. Beginning at 08:00, tests were administered every hour through 18:00.
- Baseline day (B). Timing of lights out was based on participants' average TIB the previous week with baseline testing the following day.
- 7-day Sleep Restriction phase (SR1-SR7). Following the baseline testing day, participants began the 7-day Sleep Restriction phase, in which nightly TIB was 04:00–07:00 followed by daytime testing from 08:00 through 18:00.
- 5-day Recovery phase (R1-R5). Following the last Sleep Restriction testing day, participants began the 5-day Recovery phase, in which nightly TIB was 23:00–07:00 followed by daytime testing from 08:00 through 18:00 (TIB was set at 8 h to maintain consistency with 2 prior studies2,7,8 to which this is a follow-up).
Table 2.
Study Design
| PHASE | STUDY DAY | DURATION (days) | TIME IN BED (h) | MEASURES |
|---|---|---|---|---|
| At-home | — | 14 | “Usual” | actigraphy, sleep diary, call-in |
| In-Laboratory | ||||
| OVERNIGHTS | O1-O7 | 7 | Extended (10) or Habitual | actigraphy, sleep diary |
| Full-time In-Laboratory, | ||||
| BASELINE | B | 1 | Extended (10) or Habitual | actigraphy, PSG, PVT, MWT, SSS |
| Full-time In-Laboratory, | ||||
| RESTRICTION | SR1-SR7 | 7 | 3 | actigraphy, PSG, PVT, MWT, SSS |
| Full-time In-Laboratory, | ||||
| RECOVERY | R1-R5 | 5 | 8 | actigraphy, PSG, PVT, MWT, SSS |
Abbreviations: TIB = time in bed; PSG = polysomnogram; PVT = Psychomotor Vigilance Task; MWT = maintenance of wakefulness; SSS = Stanford Sleepiness Scale
At the end of the fifth recovery day, vital signs were measured, all recording equipment removed, and a medical examination was performed. Participants were then debriefed and released from the study.
MEASURES
Actigraphy
Wrist movements were recorded using the Mini Motionlogger BMA-32 (Ambulatory Monitoring, Inc., Ardsley, NY). Data were scored for estimated total sleep time ([TST] minutes of sleep within the identified sleep period [elapsed time from the start of sleep to sleep end time]) using a validated scoring algorithm13 and actigraphic scoring used methods described previously,14 based on correspondence between participants' sleep diary, answering machine call-in times, and actigraphic record.
To determine Habitual Sleep schedules for volunteers assigned to the Habitual Group, actigraphic estimated total sleep time on weeknights (Sunday through Thursday nights; nights excluded if followed by a holiday or weekend) was determined, and 15 min were added to the average (to avoid inadvertently sleep restricting volunteers) and rounded up to the nearest 5 min for the final TIB amount. Bedtime was determined by subtracting TIB from the 07:00 rise time (i.e., a subject averaging 7 hours 15 min of sleep would have a bedtime of 23:45).
Polysomnography
Polysomnographic measurements included electroencephalogram (EEG [C3 and C4]), electrooculogram (EOG [outer canthus of each eye]), and electromyogram (EMG [mental/submental]). Contralateral mastoid leads served as references for all unipolar measurements (EEG and EOG). PSG data were scored by a trained research technician in accordance with Rechtschaffen and Kales criteria,15 using Alice 4 Sleepware software (Respironics, Inc., Murraysville, PA). Dependent measures for nighttime sleep periods (defined as lights out to lights on) included minutes of individual stages (1, 2, slow wave sleep [SWS; stages 3 and 4 combined] and REM) and TST (sum of minutes spent in all sleep stages). The dependent measure for the modified maintenance of wakefulness test (described next) was latency (in minutes) to the first 30-sec epoch of sleep.
Modified Maintenance of Wakefulness Test (MWT)
For the modified MWT, participants were escorted to their individual darkened, sound-attenuated bedrooms and allowed to lie down on their beds. They were instructed to close their eyes and to try to remain awake. PSG was monitored online. Participants were awakened at the onset of stage 2 sleep. If participants did not fall asleep after 20 min, the test was terminated.
Psychomotor Vigilance Task (PVT)
A 5-min version of the Psychomotor Vigilance Task (PVT)16 was administered on a personal digital assistant (PDA). PVT was analyzed for speed (1/reaction time*1000), and number of lapses (reaction times > 500 msec).
Stanford Sleepiness Scale (SSS)
Participants using the SSS17 selected which of 7 statements best described their current state of alertness ranging from “1–feeling active and vital; alert; wide awake” to “7–almost in reverie; sleep onset soon; losing struggle to remain awake.” The dependent variable was self-rated sleepiness score.
Analyses
Nighttime Sleep
Nighttime sleep data (actigraphy and PSG) were analyzed using a mixed-model analysis of variance (ANOVA) in SPSS Version 12.0 for PC (SPSS Inc., Chicago, IL). For nighttime actigraphically estimated sleep data, the model included fixed effects for Group (Extended v. Habitual) and Day (14 levels during the “at-home” sleep schedule assessment phase; 7 levels during the in-laboratory, overnight phase: O1-O7). For nighttime PSG sleep data, the model included fixed effects for Group and Day (13 levels across the full-time, in-laboratory sub-phases: B, SR1-SR7, R1-R5). Significant interactions were followed by post-hoc t-tests (Bonferroni correction). Greenhouse-Geisser corrections were applied to repeated measures effects. Statistical significance was P < 0.05.
