Abstract
Study Objectives:
Many patients with obstructive sleep apnea (OSA) have spontaneous periods of stable flow limited breathing during sleep without respiratory events or arousals. In addition, OSA is often more severe during REM than NREM and more severe during stage 2 than slow wave sleep (SWS). The physiological mechanisms for these observations are unknown. Thus we aimed to determine whether the activity of two upper airway dilator muscles (genioglossus and tensor palatini) or end-expiratory lung volume (EELV) differ between (1) spontaneously occurring stable and cyclical breathing and (2) different sleep stages in OSA.
Design:
Physiologic observation.
Setting:
Sleep physiology laboratory.
Study Participants:
15 OSA patients with documented periods of spontaneous stable breathing.
Intervention:
Subjects were instrumented with intramuscular electrodes for genioglossus and tensor palatini electromyograms (EMGGG and EMGTP), chest and abdominal magnetometers (EELV measurement), an epiglottic pressure catheter (respiratory effort), and a mask and pneumotachograph (airflow/ventilation). Patients slept supine overnight without CPAP.
Measurements and Results:
Peak and Tonic EMGGG were significantly lower during cyclical (85.4 ± 2.7 and 94.6 ± 4.7 % total activity) than stable breathing (109.4 ± 0.4 and 103 ± 0.8 % total activity, respectively). During respiratory events in REM, tonic EMGGG activity was lower than during respiratory events in stage 2 (71.9 ± 5.1 and 119.6 ± 5.6 % total activity). EMGGG did not differ between stable stage 2 and stable SWS (98.9 ± 3.2 versus 109.7 ± 4.4 % total activity), nor did EMGTP or EELV differ in any breathing condition/sleep stage.
Conclusions:
Increased genioglossus muscle tone is associated with spontaneous periods of stable flow limited breathing in the OSA subjects studied. Reductions in genioglossus activity during REM may explain the higher severity of OSA in that stage. Increased lung volume and tensor palatini activity do not appear to be major mechanisms enabling spontaneous stable flow limited breathing periods.
Citation:
Jordan AS; White DP; Lo YL; Wellman A; Eckert DJ; Yim-Yeh S; Eikermann M; Smith SA; Stevenson KE; Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. SLEEP 2009;32(3):361–368.
Keywords: Genioglossus, tensor palatini, respiratory events, REM sleep, slow wave sleep
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON DISORDER, CHARACTERIZED BY REPETITIVE UPPER AIRWAY COLLAPSE DURING SLEEP, WHICH HAS IMPORTANT consequences, including increased risk for car accidents1 and cardiovascular disease.2,3 Upper airway collapse in OSA is thought to occur at sleep onset because of the reduction of activity of several upper airway dilator muscles, which then do not hold the anatomically vulnerable airway open. However, some upper airway dilator muscles, including the genioglossus, can increase activity during sleep in response to respiratory stimuli,4–6 potentially counteracting some of these changes at sleep onset.
The severity of OSA varies throughout the night and between sleep stages. Generally, obstructive respiratory events are more common and longer in REM than NREM sleep. In contrast, respiratory events are uncommon during slow wave sleep (SWS). Younes reported that most patients with OSA have periods of sleep which are free from respiratory events during the night, although these stable breathing periods are likely accompanied with snoring and flow limitation.7 The mechanisms that enable stable breathing to occur spontaneously during sleep in OSA are currently unknown. However, these are of interest because therapies designed to augment these mechanisms may provide an alternative to CPAP.
Previous studies have indicated that increasing either end-expiratory lung volume (EELV) or dilator muscle activity during sleep reduces airway collapsibility/apnea severity.8–13 Despite the well-recognized effects of manipulating these variables on airway collapsibility, it is not known whether spontaneous changes in either of these variables are what enables periods of stable breathing to occur in OSA patients. Therefore we aimed to conduct an observational study of muscle activity and EELV during spontaneously occurring stable and cyclical breathing in OSA. We recognized that this study would, by design, show only associations and not causality. However, before a study can be designed to assess causality, we first need to determine which of these factors, if any, are altered during stable breathing.
