Abstract
Study Objectives:
Long-duration ( ≥ 6 months) polysomnographic studies of insomnia medications are lacking. This study evaluated the long-term efficacy of ramelteon, a selective MT1/MT2 melatonin-receptor agonist used for insomnia treatment.
Design:
Six-month, randomized, double-blind, placebo-controlled study.
Setting:
Forty-six investigative sites in the United States, Europe, Russia, and Australia.
Participants:
Four hundred fifty-one adults (age ≥ 18 years) with chronic primary insomnia.
Interventions:
Ramelteon, 8 mg, or placebo 30 minutes before bedtime nightly for 6 months.
Measurements:
Sleep was evaluated by polysomnography and morning questionnaires on the first 2 nights of Week 1; the last 2 nights of Months 1, 3, 5, and 6; and Nights 1 and 2 of the placebo run-out. Next-morning residual effects as well as adverse effects and vital signs were recorded at each visit. Rebound insomnia and withdrawal effects were evaluated during placebo run-out.
Results:
Over the 6 months of treatment, ramelteon consistently reduced latency to persistent sleep compared with baseline and with placebo; significant decreases were observed at Week 1 and Months 1, 3, 5, and 6 (P < 0.05). Ramelteon significantly reduced subjective sleep latency relative to placebo at Week 1, Month 1, and Month 5 (P < 0.05), with reductions nearing statistical significance at Months 3 and 6 (P ≤ 0.08). No significant next-morning residual effects were detected during ramelteon treatment. No withdrawal symptoms or rebound insomnia were detected after ramelteon discontinuation. Most adverse events were mild or moderate in severity.
Conclusions:
In adults with chronic insomnia, long-term ramelteon treatment consistently reduced sleep onset, with no next-morning residual effects or rebound insomnia or withdrawal symptoms upon discontinuation.
Citation:
Mayer G; Wang-Weigand S; Roth-Schechter B; Lehmann R; Staner C; Partinen M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. SLEEP 2009;32(3):351–360.
Keywords: Melatonin receptor agonist, Polysomnography, insomnia, long-term
INSOMNIA IS BROADLY DEFINED AS DIFFICULTY INITIATING OR MAINTAINING SLEEP, WAKING TOO EARLY, OR NONRESTORATIVE SLEEP. IT IS ESTIMATED that up to 34% of adults in the United States1,2 and 37% in Europe2 have some form of insomnia.
For the majority of individuals with insomnia, the symptoms are long lasting.3 Chronic insomnia (present for 30 days or more) often requires long-term treatment, typically well beyond 4 to 5 weeks.4 The persistent nature of insomnia poses a challenge to clinicians because most medications indicated for the treatment of insomnia are limited to short-term use. In the United States and in Europe, benzodiazepine receptor agonists (BzRAs) are the most commonly prescribed medications for the treatment of insomnia (eg, zolpidem, zolpidem MR, zaleplon, eszopiclone, and zopiclone). These medications achieve their effect through action at GABAA receptors, which are widely distributed throughout the brain. BzRAs are considered controlled substances, and the majority (ie, zopiclone, zolpidem, zaleplon) are indicated for a maximum of 4 weeks of use.5–9 BzRAs have also been associated with memory and balance impairment,10 rebound insomnia, withdrawal symptoms, and abuse potential.11,12 Short-term studies have demonstrated disruptions in sleep architecture (as well as increases in sleep latency) immediately after discontinuation of BzRA treatment, which makes withdrawing from these types of treatments difficult.13,14
Though not indicated for insomnia, antidepressants (such as trazodone) and alternative treatments like valerian are often used to treat insomnia as well. The data regarding efficacy for treatment of sleep disorders with these agents are inconsistent, and the majority of studies are limited by sample size, lack of diagnostic criteria, and the absence of a placebo control.15,16
Some newer pharmacologic agents that target melatonin receptors have been developed based on the evidence that certain melatonin receptors are involved in the control of sleep. In the 2-process model of sleep, there is a drive for sleep that accumulates during wakefulness and decreases during sleep (homeostatic process) and a 24-hour oscillatory rhythm that guides sleep and wake propensity (circadian process).17 Combined, the homeostatic and circadian processes influence the duration and timing of sleep and wakefulness. The circadian process is driven by the suprachiasmatic nucleus (SCN), which contains a number of high-affinity melatonin receptors. During the day, the SCN actively produces an arousal signal that maintains wakefulness and opposes the sleep drive. In response to darkness, the SCN works on a feedback loop, first signaling the release of melatonin (a chemical expression of darkness), which feeds back to inhibit SCN activity. In vitro studies indicate that the MT1 receptor mediates the acute inhibition of SCN firing by melatonin18 and the MT2 receptor is involved with the phase-shifting effects of melatonin on circadian rhythms.19–21 However, in vivo studies have further complicated the issue, indicating that the MT1 receptor may have a role in mediating phase-shifts in conjunction with the MT2 receptor.22
Ramelteon is the first melatonin-receptor agonist approved in the United States for the treatment of insomnia and has a mechanism of action that differs considerably from commonly used BzRAs. Ramelteon is an MT1/MT2 melatonin-receptor agonist with negligible affinity for either the MT3 binding site or other neuronal receptors.23 The actions of ramelteon on the MT1 receptor are thought to inhibit the neuronal firing in the SCN, effectively turning off the arousal signal and allowing sleep to occur. Previous randomized, placebo-controlled studies have demonstrated the efficacy and safety of ramelteon in subjects with chronic insomnia.24–27 These trials also demonstrated that there was neither evidence of next-day residual effects nor evidence of withdrawal or rebound insomnia when ramelteon use was discontinued. Ramelteon also lacks abuse potential.28
The National Institutes of Health State-of-the-Science Statement on manifestations and management of insomnia highlighted the need for long-term studies of primary insomnia.3 Not only is information about the incidence and duration of chronic insomnia scarce, but data regarding the efficacy and adverse effects with long-term pharmacologic treatment of insomnia are lacking. Lastly, no objective polysomnographic study of long duration is available. This is particularly important because a recent study demonstrated that self-reported total sleep times and sleep latencies are subjectively overestimated, even on the morning following overnight polysomnography.29 Therefore, this study was designed to evaluate objective and subjective efficacy and safety of ramelteon when administered nightly for 6 months in adults with chronic insomnia.
METHODS
Design
This randomized, double-blind, placebo-controlled, phase III study evaluated the efficacy and safety of ramelteon, 8 mg, administered nightly for 6 months in adults in the United States, Europe, Russia, and Australia. Following an initial 1-week screening period, eligible subjects underwent a single-blind placebo run-in for 2 weeks. Thereafter, randomized subjects underwent double-blind treatment with ramelteon, 8 mg, or placebo administered 30 minutes before habitual bedtime every night for 6 months. A 2-week, single-blind, placebo run-out period followed.