Performance and Sleepiness
Responses at each time of day for PVT, modified MWT, and SSS variables were collapsed to obtain daily mean values. Time-of-day effects will be examined separately and presented elsewhere.
For PVT, modified MWT, and SSS variables, discontinuous growth modeling (DGM)18 was used to examine patterns of responses across days of sleep restriction and recovery. DGM provides the ability to describe change in terms of 3 distinct parameters—a sleep restriction slope (RESTRICT), a recovery transition parameter (TRANS), and a recovery slope (RECOV). Analyses were conducted using the open-source platform R19 and the nonlinear and linear mixed effect model (NLME) package for R.20 The growth modeling strategy used in the analyses is similar to that described previously,21–23 and consists of several steps. During the first steps, the nature of the intraindividual growth trajectories were identified for each variable, and the extent to which the growth trajectories contain reliable individual differences was determined. Identification of individual factors (i.e., group and age) that explain individual differences in the intraindividual growth trajectories were identified in subsequent steps.
In the DGM, effects of experimental day were captured using 3 level 1 predictors. The first predictor (RESTRICT) was a vector of sequential whole numbers ranging from zero (baseline) to 12 (final recovery day) used to model the slope of the change starting at baseline and extending through the sleep restriction phase. The second predictor (TRANS) was a dummy coded variable vector containing a value of zero for data collected during the baseline day and the sleep restriction phase, and a value of 1 for measures collected during the recovery phase. TRANS was used to represent the abrupt discontinuity between the baseline/sleep restriction phase and the recovery phase. The third predictor (RECOV) was a vector containing zeros for measures collected during the baseline and sleep restriction phase and sequential numbers from zero to 4 for measures collected across the 5 recovery days. RECOV captured the degree to which the recovery slope differed from the slope originating at baseline and extending through the sleep restriction phase. Age and group (Extended vs Habitual) were included as level 2 predictors of individual differences in the level 1 predictors. The following steps were performed in the development of the final model.
Step 1
A null random coefficient model (RCM) was estimated for each variable as the basis for calculating the intraclass correlation coefficient (ICC). The ICC represents the degree to which reliable individual differences exist among participants (details on estimating ICC can be found in Bliese24). In experimental settings, it is also common to estimate an ICC reflecting the experimental design; thus the ICC was also estimated conditional on the design elements (RESTRICT, TRANS, and RECOV).
Step 2
The second step of the model regressed the outcome on the 3 predictors (RESTRICT, TRANS, RECOV) to capture the discontinuous nature of the experimental design. In this step, an examination of the within-individual error structure was conducted.21 These analyses suggested significant lag 1 serial autocorrelation in the repeated measures for lapses (−2 log-likelihood ratio = 21.75, P < 0.0001), speed (−2 log-likelihood ratio = 15.08, P = 0.0004), sleep onset latency (−2 log-likelihood ratio = 4.03, P = 0.045), and SSS score (−2 log-likelihood ratio = 6.28, P = 0.01); consequently a lag 1 within-individual error structure term was included for all models.
Step 3
Step 3 tested for interindividual variability in the sleep restriction slope, the transition, and the recovery slope (RESTRICT, TRANS, RECOV, respectively) by contrasting 4 models. The first model restricted all 3 predictors to be equal across respondents; the second allowed individual slopes to vary for the restriction phase slope (RESTRICT); the third allowed for variability in both the restriction (RESTRICT) and the transition (TRANS) parameter, and the fourth allowed for individual variability in all 3 parameters. In all 4 models, age was included as an individual-level predictor of the intercept and slope parameters, so the reported tests reveal the extent to which residual variability is evident after the effects of age are controlled. This was done because Bliese and colleagues have previously identified age as an individual difference that affects response to sleep restriction.22 Table 3 provides degrees of freedom, model fit indices, log-likelihood ratios, and P-values for the model contrasts for (a) lapses, (b) speed, (c) sleep onset latency, and (d) SSS. For all variables, the best fitting model allowed for individual variability in the transition slope (TRANS) even after the effects of age were controlled. For speed, the fourth model failed to converge, suggesting near zero residual variances. Although residual individual differences in the restriction and recovery slopes were not significant for all variables based on the log-likelihood test, this test tends to be conservative,20 therefore the potential role of group (Extended versus Habitual) on variability in all phases' slopes was examined.
Table 3.