Thus the primary aim of this study was to determine whether the activity of 2 upper airway dilator muscles, the genioglossus (EMGGG) and tensor palatini (EMGTP), or end-expiratory lung volume differ between spontaneous periods of stable and cyclical breathing in OSA. In addition, we sought to determine whether sleep stage (SWS or REM) influenced muscle activity and lung volume independent of respiratory events. We hypothesized that: (1) muscle activity and/or lung volume would be increased during stable breathing when compared to cyclical breathing; (2) respiratory events during REM sleep would be associated with lower muscle activity and/or lung volume than respiratory events during stage 2; and (3) muscle activity would be similar during stable SWS compared to stable stage 2 sleep, but higher in SWS than when all stage 2 sleep is considered. Some of the results of this study have been previously reported in the form of abstracts.14,15
METHODS
Subjects
Fifteen patients with previously diagnosed OSA (AHI > 15) who had documented periods of stable breathing without respiratory events during supine sleep on diagnostic polysomnography participated in the study after giving informed consent. All patients were non-smokers and were free of known cardiorespiratory disease and sleep disorders other than OSA.
Procedures
Patients arrived at the laboratory 2 h before their habitual bedtime and were instrumented with electroencephalogram (C3-A1, Oz-A2), submental electromyogram (EMG), left and right electro-oculogram, and electrocardiogram with surface electrodes. Epiglottic pressure was measured as previously described16 with a pressure-tipped catheter placed after decongestion (0.05% oxymetazoline HCl) and topical anaesthesia (4% lidocaine HCl). The EMG of the tensor palatini (EMGTP) and genioglossus (EMGGG) were recorded with intramuscular fine-wire electrodes as previously described.5,17 Subjects were fitted with a mask (nasal or full face) and pneumotachograph with differential pressure transducer for measurement of airflow and calculation of ventilation. End tidal CO2 (PETCO2) was also monitored from one nostril and arterial oxygen saturation (SpO2) measured with finger pulse oximetry. Magnetometer coils (EOL Eberhard, Oberwil, Switzerland) were placed on the chest (mid-sternum) and abdomen (umbilicus) and were calibrated over a range of tidal volumes against the pneumotachograph calculated volume. Following instrumentation and recording of 5 min of wakefulness, subjects were allowed to fall asleep without CPAP. Subjects were asked to remain in the supine position for the duration of the study (confirmed with video monitoring). A minimum of 4 h of data were recorded with Spike2 acquisition software (1401plus, CED, UK) sampled at 1000 Hz (EMGGG and EMGTP) or 250 Hz (all other signals).
Data Analysis
Sleep studies were staged in the standard manner18,19 by a registered technician with airflow/tidal volume used in place of nasal pressure/ thermistor and epiglottic pressure used for respiratory effort. The technician was blinded to EMGGG, EMGTP and lung volume signals during staging. Periods of stable breathing were identified as > 3 min periods of sleep without any respiratory events or arousal from sleep. Snoring/flow limitation did not preclude inclusion into stable breathing periods. Periods of cyclical breathing were identified as > 3 min periods with ≥ 1 obstructive apnea/hypopnea per min. When multiple > 3 min periods of data were available for analysis, the data periods closest temporally were analyzed. During respiratory events, all data were averaged on the breath prior to the respiratory event (Prior), the first 3 (+1 to +3) and last 3 (−3 to −1) respiratory efforts during the event and on the first breath after termination of the event (Post). During stable breathing each breath was numbered from 1 to 8 and all data averaged by breath number such that similar comparisons to cyclical breathing were possible. In addition, the epiglottic pressure was plotted against the EMGGG for every breath during sleep in a given sleep stage/breathing condition (cyclical or stable breathing) and the slope and intercept of the relationship determined for each individual. This allowed assessment of the responsiveness of the genioglossus to negative pressure in the different sleep stages/conditions.