Participants
Men and nonpregnant nonlactating women who were 18 years of age or older with a body mass index between 18 and 34 (inclusive) were enrolled. Subjects provided information on medical and sleep history, demographics, prior medication history, concurrent medical conditions, and current medication information. Eligible subjects were required to have chronic insomnia (difficulty initiating or maintaining sleep or nonrestorative sleep lasting at least 3 months), a sleep disturbance that caused clinically significant distress or impairment, a self-reported total sleep time (sTST) of less than 6.5 hours, a self-reported sleep latency (sSL) of at least 45 minutes per night, and a habitual bedtime between 22:00 and 01:00. Approximately 20% of enrolled subjects reported using medication to fall asleep (1–4 times/week). All sleep-related medications were discontinued at least 1 week before the start of the study. Subjects were excluded from participating if they had narcolepsy, a sleep-related breathing disorder, a circadian rhythm sleep disorder, a parasomnia, or if they had a sleep-schedule change (within 3 months), had traveled across more than 3 times zones (within 7 days), or had participated in a weight-loss program or altered their exercise routine (within 30 days). Other reasons for exclusion were a history of seizures, restless legs syndrome, periodic leg movement syndrome, fibromyalgia, a psychiatric disorder (within 6 months), or drug or alcohol abuse (within 12 months); a significant neurologic, hepatic, renal, endocrine, cardiovascular, gastrointestinal, pulmonary, hematologic, or metabolic disease (within 30 days); or a clinically abnormal finding as determined by medical history, physical examination, electrocardiogram, or clinical laboratory tests. Other reasons for exclusion included a known hypersensitivity to ramelteon or related compounds; any condition or use of a drug or supplement that affected the sleep-wake function (including tobacco products and central nervous system medications) and prohibited the subject from completing the study or was not in the best interest of the subject; or participation in an investigational study in the previous 30 days. Any use of concomitant medications (excluding drugs or supplements known to affect sleep or central nervous system functioning) was evaluated on a case-by-case basis throughout the study.
Following initial screening, subjects received single-blind placebo and underwent polysomnography screening on 2 consecutive nights in the sleep laboratory. Subjects were required to have a mean latency to persistent sleep (LPS) measured by polysomnography of greater than 20 minutes (neither night could have been < 15 minutes), an apnea-hypopnea index (per hour of sleep) of less than 10, and a periodic leg movements with arousal index (per hour of sleep) of less than 10. Subjects were also required to have a negative urine drug test.
The Institutional Review Board at each study site approved the study procedures and informed-consent forms. The study was conducted according to applicable Food and Drug Administration laws and regulations, the World Medical Association Declaration of Helsinki (1989), and the International Conference for Harmonisation (ICH) Harmonised Tripartite Guideline for Good Clinical Practice.
Procedure
Subjects completed an initial 1-week screening period during which informed consent was obtained and medical history, physical examination, vital signs, 12-lead electrocardiograms, and clinical laboratory test data were collected. Subjects who qualified for the study based on initial screening criteria underwent polysomnography screening on the first 2 nights of the 2-week placebo run-in period, during which they received single-blind placebo 30 minutes prior to their habitual bedtime. Any excluded medications were discontinued 1 week prior to the 2-night polysomnography screening. Subjects practiced the Digit Symbol Substitution Test (DSST), immediate and delayed memory recall tests, and the visual analog scale (VAS) for mood and feelings on the first night of polysomnography screening. Subjects were awakened after 8 hours of polysomnography recording and completed a postsleep questionnaire, the DSST, memory recall tests, the VAS for mood and feelings, and a Tyrer Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ). Subjects were asked to take single-blind placebo every night for the remainder of the 2-week run-in.
Subjects meeting screening entry criteria were randomly assigned to receive ramelteon, 8 mg, or placebo nightly 30 minutes before bedtime for 6 months (168 consecutive nights). When in the sleep laboratory, sleep was monitored by PSG during the first 2 nights of Week 1 and the last 2 nights of Months 1, 3, 5, and 6. Subjects were awakened after 8 hours of polysomnography recording and asked to complete a postsleep questionnaire, the DSST, immediate and delayed memory recall tests, the VAS for mood and feelings, and the BWSQ 45 to 60 minutes after waking to evaluate subjective sleep parameters, next-day residual effects, and withdrawal symptoms. On all other nights, when at home, subjects were instructed to take their study medication nightly as directed. Safety was assessed by vital signs and clinical laboratory evaluations as well as adverse-event monitoring.
Subjects received single-blind placebo during a 2-week washout period to evaluate rebound effects. Sleep was monitored by polysomnography in the sleep laboratory on the first 2 nights of the washout period. Subjects were instructed to take placebo medication at home nightly for the remaining 12-day period.
Sleep-Related Measures
The prospectively defined primary efficacy endpoint was LPS measured by polysomnography, and TST was a secondary polysomnography efficacy variable. Analysis of sleep architecture included total time spent in each sleep stage (Stage 1, Stage 2, Stage 3/4, and rapid eye movement [REM]) and latency to REM. Self-reported efficacy was assessed by a postsleep questionnaire the morning after polysomnography recordings in the sleep laboratory, which evaluated mean sSL, mean sTST, mean subjective number of awakenings, mean subjective wake time after sleep onset, and mean sleep quality (evaluated on a 7-point Likert scale on which 1 = excellent and 7 = extremely poor). The postsleep questionnaire was also used to evaluate morning level of alertness and ability to concentrate, using the same 7-point Likert scale.
Next-Morning Residual Measures
For the DSST,30 subjects were given a set of symbols with corresponding single-digit numbers and were asked to make as many symbol-for-digit substitutions as possible, working from left to right without skipping any boxes within a 90-second period. The number of correct substitutions in the 90-second period was recorded.
Memory recall tests were used to assess effects on memory. Approximately 45 to 60 minutes after waking, subjects were read a list of words. Immediately afterward, subjects were given 2 minutes to record as many words as they remembered (Immediate Recall). Subjects waited another 25 minutes and were again given 2 minutes to recall as many words as possible from the list (Delayed Recall).
The VAS for mood and VAS for feeling were used to assess next-day residual effects.31–33 The VAS for mood consisted of 12 items: drowsy, slowed down, sleepy, sedated, tired, worn out, listless, fatigued, exhausted, sluggish, weary, and bushed. The VAS for feelings included 8 items: calm/anxious, energetic/fatigued, thinking slowed down/thinking speeded up, peaceful/tense, normal/spacey, at ease/nervous, relaxed/excited, and normal/easily irritated. For each item, subjects graded their subjective states using a scale of 0 (a little) to 100 (a lot).
Treatment-Discontinuation Measures
LPS was measured by polysomnography on the first 2 nights of Month 7 (the placebo run-out period) to evaluate rebound insomnia. Rebound was defined as mean LPS during placebo run-out that was equal to or worse than mean LPS at baseline.