Tests for Slope Variability in Design Effect Model for a) PVT Lapses, b) PVT Speed, c) MWT Sleep Onset Latency, and d) SSS Score
| Model | Random parameter | d.f. | AIC | log Lik | Test | L. ratio | P-value |
|---|---|---|---|---|---|---|---|
| a) lapses | |||||||
| 1 | Intercept | 12 | 1390.80 | −683.40 | |||
| 2 | RESTRICT | 14 | 1391.66 | −681.83 | 1 vs. 2 | 3.13 | 0.21 |
| 3 | RESTRICT and TRANS | 17 | 1361.24 | −663.62 | 2 vs. 3 | 36.43 | 0.00 |
| 4 | RESTRICT, TRANS and RECOV | 21 | 1355.82 | −656.91 | 3 vs. 4 | 13.42 | 0.01 |
| b) speed | |||||||
| 1 | Intercept | 12 | 107.27 | −41.63 | |||
| 2 | RESTRICT | 14 | 105.89 | −38.95 | 1 vs. 2 | 5.38 | 0.07 |
| 3 | RESTRICT and TRANS | 17 | 102.68 | −34.34 | 2 vs. 3 | 9.21 | 0.03 |
| 4 | RESTRICT, TRANS and RECOV | — | — | — | — | — | — |
| c) sleep onset latency | |||||||
| 1 | Intercept | 12 | 1570.67 | −773.33 | |||
| 2 | RESTRICT | 14 | 1574.67 | −773.33 | 1 vs. 2 | 0.0005 | 0.10 |
| 3 | RESTRICT and TRANS | 17 | 1565.67 | −765.83 | 2 vs. 3 | 15.00 | 0.002 |
| 4 | RESTRICT, TRANS and RECOV | 21 | 1567.05 | −762.53 | 3 vs. 4 | 6.62 | 0.16 |
| d) SSS | |||||||
| 1 | Intercept | 12 | 718.22 | −347.11 | |||
| 2 | RESTRICT | 14 | 719.43 | −345.72 | 1 vs. 2 | 2.79 | 0.25 |
| 3 | RESTRICT and TRANS | 17 | 715.45 | −340.72 | 2 vs. 3 | 9.96 | 0.02 |
| 4 | RESTRICT, TRANS and RECOV | 21 | 719.74 | −338.87 | 3 vs. 4 | 3.71 | 0.45 |
Abbreviations: RESTRICT = Sleep Restriction slope; TRANS = recovery Transition parameter; RECOV = recovery slope
Step 4
In the final step of modeling, the tests for variability in the 3 slope parameters were repeated; however, in these analyses both group and age were included as individual-level predictors. The purpose of these tests was to determine if residual variability existed after both group and age had been included as explanatory variables. For lapses, sleep onset latency, and SSS, allowing for variability in the TRANS parameter significantly improved model fit (lapses, −2 log-likelihood ratio = 6.33, P = 0.04; sleep onset latency, −2 log-likelihood ratio = 8.05, P = 0.02; SSS, −2 log-likelihood ratio = 9.55, P = 0.01) suggesting that the individual differences still remain even after including age and group as predictors; therefore, in the final models a random term for individual variability in the TRANS parameter was included. For speed, allowing for variability in any parameter did not improve the fit to the model, so individual variability was only allowed for the intercept.
RESULTS
Due to technical difficulties, one or more sessions of the various dependent measures were lost from some subjects from each sleep group (< 1% for each variable for each group). Because the analytical methods are robust to missing values, these subjects are included in the analyses but with consequently reduced degrees of freedom.
Nighttime Sleep
At-Home Phase
Actigraphically estimated nightly total sleep time collapsed across all nights did not differ between Extended (mean [SD] TST = 361 [113] min) and Habitual (mean [SD] TST = 399 [86] min) groups during the initial 2-week “At-home” phase (P = 0.095). Only TST on nights 6 and 8 differed between groups, with the Habitual group obtaining more sleep (Day 6: Extended mean [SD] TST = 382 [29] and Habitual mean [SD] TST = 469 [30]; Day 8: Extended mean [SD] TST = 329 [29] and Habitual mean [SD] TST = 428 [30]).
Mean differences between usual rise times during the At-home phase and 07:00 (rise time for the subsequent study phases) did not differ between Extended (mean [SD) difference in rise time = 65 [133] min) and Habitual (mean [SD] difference in rise time = 60 [78] min) groups (P = 0.91).
In-Laboratory Overnight Phase
With the start of randomization to Extended v. Habitual groups, actigraphically estimated TST differed between groups (mean [SD] TST minutes collapsed across nights = 479.5 (71.5) for Extended and 363.2 (63.9) for Habitual; Group main effect F1,21 = 49.12, P < 0.001). The Day and Group × Day effects were not significant (P > 0.05).
Full-time In-Laboratory Phase
Differences across days.
Table 4 lists mean minutes of each PSG sleep variable as a function of Group and Day. In general, sleep amounts decreased from Baseline to the sleep restriction phase, then increased from sleep restriction to recovery.
Table 4.
Mean (SE) Minutes of Various Sleep Stages and TST Across Full-Time, In-Laboratory Sub-Phases for a) Extended and b) Habitual Sleep Groups
| Variable | B | SR1 | SR2 | SR3 | SR4 | SR5 | SR6 | SR7 | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a) Extended | |||||||||||||
| TST | 521 (5) | 167 (5) | 175 (5) | 176 (5) | 173 (5) | 173 (5) | 177 (5) | 176 (5) | 465 (5) | 446 (5) | 447 (5) | 440 (6) | 432 (5) |
| Stg 1 | 63 (4) | 13 (5) | 8 (4) | 7 (4) | 6 (4) | 6 (4) | 6 (4) | 6 (5) | 23 (5) | 30 (5) | 31 (5) | 39 (5) | 37 (5) |
| Stg 2 | 252 (7) | 58 (7) | 57 (7) | 56 (7) | 51 (7) | 51 (7) | 55 (7) | 55 (7) | 230 (7) | 213 (7) | 223 (7) | 210 (7) | 214 (7) |
| SWS | 86 (8) | 67 (8) | 80 (8) | 80 (8) | 81 (8) | 88 (8) | 83 (8) | 82 (8) | 115 (8) | 99 (8) | 92 (9) | 94 (9) | 92 (9) |
| REM | 121 (5) | 31 (5) | 32 (5) | 33 (5) | 35 (5) | 30 (5) | 34 (5) | 33 (5) | 95 (5) | 102 (5) | 100 (5) | 99 (5) | 91 (5) |
| b) Habitual | |||||||||||||
| TST | 407 (5) | 174 (5) | 177 (5) | 176 (5) | 175 (5) | 176 (5) | 176 (5) | 176 (5) | 462 (5) | 459 (5) | 452 (5) | 448 (6) | 444 (5) |
| Stg 1 | 26 (5) | 7 (4) | 5 (4) | 5 (4) | 4 (4) | 6 (4) | 4 (4) | 5 (4) | 17 (4) | 24 (4) | 30 (4) | 30 (4) | 31 (4) |
| Stg 2 | 192 (7) | 63 (7) | 58 (7) | 55 (7) | 53 (7) | 60 (7) | 55 (7) | 62 (7) | 219 (7) | 218 (7) | 210 (7) | 219 (7) | 207 (7) |
| SWS | 96 (9) | 77 (8) | 87 (8) | 83 (8) | 85 (8) | 76 (8) | 81 (8) | 79 (8) | 130 (8) | 113 (8) | 107 (9) | 100 (8) | 105 (8) |
| REM | 94 (5) | 29 (5) | 28 (5) | 34 (5) | 34 (5) | 36 (5) | 37 (5) | 31 (5) | 97 (5) | 106 (5) | 109 (5) | 103 (5) | 104 (5) |
Abbreviations: B = Baseline, SR = Sleep Restriction, R = Recovery
Differences between Extended versus Habitual groups.