The Tyrer BWSQ was used to assess withdrawal effects after the treatment period.34 Subjects were presented with 20 possible symptoms and were asked to rate each symptom on a 3-point scale with 0 equal to no experience; 1, moderate experience; and 2, severe experience.
Safety Assessments
The incidence of adverse events, including severity (mild, moderate, or severe) and the relationship to study drug, were recorded at each polysomnography visit. Vital-sign assessment and clinical laboratory tests were also obtained the morning following polysomnography recordings. Follow-up clinical laboratory tests, a physical exam, and adverse event monitoring were conducted 7 days after the final treatment.
Statistical Analysis
All statistical analyses were performed using SAS® version 8.2 (SAS, Inc., Cary, NC). Descriptive statistics were used to summarize continuous variables. Frequency counts and percentages were calculated for categorical data. All statistical analyses were 2 sided and performed at the 5% significance level. All subjects who were randomly assigned and who received at least 1 dose of study medication were included in each analysis. The primary efficacy variable was the mean LPS from 2 consecutive nights of polysomnography at Month 3 and then Month 6. All polysomnography variables were scored by a central reader. The ramelteon and placebo groups were compared using an analysis of covariance (ANCOVA) model with treatment group as a factor and the baseline LPS as a covariate. Treatment comparison at Month 6 was contingent on statistical significance over placebo at the 0.05 significance level for the Month-3 data. Baseline polysomnography variables were defined as the average of nonmissing observations from the single-blind placebo lead-in period. P values for comparisons were obtained using t tests from the ANCOVA model of the overall treatment comparison. All secondary efficacy endpoints were analyzed in the same manner as the primary endpoint, with the parameter of interest as a covariate. All primary and secondary variables were based on last observation carried forward. Analyses of exploratory variables (ie, rebound insomnia, BWSQ) and safety variables were based on observed data only.
RESULTS
Disposition and Baseline Characteristics
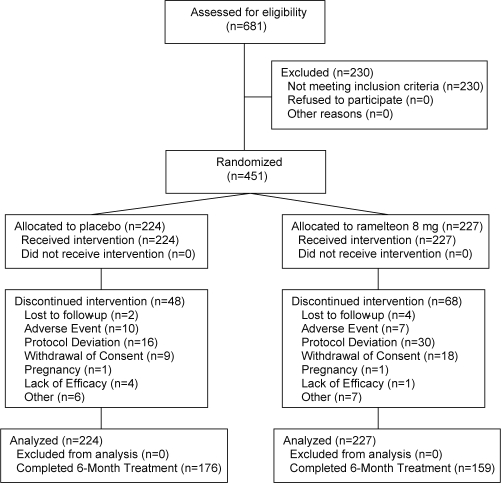
A total of 451 subjects met entry criteria and were randomly assigned to receive ramelteon, 8 mg, (n = 227) or placebo (n = 224). Of these subjects, 335 (74.3%) completed double-blind treatment: 159 (70.0%) in the ramelteon group and 176 (78.6%) in the placebo group (Figure 1). Subject demographics were similar between groups. Overall, the majority of subjects were women (63.2%), and 85.4% were Caucasian, 13.3% were black or African American, 1.6% were Asian, and 0.2% were American Indian or Alaskan Native. The average age of the participants was 46.2 years (14.80 SD, range: 18 to 79 years). On average, subjects reported that it usually took 83.1 minutes (36.94 SD) to fall asleep, and they slept 4.9 hours (0.88 SD). The majority (96.7%) reported that their lack of sleep was associated with a decreased ability to function.
Figure 1.
Subject disposition diagram.
Polysomnographic Sleep Measures
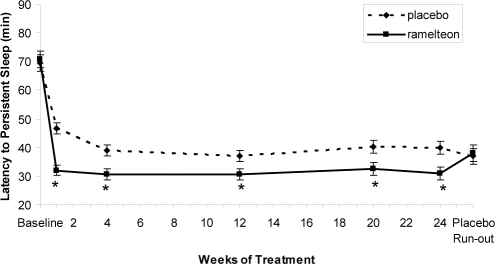
Mean baseline LPS and TST were similar in the ramelteon, 8 mg, (70.75 and 329.28 minutes, respectively) and placebo groups (69.53 and 329.72 minutes, respectively). Significantly greater reductions of LPS were observed with ramelteon compared with placebo throughout treatment (Week 1 and Months 1, 3, 5, and 6; P < 0.05 each) (Figure 2). Similarly, a consistently greater change from baseline was observed in the ramelteon group (ranged from 54%-56%) compared with the placebo group (ranged from 30%–47%). A significantly greater increase of TST was observed with ramelteon compared with placebo at Week 1 (ramelteon: 381.1 minutes, placebo: 365.7 minutes; P < 0.001) but not at any other time point (Table 1).
Figure 2.
Polysomnography-measured latency to persistent sleep over 6 months of nightly ramelteon, 8 mg, or placebo treatment. Data are least-squares means with standard error bars. Last observation carried forward data were used at each time point except placebo run-out, which was observed data only. *P < 0.05 versus placebo, obtained from t tests from an analysis of covariance model of overall treatment comparison.
Table 1.
Polysomnography and Self-Reported Sleep Measures Over 6 Months of Treatment
| Baseline | Week 1 | Month 1 | Month 3 | Month 5 | Month 6 | Placebo Run-out | |
|---|---|---|---|---|---|---|---|
| PSG-Recorded Sleep Measures | |||||||
| TST, minutes | |||||||
| Placebo | 329.72 (3.957) | 365.73 (3.073) | 373.79 (3.049) | 379.31 (3.134) | 379.07 (3.357) | 380.11 (3.255) | 383.00 (3.957) |
| Ramelteon | 329.28 (3.931) | 381.08 (3.059)a | 380.01 (3.028) | 382.46 (3.113) | 380.81 (3.335) | 381.39 (3.233) | 372.87 (4.148) |
| Self-Reported Sleep Measures | |||||||
| sTST, minutes | |||||||
| Placebo | 303.14 (4.393) | 330.24 (3.673) | 340.33 (4.040) | 344.90 (4.160) | 347.34 (4.412) | 349.49 (4.346) | 359.31 (4.475) |
| Ramelteon | 303.77 (4.374) | 337.00 (3.665) | 342.24 (4.040) | 352.30 (4.160) | 351.56 (4.402) | 345.39 (4.336) | 352.79 (4.698) |
| sNAW | |||||||
| Placebo | 3.83 (0.164) | 3.51 (0.140) | 3.30 (0.115) | 3.42 (0.139) | 3.17 (0.137) | 3.20 (0.154) | 3.41 (0.284) |
| Ramelteon | 3.73 (0.163) | 3.59 (0.140) | 3.58 (0.115) | 3.24 (0.139) | 3.25 (0.137) | 3.32 (0.154) | 2.88 (0.299) |
| sWASO, minutes | |||||||
| Placebo | 105.21 (4.595) | 91.33 (3.510) | 84.88 (3.718) | 80.72 (3.544) | 79.51 (3.874) | 79.54 (3.822) | 72.36 (3.661) |
| Ramelteon | 101.42 (4.574) | 94.22 (3.494) | 90.82 (3.710) | 83.47 (3.536) | 84.68 (3.856) | 90.89 (3.796)a | 78.32 (3.854) |
| Sleep qualityb | |||||||
| Placebo | 4.61 (0.066) | 4.33 (0.061) | 4.17 (0.066) | 4.06 (0.067) | 4.07 (0.069) | 4.01 (0.065) | 3.87 (0.071) |
| Ramelteon | 4.72 (0.066) | 4.21 (0.061) | 4.18 (0.066) | 4.04 (0.067) | 3.94 (0.069) | 4.01 (0.065) | 3.86 (0.075) |
Data are reported as least-square means (SE). The number of subjects was 224 in the Placebo group and 227 in the Ramelteon group. The number for each time point may be slightly less in either group due to available data.
Abbreviations: PSG, polysomnography; TST, total sleep time; sTST, subjective total sleep time; sNAW, subjective number of awakenings; sWASO, subjective wake time after sleep onset.
P < 0.05 vs placebo (obtained using t-tests from the analysis of covariance model of the overall treatment comparison).
Evaluated on a 7-point Likert scale on which 1 = excellent and 7 = extremely poor.
Sleep Architecture
There were no statistically significant changes in percentage of time spent in Stage 1 or REM sleep with ramelteon versus placebo except for a small but statistically significant change in Stage 1 at the placebo run-out (Table 2). There was a small but statistically significant increase in percentage of time spent in Stage 2 sleep with ramelteon compared with placebo at each time point (Week 1, Month 1, 3, 5, and 6) and a small but statistically significant decrease in the percentage of time spent in Stage 3/4 sleep with ramelteon compared with placebo at each time point (Week 1, Month 1, 3, 5, and 6).
Table 2.
Sleep Architecture
| Baseline | Week 1 | Month 1 | Month 3 | Month 5 | Month 6 | Placebo Run-out | |
|---|---|---|---|---|---|---|---|
| Stage 1, % TST | |||||||
| Placebo | 11.51 (0.364) | 10.61 (0.234) | 10.44 (0.257) | 9.97 (0.241) | 10.01 (0.226) | 10.07 (0.273) | 10.09 (0.258) |
| Ramelteon | 11.33 (0.362) | 10.60 (0.233) | 10.05 (0.255) | 10.19 (0.239) | 10.13 (0.224) | 9.96 (0.271) | 9.32 (0.271)a |
| Stage 2, % TST | |||||||
| Placebo | 59.56 (0.770) | 57.78 (0.402) | 57.34 (0.410) | 57.68 (0.436) | 58.10 (0.450) | 57.89 (0.475) | 57.84 (0.532) |
| Ramelteon | 57.09 (0.764) | 59.93 (0.400)a | 59.01 (0.407)a | 59.55 (0.433)a | 60.17 (0.447)a | 60.31 (0.472)a | 58.59 (0.557) |
| Stage 3/4, % TST | |||||||
| Placebo | 10.37 (0.697) | 12.72 (0.339) | 12.68 (0.353) | 12.27 (0.362) | 11.94 (0.358) | 12.37 (0.378) | 12.38 (0.455) |
| Ramelteon | 12.89 (0.692) | 9.76 (0.337)a | 10.81 (0.351)a | 10.45 (0.360)a | 10.05 (0.356)a | 10.45 (0.376)a | 11.77 (0.477) |
| REM, % TST | |||||||
| Placebo | 18.56 (0.390) | 18.92 (0.290) | 19.61 (0.293) | 20.10 (0.315) | 19.96 (0.316) | 19.67 (0.323) | 19.76 (0.335) |
| Ramelteon | 18.69 (0.387) | 19.69 (0.289) | 20.07 (0.291) | 19.78 (0.313) | 19.65 (0.314) | 19.27 (0.321) | 20.23 (0.352) |
| Latency to REM, min | |||||||
| Placebo | 80.50 (2.766) | 82.19 (2.595) | 79.41 (2.339) | 79.97 (2.640) | 80.69 (2.846) | 79.10 (2.705) | 78.70 (2.738) |
| Ramelteon | 79.61 (2.747) | 74.73 (2.584)a | 71.49 (2.323)a | 73.16 (2.622) | 74.25 (2.827) | 75.00 (2.687) | 76.69 (2.870) |
Data are reported as LS Mean (SE). The number of subjects was 224 in the Placebo group and 227 in the Ramelteon group. The number for each time point may be slightly less in either group due to available data.
Abbreviations: TST, total sleep time; REM, rapid eye movement.
P < 0.05 vs placebo (obtained using t-tests from the analysis of covariance model of the overall treatment comparison).
Self-Reported Sleep Measures
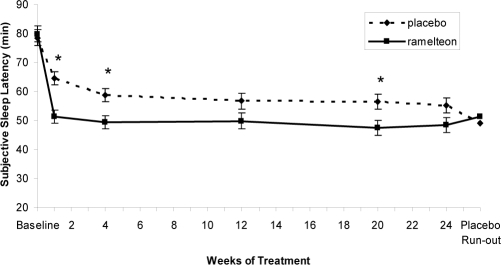
Mean baseline sSL and sTST was similar in the ramelteon, 8 mg, (79.76 and 303.77 minutes, respectively) and placebo groups (78.53 and 303.14 minutes). Significantly greater reductions in sSL were observed with ramelteon compared with placebo at Week 1 and Months 1 and 5 (P < 0.05 each) (Figure 3). Reductions in sSL were also observed at Months 3 and 6, though they did not reach statistical significance (P < 0.08). There were consistently greater improvements from baseline in the ramelteon group (ranged from 35%–40%) compared with the placebo group (ranged from 18%-30%) throughout the entire treatment period. There were no statistically significant differences between ramelteon and placebo at any time point on the following measures: sTST, subjective number of awakenings, and sleep quality (Table 1). No significant differences in subjective wake time after sleep onset was observed between ramelteon and placebo at any time point except Month 6 (ramelteon: 90.89 minutes, placebo: 79.54 minutes; P = 0.036) (Table 1).
Figure 3.
Subjective sleep latency measured over 6 months of nightly ramelteon, 8 mg, or placebo treatment. Data are least-squares means with standard error bars. Last observation carried forward data were used at each time point except placebo run-out, which was observed data only. *P < 0.05 versus placebo, obtained from t tests from an analysis of covariance model of overall treatment comparison.