At Baseline, the Extended group obtained more TST (Day × Group, F12,85 = 26.18, P < 0.001; mean [SD], Extended = 520.5 [11.3], Habitual = 406.6 (12.1]), more REM (Day × Group, F12,156 = 2.83, P = 0.002), more stage 1 (Day × Group, F12,55 = 5.79, P < 0.001), and more stage 2 (Day × Group, F12,86 = 6.50, P < 0.001) than did the Habitual group. No other effects were significant (P > 0.05).
Discontinuous Growth Modeling (PVT, Modified MWT, SSS).
Intraclass Correlation Coefficients
The null model values for the ICC are 0.57, 0.72, 0.24, and 0.28 for lapses, speed, sleep latency, and self-rated sleepiness (SSS), respectively. ICC estimates based on the design elements are 0.70, 0.85, 0.45, and 0.44 for lapses, speed, sleep latency, and self-rated sleepiness (SSS) respectively. These values indicate a high degree of consistency in responses for individuals and significant differences among individuals. Both null model and design based ICCs were highest for both aspects of PVT performance.
Final Model Estimates
The final model estimates for each variable are provided in tables 5–7. The variance components provided in Tables 5–7 provide an estimate of the residual intra-class correlation coefficient (ICC) and the Akaike information criterion (AIC) and Bayesian information criterion (BIC) provide fit indices for model comparison (smaller values indicate better fit). As expected, age effects were significant and similar to effects reported by Bliese and colleagues (better performance in older subjects),22 and will be discussed below.
Table 5.
Model Estimates for PVT a) Lapses and b) Speed
| Parameter | SE | d.f. | t-value | P-value | |
|---|---|---|---|---|---|
| a) lapses | |||||
| Fixed effects | |||||
| Intercept (ms) | 0.23 | 3.35 | 273 | 0.07 | 0.47 |
| Restriction slope (RESTRICT)* | 2.70 | 0.40 | 273 | 6.80 | 0.00 |
| Age | −0.05 | 0.11 | 21 | −0.40 | 0.35 |
| Transition to recovery (TRANS)* | −15.33 | 2.70 | 273 | −5.67 | 0.00 |
| Recovery slope (RECOV)* | −3.14 | 0.88 | 273 | −3.54 | 0.00 |
| Prior sleep group (Group) | 1.03 | 1.43 | 21 | 0.72 | 0.24 |
| RESTRICT X Age* | −0.09 | 0.01 | 273 | −6.75 | 0.00 |
| Age X TRANS* | 0.44 | 0.09 | 273 | 4.86 | 0.00 |
| Age X RECOV* | 0.12 | 0.03 | 273 | 4.02 | 0.00 |
| RESTRICT X Group* | 0.40 | 0.17 | 273 | 2.35 | 0.01 |
| TRANS X Group | −0.85 | 1.16 | 273 | −0.73 | 0.23 |
| RECOV X Group* | −0.75 | 0.39 | 273 | −1.94 | 0.03 |
| Correlations | |||||
| Variance components | |||||
| Intercept | 8.70 | ||||
| Transition to recovery | 2.45 | −0.66 | |||
| Residual | 4.75 | ||||
| Fit indices | |||||
| Deviance (−2 Log-likelihood) | −677.96 | ||||
| AIC | 1389.91 | ||||
| BIC | 1452.53 | ||||
| b) speed | |||||
| Fixed effects | |||||
| Intercept (ms)* | 3.68 | 0.53 | 273 | 6.88 | 0.00 |
| Restriction slope (RESTRICT)* | −0.34 | 0.05 | 273 | −7.34 | 0.00 |
| Age | 0.02 | 0.02 | 21 | 1.03 | 0.16 |
| Transition to recovery (TRANS)* | 1.72 | 0.26 | 273 | 6.66 | 0.00 |
| Recovery slope (RECOV)* | 0.33 | 0.10 | 273 | 3.22 | 0.00 |
| Prior sleep group (Group) | −0.20 | 0.23 | 21 | −0.89 | 0.19 |
| RESTRICT X Age* | 0.01 | 0.00 | 273 | 4.81 | 0.00 |
| Age X TRANS* | −0.03 | 0.01 | 273 | −3.22 | 0.00 |
| Age X RECOV* | −0.01 | 0.00 | 273 | −2.71 | 0.00 |
| RESTRICT X Group | 0.01 | 0.02 | 273 | 0.64 | 0.26 |
| TRANS X Group* | −0.23 | 0.11 | 273 | −2.10 | 0.02 |
| RECOV X Group | 0.05 | 0.05 | 273 | 1.12 | 0.13 |
| Variance components | |||||
| Intercept | 0.26 | ||||
| Residual | 0.06 | ||||
| Fit indices | |||||
| Deviance (−2 Log-likelihood) | −45.90 | ||||
| AIC | 121.81 | ||||
| BIC | 177.06 | ||||
Abbreviations: RESTRICT = Sleep Restriction slope; TRANS = recovery Transition parameter; RECOV = recovery slope;
P < 0.05, one-tailed
Table 6.