Measures of Next-Day Residual Effects
There were no statistically significant differences between ramelteon and placebo at any time point on measures of morning level of alertness and ability to concentrate, the DSST, or immediate and delayed memory recall (Table 3). With the exception of sporadic significant differences on individual items of the VAS, no consistent statistically significant differences were observed on the various VAS assessments for mood and feeling throughout the 6-month treatment.
Table 3.
Next-Day Residual Measures
| Baseline | Week 1 | Month 1 | Month 3 | Month 5 | Month 6 | Placebo Run-out | |
|---|---|---|---|---|---|---|---|
| Morning alertness | |||||||
| Placebo | 4.09 | 3.88 | 3.90 | 3.75 | 3.80 | 3.70 | 3.67 |
| Ramelteon | 4.12 | 4.00 | 3.86 | 3.79 | 3.79 | 3.78 | 3.67 |
| Ability to concentrate | |||||||
| Placebo | 4.10 | 3.89 | 3.96 | 3.79 | 3.85 | 3.75 | 3.67 |
| Ramelteon | 4.15 | 3.95 | 3.83 | 3.73 | 3.77 | 3.79 | 3.67 |
| DSST | |||||||
| Placebo | 36.00 | 38.31 | 40.51 | 38.36 | 40.27 | 41.72 | 41.49 |
| Ramelteon | 35.27 | 38.08 | 40.48 | 39.29 | 40.77 | 41.53 | 42.27 |
| Immediate memory recall | |||||||
| Placebo | 6.78 | 8.13 | 7.60 | 8.98 | 8.81 | 8.46 | 8.43 |
| Ramelteon | 6.89 | 8.11 | 7.50 | 9.12 | 8.67 | 8.36 | 8.09 |
| Delayed memory recall | |||||||
| Placebo | 4.87 | 6.44 | 6.30 | 7.79 | 7.45 | 7.42 | 6.95 |
| Ramelteon | 5.04 | 6.41 | 6.32 | 7.92 | 7.46 | 7.24 | 6.52 |
| VAS for feelings – energetic/fatigued | |||||||
| Placebo | 47.99 | 42.53 | 41.77 | 40.72 | 39.08 | 39.22 | 38.14 |
| Ramelteon | 47.83 | 44.67 | 42.66 | 39.45 | 40.93 | 43.26a | 42.22a |
| VAS for mood - drowsy | |||||||
| Placebo | 36.06 | 31.11 | 30.22 | 29.61 | 30.62 | 28.65 | 27.76 |
| Ramelteon | 35.22 | 34.46a | 31.96 | 29.69 | 30.24 | 30.69 | 28.86 |
| VAS for mood – slowed down | |||||||
| Placebo | 34.43 | 30.12 | 28.87 | 29.11 | 29.28 | 28.11 | 26.25 |
| Ramelteon | 33.85 | 33.27a | 30.87 | 28.78 | 29.88 | 30.41 | 28.42 |
| VAS for mood - sleepy | |||||||
| Placebo | 37.86 | 33.17 | 31.78 | 30.41 | 31.23 | 30.12 | 28.62 |
| Ramelteon | 37.68 | 36.42a | 33.08 | 31.12 | 31.58 | 31.94 | 29.48 |
| VAS for feelings – thinking slowed down/speeded up | |||||||
| Placebo | 39.08 | 39.91 | 38.88 | 39.25 | 39.28 | 40.90 | 40.79 |
| Ramelteon | 39.31 | 38.39 | 40.59 | 40.58 | 39.46 | 37.34a | 37.94 |
Data are reported as least-square mean. The number of subjects was 224 in the Placebo group and 227 in the Ramelteon group. The number for each time point may be slightly less in either group due to available data. DSST refers to Digit Symbol Substitution Test.
Subjective morning alertness and ability to concentrate are evaluated on a 7-point scale in which 1 = excellent and 7 = extremely poor. Visual analog scale (VAS) for Feelings: 0 = energetic, 100 = fatigued; VAS for Mood: 0 = a little, 100 = a lot;
P < 0.05 vs placebo (obtained using t-tests from the analysis of covariance model of the overall treatment comparison).
Measure of Rebound Insomnia
No rebound insomnia (defined as mean LPS during placebo run-out that was equal to or worse than mean LPS at baseline) was observed during the single-blind, placebo run-out period (Figure 2). LPS did not return to baseline levels in either group. There was no significant difference between ramelteon and placebo in LPS.
Measures of Withdrawal
Baseline BWSQ scores (assessed at the end of Month 6) were similar between groups (ramelteon: 1.8; placebo: 1.4). There was no statistically significant difference between ramelteon and placebo in BWSQ scores on placebo run-out Day 2 (P = 0.711) or Day 3 (P = 0.679).
Safety
The overall incidence of adverse events was similar in the ramelteon (51.8%) and placebo groups (50.7%). The most frequently occurring adverse events are shown in Table 4. A total of 18 (10 in placebo group, 8 in ramelteon group) subjects discontinued treatment due to an adverse event. Seven subjects reported a total of 9 serious adverse events (2 in placebo group, 5 in ramelteon group) during the 6 months of this study; only leukopenia was considered to be possibly treatment related. A 64-year-old woman in the ramelteon group was diagnosed with leukopenia on Day 78 of treatment and was withdrawn from the study on Day 79. Blood levels were back to normal by Day 84, and no other clinical abnormalities were detected. The adverse event was considered to be possibly treatment related due to the appearance of symptoms during treatment and the resolution of symptoms upon drug discontinuation. No deaths were reported during this study. No clinically significant changes in vital signs or physical exams were reported.
Table 4.
Incidence of Adverse Events Occurring in more than 3% of Subjects
| Adverse Event | Placebo (n = 223) | Ramelteon (n = 228) |
|---|---|---|
| Headache | 18 (8.1) | 18 (7.9) |
| Upper respiratory tract infection | 6 (2.7) | 11 (4.8) |
| Nasopharyngitis | 11 (4.9) | 10 (4.4) |
| Urinary tract infection | 9 (4.0) | 9 (3.9) |
| Dizziness | 5 (2.2) | 7 (3.1) |
| Nausea | 8 (3.6) | 7 (3.1) |
Data are presented as number (%).