Model Estimates for Modified MWT Sleep Latency
| Parameter | SE | d.f. | t-value | P-value | |
|---|---|---|---|---|---|
| Fixed effects | |||||
| Intercept (ms) | 4.35 | 2.63 | 276 | 1.65 | 0.05 |
| Restriction slope (RESTRICT)* | −0.91 | 0.48 | 276 | −1.90 | 0.03 |
| Age* | 0.38 | 0.09 | 21 | 4.31 | 0.00 |
| Transition to recovery (TRANS) | 0.66 | 3.25 | 276 | 0.20 | 0.42 |
| Recovery slope (RECOV)* | 3.11 | 1.07 | 276 | 2.90 | 0.00 |
| Prior sleep group (Group)* | −3.56 | 1.13 | 21 | −3.15 | 0.00 |
| RESTRICT × Age* | −0.05 | 0.02 | 276 | −3.05 | 0.00 |
| Age × TRANS* | 0.34 | 0.11 | 276 | 3.10 | 0.00 |
| Age × RECOV | −0.00 | 0.04 | 276 | −0.03 | 0.49 |
| RESTRICT × Group* | 0.68 | 0.20 | 276 | 3.32 | 0.00 |
| TRANS × Group | −1.83 | 1.39 | 276 | −1.31 | 0.10 |
| RECOV × Group | −0.48 | 0.46 | 276 | −1.04 | 0.15 |
| Correlations | |||||
| Variance components | |||||
| Intercept | 2.90 | ||||
| Transition to recovery | 1.73 | 0.99 | |||
| Residual | 7.53 | ||||
| Fit indices | |||||
| Deviance (−2 Log-likelihood) | −763.20 | ||||
| AIC | 1560.40 | ||||
| BIC | 1623.20 | ||||
Abbreviations: RESTRICT = Sleep Restriction slope; TRANS = recovery Transition parameter; RECOV = recovery slope;
P < 0.05, one-tailed
Table 7.
Model Estimates for SSS Scores
| Parameter | SE | d.f. | t-value | P-value | |
|---|---|---|---|---|---|
| Fixed effects | |||||
| Intercept (ms)* | 2.06 | 0.89 | 276 | 2.32 | 0.01 |
| Restriction slope (RESTRICT) | 0.15 | 0.11 | 276 | 1.30 | 0.10 |
| Age | −0.02 | 0.03 | 21 | −0.57 | 0.29 |
| Transition to recovery (TRANS) | −0.51 | 0.89 | 276 | −0.57 | 0.29 |
| Recovery slope (RECOV) | −0.09 | 0.26 | 276 | −0.35 | 0.36 |
| Prior sleep group (Group) | 0.34 | 0.38 | 21 | 0.88 | 0.20 |
| RESTRICT × Age | 0.00 | 0.00 | 276 | 1.00 | 0.16 |
| Age × TRANS | −0.04 | 0.03 | 276 | −1.17 | 0.12 |
| Age × RECOV | −0.00 | 0.01 | 276 | −0.14 | 0.45 |
| RESTRICT × Group | −0.02 | 0.05 | 276 | −0.37 | 0.36 |
| TRANS × Group | −0.35 | 0.38 | 276 | −0.91 | 0.18 |
| RECOV × Group | −0.09 | 0.11 | 276 | −0.85 | 0.20 |
| Correlations | |||||
| Variance components | |||||
| Intercept | 0.59 | ||||
| Transition to recovery | 0.32 | −0.77 | |||
| Residual | 0.42 | ||||
| Fit indices | |||||
| Deviance (−2 Log-likelihood) | −342.50 | ||||
| AIC | 719.00 | ||||
| BIC | 781.79 | ||||
Abbreviations: RESTRICT = Sleep Restriction slope; TRANS = recovery Transition parameter; RECOV = recovery slope;
P < 0.05, one-tailed
Psychomotor Vigilance Task
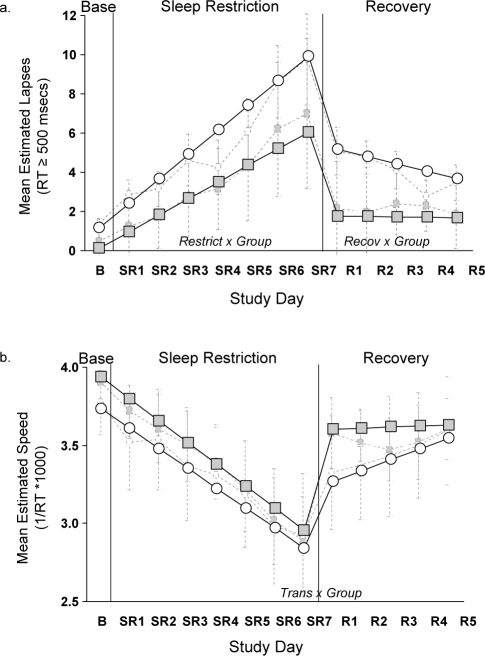
The model estimates for PVT (a) lapses and (b) speed, controlling for age, are displayed in Table 5. Figure 1 uses the parameter estimates from Table 5 to illustrate the experimental design effects for lapses and speed. The interactions between Group and each of 3 parameters (RESTRICT, TRANS, and RECOV) are illustrated in the figures. During sleep restriction, the Habitual group showed a steeper slope of PVT performance deterioration for lapses compared to the Extended group (RESTRICT × Group). At the transition from the sleep restriction to recovery phase, there were no differences between the groups for lapses. During the 5-day Recovery phase the groups showed significantly different patterns of performance for lapses (RECOV × Group). Specifically, the Extended group recovered significantly after one night and maintained a stable level of improved performance, while the Habitual group gradually improved across the 5 Recovery days.