DISCUSSION
To date, other than this study, no long-term ( ≥ 6 months), polysomnographic, placebo-controlled studies of medications indicated for insomnia have been reported in adults with primary insomnia. A few long-term studies of BzRAs have evaluated subjective efficacy, but no objective parameters were measured.35–37 This lack of long-term, objective sleep studies is significant because subjective outcome measures have been shown to be unreliable relative to polysomnographic measures.29
On polysomnographic recordings, ramelteon reduced LPS from 70.75 minutes at baseline to 32.02 minutes at Week 1. Reductions of this magnitude were consistently observed at each subsequent visit over a period of 6 months, which implies sustained efficacy with no evidence of tolerance. The differences in LPS relative to placebo in the present study are comparable to those found with other sleep aids. The LPS mean difference from placebo was approximately 15 minutes at Week 1 and 9 minutes at Month 6. In a recent meta-analysis, the mean difference from placebo on polysomnographically recorded LPS with traditional BzRAs (eg, triazolam, temazepam, nitrazepam) was 10 minutes and with newer BzRAs (eg, zopiclone, zolpidem, eszopiclone, zaleplon) was 12.8 minutes.38
Clinical significance is difficult to determine in sleep studies, especially when the objectively measured differences from placebo are in the 10- to 15-minute range. Two indicators of clinically meaningful effect are either a 50% or greater improvement in the primary symptom or, specifically for insomnia studies, a reduction in LPS to at or near 30 minutes.39 In this study, the mean percentage change in LPS from baseline for subjects in the ramelteon group was greater than 50% for all time points, whereas the mean percentage change for subjects in the placebo group was consistently less than 50% improvement (Figure 2). Additionally, the mean LPS for subjects in the ramelteon group were at or near 30 minutes for all time points (Figure 1). This suggests that the reductions in LPS for ramelteon subjects in this study may be considered clinically meaningful based on current recommendations for assessing clinically significant effects.
Self-reported sleep latency showed results similar to polysomnographic recordings, with a statistically significant reduction of sSL at Weeks 1 and 4. Thereafter, the ramelteon treatment effect remained remarkably constant, although not all time points reached statistical significance when compared with placebo. No differences between ramelteon and placebo were observed on other subjective sleep parameters. It is noteworthy that this study was powered for polysomnography-derived sleep measures, (ie, it was not powered to detect differences on subjective measures of sleep). Generally, sSL does not show the same magnitude of improvement as do polysomnographic measures.40,41 The discrepancy between the polysomnography outcomes and those on self-reported measures may also be the result of sleep-time misperception.29,42 A number of studies have demonstrated that subjects tend to underestimate their total sleep time and overestimate their time to sleep onset.29,42,43
Overall, various sleep parameters improved considerably from baseline with ramelteon, although improvements from baseline were also observed in the placebo group. In this study, sleep latency decreased from baseline by approximately 30 minutes in the placebo group and 40 minutes in the ramelteon group at the 6-month visit. These improvements with placebo are comparable to those found in other sleep studies. In a placebo-controlled study of the BzRA eszopiclone in individuals with chronic insomnia, the placebo group showed a 30-minute decrease in sSL from baseline at 6 months.35 Similarly, studies of medications not indicated for the treatment of insomnia, the antidepressant trazodone44 and the herbal supplement valerian,45 have reported improvements in sleep under the placebo condition. An improvement on sleep parameters in placebo groups is common in sleep studies46 and may be due to a combination of the general placebo effect and improved sleep hygiene resulting from study participation.47 However, it should be noted that subjects in the ramelteon group demonstrated significant improvements in sleep latency over and above placebo at all time points.
At Week 1, TST measured by polysomnography was significantly increased in the ramelteon group compared with the placebo group; however, no significant differences were detected at any other time points. When sleep time was examined by sleep stage, subjects in the ramelteon group spent a greater percentage of TST in Stage 2 sleep and a lower percentage of TST in Stage 3/4 sleep. The changes in Stage 2 and 3/4 sleep are consistent with previous studies of ramelteon, although these changes are not likely to be clinically meaningful given the small magnitude of the change.25–27,48
No next-day effects were observed on measures of psychomotor function, immediate and delayed memory recall, level of alertness, and ability to concentrate over 6 months of treatment. The VAS for mood and feeling were similar between the ramelteon and placebo groups as well. The overall lack of next-day impairment with ramelteon is consistent with previous placebo-controlled studies of up to 35 days of ramelteon treatment in adults with chronic insomnia.24–27 These findings are in contrast to some studies of BzRAs, which achieve their effect through action at the α1 subunit of the GABAA receptor. Action at this subunit has been associated with retrograde amnesia.49–51 Many commonly prescribed sleep medications (eg, BzRAs, trazodone) are also associated with psychomotor and cognitive impairment.49,51–54
Ramelteon was well tolerated with an adverse-event profile similar to that of placebo, which is consistent with previous clinical studies. For instance, 2 long-term safety studies extensively evaluated the effects of ramelteon on numerous safety variables and found no clinically meaningful changes on a range of variables, including vital signs, findings on physical exam, clinical chemistry, hematology, urinalysis, and electrocardiogram trends.55,56 It is notable that this study also had a relatively low dropout rate, which may speak to tolerability associated with the use of ramelteon. Previous efficacy and safety studies with ramelteon have had low dropout rates, very similar to those of placebo.25,26
When subjects discontinued using ramelteon after 6 months of treatment, no rebound insomnia and no withdrawal effects were observed. However, there was no continuation of treatment effect upon discontinuation of ramelteon, and LPS and sSL were similar to those of placebo during the run-out period. These findings are in line with other clinical trials of ramelteon.24,26,27,57 In a study of substance abusers given ramelteon or the benzodiazepine triazolam, ramelteon did not demonstrate any potential for abuse liability, unlike triazolam.28 In contrast, BzRAs are controlled substances with the potential for abuse.5,58–60 For instance, in a 4-week study of zaleplon and zolpidem in adults with chronic insomnia, significant rebound and withdrawal effects were observed in the zolpidem group.61
A limitation of this study is that no subjective measures were recorded in the at-home setting, and the inclusion and exclusion criteria were quite strict. Together, these factors may limit the external validity of this study, although this limitation is true of all well-controlled clinical trials. Another limitation is that few assessments of daytime function or quality of life were done in this study. Impairment in daytime functioning is a common complaint in patients with insomnia (as reflected in the large percentage of subjects in this study reporting a decreased ability to function) and should be taken into account when treating the disease. However, this study focused primarily on the objective sleep-latency effects, and a more comprehensive analysis of improvements in daytime function is needed. Although sleep onset was consistently improved in the present study, a potential limitation of these data is that efficacy was only evaluated across 2 nights in the sleep laboratory, and, thus, no conclusion about the sustainability of these results in the at-home setting can be made from this study. Also, there was no active control group, such as a BzRA group or a melatonin group. Future studies using a head-to-head design will be particularly important when evaluating differences on measures of tolerance, rebound, and withdrawal. The ability to measure withdrawal was somewhat limited by the standard measure that is available (BWSQ). The BWSQ is validated based on a 1-week assessment and is intended to measure symptoms associated with the use of benzodiazepines. It has not been validated using a shorter assessment period or with an insomnia medication with a mechanism of action similar to that of ramelteon.
The mechanism of action of ramelteon is unlike that of any of the traditional sleep medications in which the hypnotic effect is mediated through the GABAA receptor. Ramelteon acts as an agonist at the MT1/MT2 melatonin receptors, which are not involved in general central nervous system sedation but, instead, influence the body's sleep-wake cycle. Activation of the MT1 and MT2 receptors in the SCN may both precipitate the acute hypnotic effects of ramelteon as well as shift the endogenous melatonin phase. Previous studies have shown that ramelteon is capable of inducing a significant phase advance.62,63 Melatonin studies have shown that the optimal timing for exogenous melatonin to cause a phase advance (based on the melatonin response curve) is during the afternoon or early evening.64,65 Based on these data, all of the phase-shift studies of ramelteon have administered medication in the afternoon. In addition, these studies have been in healthy adults with no evidence of sleep disorders. There have been no studies of the phase-shifting effects of ramelteon when given at bedtime in adults with insomnia. The effects of ramelteon in insomnia have been on time to sleep onset without determining the melatonin phase of the subjects. Although it is possible that the sleep-promoting effects of ramelteon are partly a result of phase shifts mediated by the MT2 receptor, it is also possible that sleep promotion is a result of the acute hypnotic effects of ramelteon via activation of the MT1 receptor. At the current time, there have not been enough studies of both the phase-shifting and sleep-promoting effects of ramelteon in adults with insomnia to establish the exact mechanisms responsible for its effects. It is possible that both pathways play a role in reducing the time to sleep onset.
In conclusion, in adults with chronic primary insomnia, long-term treatment with ramelteon, 8 mg, significantly reduced sleep onset at all measured time points over 6 months of nightly administration. There were no significant next-day residual effects and no rebound insomnia or withdrawal symptoms upon discontinuation. Ramelteon was well tolerated, with a low incidence of adverse events that was similar to that of placebo.
DISCLOSURE STATEMENT
This study was supported by the Takeda Pharmaceutical Company, Ltd. Dr. Mayer has participated in speaking engagements for Cephalon, UCB, and Sanofi-Aventis and has consulted for UCB. Dr. Wang-Weigand is an employee of Takeda. Dr. Roth-Schechter has consulted for TransOral and Takeda. Dr. Lehmann has participated in industry sponsored trials for Astellas, AstraZeneca, Gilead, GlaxoSmithKline, Grunenthal, Merck Sharp – Dohme, Novartis, Pfizer, Sanofi-Aventis, Takeda, UCB, Wyeth, Menarini, Almirall, and Johnson – Johnson. Dr. Staner is an employee of Forenap Pharma a contract research organization. Dr. Partinen has participated in speaking engagements for Boehringer Ingelheim, GlaxoSmithKline, Servier, UCB, and Organon.
ACKNOWLEDGMENTS
The authors would like to thank Maria-Antonia Quera-Salva and Ral Antic, MD, for their contributions to this study and Kelly Guerrettaz, MS, and Sara Sarkey, PhD, from Takeda Pharmaceuticals North America for their assistance with manuscript preparation.
REFERENCES
- 1.Drake C, Kryger M, Phillips B. National Sleep Foundation 2005 Sleep in America Poll. [Accessed February 4, 2008]. http//www.sleepfoundation.org/site/c.huIXKjM0IxF/b.2417353/ [Google Scholar]
- 2.Leger D, Poursain B. An international survey of insomnia: under-recognition and under-treatment of a polysymptomatic condition. Curr Med Res Opin. 2005;21:1785–92. doi: 10.1185/030079905X65637. [DOI] [PubMed] [Google Scholar]
- 3.NIH state-of-the-science conference statement on manifestations and management of chronic insomnia in adults. NIH Consens Sci Statements. 2005;22:1–30. [PubMed] [Google Scholar]
- 4.Roth T. Prevalence, associated risks, and treatment patterns of insomnia. J Clin Psychiatry. 2005;66:10–3. [PubMed] [Google Scholar]
- 5.New York, NY: Sanofi-Aventis Inc; 2007. Zolpidem [package insert] [Google Scholar]
- 6.Bristol, TN: King Pharmaceuticals, Inc; 2006. Zaleplon [package insert] [Google Scholar]
- 7.Marlborough, MA: Sepracor, Inc; 2007. Lunesta [package insert] [Google Scholar]
- 8.Bridgewater, NJ: Sanofi-Aventis, Inc; 2007. Zolpidem MR [package insert] [Google Scholar]
- 9.Barnstaple, UK: Actavis; 2007. Zopiclone [package insert] [Google Scholar]
- 10.Allain H, Bentue-Ferrer D, Tarral A, Gandon JM. Effects on postural oscillation and memory functions of a single dose of zolpidem 5 mg, zopiclone 3.75 mg and lormetazepam 1 mg in elderly healthy subjects. A randomized, cross-over, double-blind study versus placebo. Eur J Clin Pharmacol. 2003;59:179–88. doi: 10.1007/s00228-003-0591-5. [DOI] [PubMed] [Google Scholar]
- 11.Rush CR, Frey JM, Griffiths RR. Zaleplon and triazolam in humans: acute behavioral effects and abuse potential. Psychopharmacology. 1999;145:39–51. doi: 10.1007/s002130051030. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66:31–41. [PubMed] [Google Scholar]
- 13.Mann K, Bauer H, Hiemke C, Roschke J, Wetzel H, Benkert O. Acute, subchronic and discontinuation effects of zopiclone on sleep EEG and nocturnal melatonin secretion. Eur Neuropsychopharmacol. 1996;6:163–8. doi: 10.1016/0924-977x(96)00014-4. [DOI] [PubMed] [Google Scholar]
- 14.Hedner J, Yaeche R, Emilien G, Farr I, Salinas E. Zaleplon shortens subjective sleep latency and improves subjective sleep quality in elderly patients with insomnia. The Zaleplon Clinical Investigator Study Group. Int J Geriatr Psychiatry. 2000;15:704–12. doi: 10.1002/1099-1166(200008)15:8<704::aid-gps183>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469–76. doi: 10.4088/jcp.v66n0409. [DOI] [PubMed] [Google Scholar]
- 16.Taibi DM, Landis CA, Petry H, Vitiello MV. A systematic review of valerian as a sleep aid: safe but not effective. Sleep Med. 2007;11:209–30. doi: 10.1016/j.smrv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Richardson GS. The human circadian system in normal and disordered sleep. J Clin Psychiatry. 2005;66:3–9. [PubMed] [Google Scholar]
- 18.Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 19.Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–20. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- 20.Jin X, von Gall C, Pieschl RL, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003;23:1054–60. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–62. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- 22.Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl 3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Kato K, Hirai K, Nishiyama K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;48:301–10. doi: 10.1016/j.neuropharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Roth T, Seiden D, Sainati S, Wang-Weigand S, Zhang J, Zee P. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7:312–8. doi: 10.1016/j.sleep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Roth T, Seiden D, Wang-Weigand S, Zhang J. A 2-night, 3-period, crossover study of ramelteon's efficacy and safety in older adults with chronic insomnia. Curr Med Res Opin. 2007;23:1005–14. doi: 10.1185/030079907x178874. [DOI] [PubMed] [Google Scholar]