Figure 1.
Predicted psychomotor vigilance test a) lapses and b) speed for Extended (shaded squares) and Habitual (open circles) sleep groups controlling for age. Dashed light-gray lines indicate raw mean (SE) data for Extended (shaded squares) and Habitual (open circles) groups.
The pattern for PVT speed is illustrated in Figure 1B. For speed, only the interaction involving the transition parameter was significant (TRANS × Group). Notice in Figure 1B that the Extended group had a much larger increase in speed at the transition point than did the Habitual group. While not significant, the pattern for PVT speed in the recovery phase mirrored that of lapses with a flat slope for the Extended group and an improvement in speed for the Habitual group.
As presented in Table 5, the interactions between Age and each of 3 parameters (RESTRICT, TRANS, and RECOV) were significant for both PVT lapses and speed. For both PVT variables, younger individuals showed a steeper slope of performance deterioration during sleep restriction and steeper slopes of improvement during the transition from sleep restriction to recovery and during recovery compared to older individuals.
Modified Maintenance of Wakefulness Test
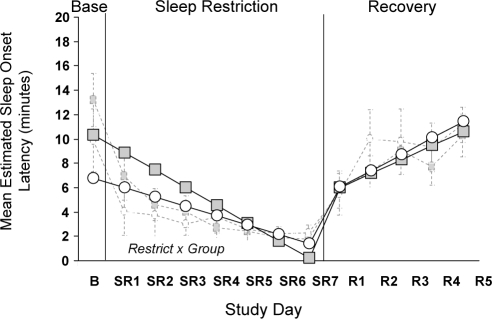
The model estimate for modified MWT sleep latency, controlling for age, is displayed in Table 6. Figure 2 uses the parameter estimates from Table 6 to illustrate the experimental design effects for sleep latency. The significant interaction between Group and RESTRICT is illustrated in the figure. During sleep restriction, the Habitual group showed shorter sleep latency compared to the Extended group (RESTRICT × Group). The groups did not differ at the transition from the sleep restriction to recovery phase, nor in patterns of sleep latency during Recovery (TRANS × Group, NS; RECOV × Group, NS).
Figure 2.
Predicted sleepiness scores for MWT sleep latency for Extended (shaded squares) and Habitual (open circles) sleep groups controlling for age. Dashed light-gray lines indicate raw mean (SE) data for Extended (shaded squares) and Habitual (open circles) groups.
A main effect of Age and significant interactions between Age and restriction (RESTRICT) and transition (TRANS) parameters are reported in Table 6. Older individuals were more alert overall, but showed steeper declines in alertness during sleep restriction and steeper slopes of improvement during the transition from sleep restriction to recovery.
Stanford Sleepiness Scale
The model estimates for SSS self-rated sleepiness score, controlling for age, are displayed in Table 7. There were no significant interactions between Group and any of the three parameters (RESTRICT, TRANS, and RECOV).
DISCUSSION
One week of sleep extension improved resilience on measures of performance and alertness during subsequent sleep restriction, and facilitated recovery thereafter. Discontinuous growth modeling was used to compare and contrast patterns of performance degradation across 7 days of sleep restriction (3 h TIB) for 2 groups of participants—one in which habitual sleep duration had been maintained for the previous 7 days (Habitual group), and the other in which TIB had been increased to 10 h for the previous 7 days (Extended group). This approach allowed: (a) examination of the trajectories (slope) of performance degradation over the sleep restriction period, and the performance improvement associated with the transition and recovery periods. It also allowed us to determine the extent to which prior sleep duration is associated with systematic differences in performance and alertness trajectories. Differences between the Extended and Habitual groups for all 3 parameters (restriction slope, transition, and recovery slope) were evident.
Because our previous work (and that of others) shows that age accounts for a significant portion of the variance in performance during sleep loss,22 this factor was controlled in the present study. As found previously,22 younger individuals in the current study showed a greater decline in PVT performance during sleep restriction. In addition, it was found that younger individuals showed greater improvement during the transition from sleep restriction to recovery than did older individuals, but older individuals maintained a generally stable level of improved PVT performance during the recovery phase. For objective sleepiness, older individuals showed a higher level of alertness initially, followed by a steeper slope of deterioration during sleep restriction and a steeper slope of improvement during the transition period compared to younger individuals. Overall, however, older individuals were objectively less sleepy than younger individuals; but age was not a significant predictor of subjective sleepiness, indicating that younger adults may not be aware of their sleepiness levels and associated performance impairment.