- 27.Zammit G, Erman M, Wang-Weigand S, Sainati S, Zhang J, Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med. 2007;3:495–504. [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch Gen Psychiatry. 2006;63:1149–57. doi: 10.1001/archpsyc.63.10.1149. [DOI] [PubMed] [Google Scholar]
- 29.Silva GE, Goodwin JL, Sherrill DL, et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler D. WAIS-R manual: Wechsler Adult Intelligence Scale-revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 31.Folstein MF, Luria R. Reliability, validity, and clinical application of the visual analog mood scale. Psychol Med. 1973;3:479–86. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- 32.Zisapel N, Nir T. Determination of the minimal clinically significant difference on a patient visual analog sleep quality scale. J Sleep Res. 2003;12:291–8. doi: 10.1046/j.0962-1105.2003.00365.x. [DOI] [PubMed] [Google Scholar]
- 33.Gift AG. Visual analog scales: measurement of subjective phenomena. Nursing Research. 1989;38:286–8. [PubMed] [Google Scholar]
- 34.Tyrer P, Murphy S, Riley P. The Benzodiazepine Withdrawal Symptom Questionnaire. J Affect Disord. 1990;19:53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 35.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 36.Erman M, Krystal A, Zammit G, Soubrane C, Roth T. Long-term efficacy of zolpidem extended release in the treatment of sleep maintenance and sleep onset insomnia with improvements in next-day functioning. Sleep. 2007;30:A241–2. [Google Scholar]
- 37.Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959–68. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–50. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morin CM, Wooten V. Psychological and pharmacological approaches to treating insomnia: Critical issues in assessing their separate and combined effects. Clin Psychol Rev. 1996;16:521–42. [Google Scholar]
- 40.Bastien CH, Fortier-Brochu E, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. J Psychosom Res. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 41.Roth T, Soubrane C, Titeux L, Walsh JK. Efficacy and safety of zolpidem-MR: a double-blind, placebo-controlled study in adults with primary insomnia. Sleep Med. 2006;7:397–406. doi: 10.1016/j.sleep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995;18:232–9. doi: 10.1093/sleep/18.4.232. [DOI] [PubMed] [Google Scholar]
- 43.McCall WV, Turpin E, Reboussin D, Edinger JD, Haponik EF. Subjective estimates of sleep differ from polysomnographic measurements in obstructive sleep apnea patients. Sleep. 1995;18:646–50. doi: 10.1093/sleep/18.8.646. [DOI] [PubMed] [Google Scholar]
- 44.Walsh JK, Erman M, Erwin CW, et al. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol. 1998;13:191–8. [Google Scholar]
- 45.Jacobs BP, Bent S, Tice JA, Blackwell T, Cummings SR. An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine (Baltimore) 2005;84:197–207. doi: 10.1097/01.md.0000172299.72364.95. [DOI] [PubMed] [Google Scholar]
- 46.McCall WV, D'Agostino R, Jr, Dunn A. A meta-analysis of sleep changes associated with placebo in hypnotic clinical trials. Sleep Med. 2003;4:57–62. doi: 10.1016/s1389-9457(02)00242-3. [DOI] [PubMed] [Google Scholar]
- 47.Miller FG, Rosenstein DL. The nature and power of the placebo effect. J Clin Epidemiol. 2006;59:331–5. doi: 10.1016/j.jclinepi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Kryger M, Wang-Weigand S, Roth T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep Breath. 2007;11:159–64. doi: 10.1007/s11325-006-0096-4. [DOI] [PubMed] [Google Scholar]
- 49.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169–73. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbee JG. Memory, benzodiazepines, and anxiety: integration of theoretical and clinical perspectives. J Clin Psychiatry. 1993;54:86–97. [PubMed] [Google Scholar]
- 51.O'Hanlon JF. Residual effects on memory and psychomotor performance of zaleplon and other hypnotic drugs. Prim Care Companion J Clin Psychiatry. 2002;4:38–44. [Google Scholar]
- 52.Rush CR, Baker RW, Wright K. Acute behavioral effects and abuse potential of trazodone, zolpidem and triazolam in humans. Psychopharmacology. 1999;144:220–33. doi: 10.1007/s002130050997. [DOI] [PubMed] [Google Scholar]
- 53.Roehrs T, Merlotti L, Zorick F, Roth T. Sedative, memory, and performance effects of hypnotics. Psychopharmacology. 1994;116:130–4. doi: 10.1007/BF02245054. [DOI] [PubMed] [Google Scholar]
- 54.Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. J Psychopharmacol. 1999;13:18–31. doi: 10.1177/026988119901300103. [DOI] [PubMed] [Google Scholar]
- 55.Richardson G, Wang-Weigand S, Zhang J, DeMicco M. Long-term safety of ramelteon treatment in adults with chronic insomnia: A 1-year study. Sleep. 2006;29:A233. [Google Scholar]
- 56.Richardson GS, Wang-Weigand S, Sainati S, Demissie S. Long-term effects of ramelteon on endocrine function in patients with chronic insomnia in a double-blind, placebo-controlled phase III study. Clin Pharmacol Ther. 2006;79:P68. [Google Scholar]
- 57.DeMicco M, Wang-Weigand S, Zhang J. Long-term therapeutic effects of ramelteon treatment in adults with chronic insomnia: a 1-year study. Sleep. 2006;29(Suppl):A234. [Google Scholar]
- 58.Bristol, TN: King Pharmaceuticals Inc; 2003. Sonata [package insert] [Google Scholar]
- 59.Eszopiclone (Lunesta), a new hypnotic. Med Lett Drugs Ther. 2005;47:17–9. [PubMed] [Google Scholar]
- 60.Ambien CR for insomnia. Med Lett Drugs Ther. 2005;47:97–98. [PubMed] [Google Scholar]
- 61.Elie R, Ruther E, Farr I, Emilien G, Salinas E. Sleep latency is shortened during 4 weeks of treatment with zaleplon, a novel nonbenzodiazepine hypnotic. J Clin Psychiatry. 1999;60:536–44. doi: 10.4088/jcp.v60n0806. [DOI] [PubMed] [Google Scholar]
- 62.Richardson GS, Zee P, Wang-Weigand S, Rodriguez L, Peng X. Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J Clin Sleep Med. 2008;4:456–61. [PMC free article] [PubMed] [Google Scholar]
- 63.Richardson G, Zammit G, Rodriguez L, Zhang J. Evaluation of circadian phase-shifting effects of ramelteon in healthy subjects. Chronobiol Int. 2005;22:1271–2. [Google Scholar]
- 64.Lewy A, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–92. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 65.Lewy AJ, Ahmed S, Sack RL. Phase shifting the human circadian clock using melatonin. Behav Brain Res. 1996;73:131–4. doi: 10.1016/0166-4328(96)00084-8. [DOI] [PubMed] [Google Scholar]