It should also be noted that the age range in the study was only 18–39 years. It is therefore not known whether older adults would demonstrate greater resilience and, conversely, if adolescents or children would show greater vulnerability. Also unknown is if these age effects reflect differences in physiological responses to sleep loss or differences in years of experience coping with, and adapting to, chronic sleep loss during adulthood. Future studies of chronic sleep restriction and recovery in children/adolescents and healthy older adults are needed to clarify age-related changes in response to sleep restriction and recovery across the lifespan.
Differences in the polysomnographically measured sleep of the Habitual and Extended groups were evident on the baseline night on all sleep measures except SWS amount. Specifically, the Extended group had greater amounts of TST, REM, NREM, stage 1, and stage 2, compared to the Habitual group (as expected, given the longer TIB of this group) on this night. No group differences in sleep architecture were found during the restriction and recovery phases. Actigraphy data from the at-home period showed that the groups did not significantly differ prior to the random assignment to groups.
The present results suggest that apparent differences in the rates of recovery of alertness/performance following sleep restriction in previous studies2,7,8 may have been due to differences in the amount of nightly sleep habitually obtained prior to the sleep restriction period (in one study, nightly pre-restriction sleep was extended, and in the other it was not). In the present study, performance deficits (PVT lapses and speed) recovered after one night of recovery sleep in the Extended Group. It should be noted that response speed failed to recover to baseline level for the Extended group. This is most likely because the baseline measures were in this case obtained following a week of extended sleep, whereas the recovery consisted of 8 h TIB per night—i.e., allowing a more typical amount of nighttime sleep and resulting in a more typical level of performance. Accordingly, one night of recovery sleep in the Extended group restored mean response speed to a stable level that was comparable to that exhibited by the Habitual group at baseline. In contrast, the Habitual group showed continuing improvement (i.e., reductions in PVT lapses) across the 5 recovery days, and performance in this group failed to improve to same extent as that of the Extended group, even after 5 nights of recovery sleep.
In many previous sleep loss studies, 1 or 2 nights of sleep extension/adaptation are administered prior to the sleep loss phase. Findings from the present study suggest that this may not be adequate—i.e., that the long-term, habitual sleep duration of study participants can mediate their sensitivity/resiliency during sleep loss and subsequent recovery, so this factor should always be controlled or taken into account when studies are performed for the purpose of documenting and (especially) quantifying the effects of sleep loss on various aspects of alertness and performance.
Of course, findings from the present study also have implications for (a) mathematical modeling efforts to predict the effects of sleep/wake schedules on performance in operational settings, and (b) our understanding of the nature of the physiological processes that underlie alertness and performance. For example, the finding that prior nightly sleep duration impacts performance and sleepiness during subsequent sleep restriction and recovery is consistent with the assertions of Johnson and colleagues25 that a simple sleep reservoir conception—in which alertness and performance vary simply as a function of the extent to which an individual's idiosyncratic and consistent need for sleep has been satisfied (combined with the circadian rhythm of alertness)—is not adequate for describing and predicting alertness and performance during sleep restriction and recovery. Indeed, the present findings are consistent with their assertion that the homeostatic process modulating sleep need varies over time, albeit with a long time constant.
As previously mentioned, recovery following sleep restriction appears to be slower than recovery following total sleep deprivation. The physiological mechanisms underlying this relatively slow recovery are unknown, but it is possible that a week of sleep restriction induces long-term neuromodulatory changes in brain physiology—changes that are not induced by shorter periods of acute, total sleep deprivation. Extrapolating from findings that suggest that the sleep homeostat may be a function of the ratio of extracellular adenosine (a homeostatic sleep factor mediating the sleep-inducing effects of prolonged wakefulness) to the density of adenosine receptors in the basal forebrain of rats,26 it is possible that the present findings reflect the behavioral consequences of an altered extracellular adenosine/adenosine receptor ratio.
While recovery to sleep restriction is generally slow compared to total sleep loss, there is, of course, variability in both recovery rate and response to sleep restriction among individuals. Van Dongen and colleagues have shown that vulnerability to the effects of total sleep loss is a trait-like characteristic and that individuals show stability in their response with repeated testing.27 In the present study, the demonstrated greater sensitivity of the Habitual versus Extended sleep group suggests the possibility that some of the observed interindividual difference may not be sensitivity or vulnerability per se, but rather habitual sleep duration. That is, habitually shorter sleepers may demonstrate increased sensitivity to sleep loss when faced with a challenge (i.e., period of sleep loss)—a vulnerability that is based as much or more on their habitual sleep behavior than on differences in their inherent vulnerability to the effects of sleep loss. In other words, the trait may be, at least in part, how much sleep is typically obtained rather than (or in addition to) how much sleep loss can be effectively tolerated. Consistent with this possibility, Klerman and Dijk have shown that habitually shorter sleepers fall asleep faster on MSLTs and obtain more “recovery” sleep in a sleep extension protocol.28
Similarly, recent evidence suggests that individuals with a PER3 clock gene polymorphism are more susceptible to sleep loss induced performance impairments.29 Like habitually short sleepers, the sleep of these susceptible individuals is also characterized by high initial values of SWA and of theta during wakefulness. Taken together, these studies suggest an overlap between habitual prior sleep duration and trait-sensitivity to sleep loss. For example, it is possible that the PER3 polymorphism actually mediates the sleep homeostat indirectly (e.g., via the timing of sleep periods and/or the level of sleep debt carried), and that it is this “behavioral” effect of the PER3 polymorphism that ultimately determines an individual's sensitivity/resilience to the effects of sleep loss.
One important, and perhaps critical, aspect of the present study that should be taken into consideration is that “recovery sleep” was restricted to 8 h TIB. It is likely that recovery would have been faster in both groups had they been afforded a longer nightly recovery sleep opportunity. Future studies varying both duration of recovery and degree of sleep restriction (e.g., 5 instead of 3 h TIB) will be needed to fully delineate the effects of prior sleep extension and to accurately determine the amount of recovery sleep needed following extended periods of sleep restriction.
Further studies will also be needed to investigate the possible role of adenosine in mediating the psychophysiological responses and behavioral capacities of humans during sleep restriction, recovery, and sleep extension. Although a recent positron emission tomography study has demonstrated that adenosine receptor binding is increased in humans during total sleep deprivation,30 the effects of longer-term sleep restriction (and/or sleep extension) on adenosine receptor binding in humans are as yet unknown.
In summary, the present study demonstrates beneficial effects of prior sleep extension on performance and alertness during sleep restriction and during subsequent recovery from that sleep restriction. One implication of this study is that habitual sleep duration needs to be taken into consideration when determining individual differences in susceptibility to sleep loss. From a practical standpoint, the present findings suggest that the “banking” of sleep prior to sleep loss may help sustain performance and alertness in operational environments and speed recovery (i.e., improve “recycle rate” of operators).
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Balkin has consulted at sleep disorders centers. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the US Army Medical Research and Materiel Command. We thank the student contractors, military personnel, on-call physicians and study participants for their dedicated work and participation in the study. Thanks also to the two anonymous reviewers for their helpful comments.
This material has been reviewed by the Walter Reed Army Institute of Research, and there is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the position of the Department of the Army of the Department of Defense.
REFERENCES
- 1.National Sleep Foundation. Sleep in America Poll. Washington DC: 2005. See http://sleepfoundation.org. [Google Scholar]
- 2.Belenky G, Wesensten, NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Lorenzo I, Ramos J, Arce C, Guevara MA, Corsi-Cabrera M. Effect of total sleep deprivation on reaction time and waking EEG activity in man. Sleep. 1995;18:346–54. [PubMed] [Google Scholar]
- 5.Corsi-Cabrera M, Arce C, Ramos J, Lorenzo I, Guevara MA. Time course of reaction time and EEG while performing a vigilance task during total sleep deprivation. Sleep. 1996;19:563–9. doi: 10.1093/sleep/19.7.563. [DOI] [PubMed] [Google Scholar]
- 6.Rosa RR, Bonnet MH, Warm JS. Recovery of performance during sleep following sleep deprivation. Psychophysiology. 1983;20:152–9. doi: 10.1111/j.1469-8986.1983.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 7.Wesensten NJ, Reichardt R, Balkin TJ. Chronic sleep restriction and resatiation, I. Recovery of psychomotor vigilance performance. Sleep. 2005;28:O396. [Google Scholar]
- 8.Balkin TJ, Reichardt R, Wesensten NJ. Chronic sleep restriction and resatiation, II. Recovery of subjective alertness. Sleep. 2005;28:O398. [Google Scholar]
- 9.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- 10.Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 11.Beck, AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–671. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 12.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness- eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 13.Sadeh A, Sharkey K, Carskadon M. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 14.Acebo CA, Sadeh A, Seifer R, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- 15.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages in human subjects. US Government Printing Office; 1968. [DOI] [PubMed] [Google Scholar]
- 16.Thorne DR, Genser SG, Sing GC, Hegge FW. The Walter Reed performance assessment battery. Neurobehav Toxicol Teratol. 1985;7:415–8. [PubMed] [Google Scholar]
- 17.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 18.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 19.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2008. R: A language and environment for statistical computing, Reference Index Version 2.7.2. ISBN 3-900051-07-0, Available at: http://www.R-project.org. [Google Scholar]
- 20.Pinheiro JC, Bates DM. Mixed-effects models in S and S-Plus. New York: Springer-Verlag; 2000. [Google Scholar]
- 21.Bliese PB, Ployhart RE. Growth modeling using random coefficient models: model building, testing, and illustrations. Org Res Methods. 2002;5:362–88. [Google Scholar]
- 22.Bliese PB, Wesensten NJ, Balkin TJ. Age and individual variability in performance during sleep restriction. J Sleep Res. 2006;15:376–85. doi: 10.1111/j.1365-2869.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 23.Bliese PD, McGurk D, Thomas JL, Balkin TJ, Wesensten NJ. “Discontinuous growth modeling of adaptation to sleep setting changes: Individual differences and age.”. Aviat Space Environ Med. 2007;78:485–92. [PubMed] [Google Scholar]
- 24.Bliese PD. Within-group agreement, non-independence, and reliability: Implications for data aggregation and analysis. In: Klein KJ, Kozowski SW, editors. Multilevel theory, research, and methods in organizations. San Francisco: Jossey-Bass; pp. 349–81... [Google Scholar]
- 25.Johnson M, Belenky G, Redmond DP, et al. Modulating the homeostatic process to predict performance during chronic sleep restriction. Aviat Space Environ Med. 2004;75:A141–6. [PubMed] [Google Scholar]
- 26.Strecker RE, Basheer R, McKenna JT, et al. Another chapter in the adenosine story. Sleep. 2006;29:426–8. doi: 10.1093/sleep/29.4.426. [DOI] [PubMed] [Google Scholar]
- 27.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 28.Klerman EB, Dijk D. Interindividual variation in sleep duration and its association with sleep debt in young adults. Sleep. 2005;28:1253–59. doi: 10.1093/sleep/28.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viola AU, Archer SN, James LM, et al. PER3 Polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 30.Elmenhorst D, Meyer PT, Winz OH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–5. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]