Both EMGGG and EMGTP were initially expressed as a percent of the highest activity observed during swallowing, tongue protrusion, sniffing or deep breaths. However, the baseline level of muscle activity varied greatly between subjects (Peak EMGGG ranged from 3.9 to 38.2 % max); therefore, each subject's muscle activity was expressed as a percent of his/her individual average level across all 16 breaths (pre, +1 to +3, −3 to −1, and post × 2 conditions) for each analysis type (stable versus cyclical breathing for example). Because genioglossus activity varies with respiratory phase, both the peak inspiratory (Peak) and expiratory minimum (Tonic) are presented.
Statistical Analyses:
A two-way repeated-measures ANOVA was used to assess the primary aim (that muscle activity and lung volume differ between stable and cyclical breathing). Repeated-measures ANOVAs were also used to compare data between respiratory events in REM and NREM. Comparisons of EMGGG-PEPI relationships and stage 2 versus SWS were performed using paired Student's t-tests. Means (SEM) are presented; P < 0.05 was considered significant. Dunn-Sidak corrected post hoc tests were used where significant main effects were found.
RESULTS
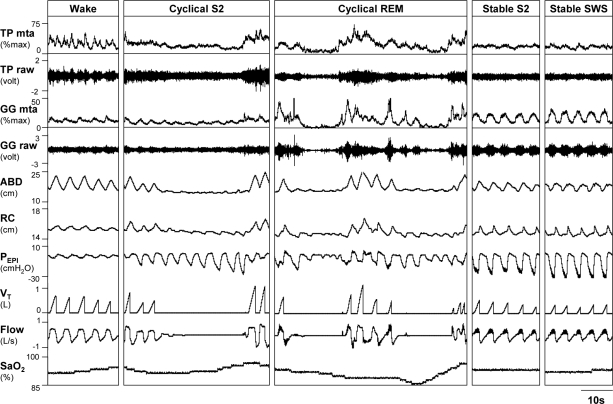
The patient characteristics and sleep study results from the night in the research laboratory are presented in Table 1. In general subjects slept well, with a sleep efficiency of approximately 80% for > 4 h of sleep. A total of 900 respiratory events (average 60 ± 9 events/subject) and equivalent time periods of stable breathing were analyzed. One subject had large mandibular tori preventing standard placement of genioglossus electrodes. Therefore only tensor palatini muscle activity is presented in this subject. One of the tensor palatini electrodes was dislodged during the overnight study in 3 subjects. As the precise time at which the electrode was displaced is not known, all tensor palatini data were discarded in these subjects. An example of the raw data during the different sleep stages and breathing conditions from a single subject is provided in Figure 1.
Table 1.
Patient Characteristics and PSG Findings on Study Night
| Mean ± SEM | Range | |
|---|---|---|
| Age (y) | 42.5 ± 2.6 | 24–56 |
| BMI (kg/m2) | 36.4 ± 2.2 | 27.3–58.4 |
| Male : Female | 10 : 5 | |
| Total sleep time (min) | 258 ± 10 | 200–307 |
| Sleep efficiency (%) | 79 ± 3 | 55–95 |
| NREM sleep time (min) | 240 ± 10 | 188–303 |
| REM sleep time (min) | 17 ± 3 | 3–31 |
| Total AHI (events/h) | 47.7 ± 6.0 | 12.5–84.7 |
| NREM AHI (events/h) | 46.7 ± 6.4 | 4.6–83.4 |
| REM AHI (events/h) | 63.1 ± 6.7 | 20–108 |
| OAI (events/h) | 13.5 ± 4.9 | 0–68 |
| HI (events/h) | 32.1 ± 4.5 | 7–59 |
Definitions of abbreviations: BMI = body mass index; AHI = apnea hypopnea index; OAI = obstructive apnea index; HI = hypopnea index.
Figure 1.
Raw data from a 34 year old male patient with OSA (AHI = 57 events/h) during wakefulness, cyclical stage 2 (S2), cyclical REM, stable stage 2 (S2), and stable slow wave sleep (SWS). Note the elevated raw and moving time averaged (MTA) activity of the genioglossus (GG) but not tensor palatini (TP) during stable breathing, which is flow limited and accompanied by snoring (high frequency oscillation visible on Flow and PEPI during inspiration). Also note the unchanged rib cage (RC) and abdominal (ABD) positions and large negative epiglottic pressures (PEPI) generated. Airflow (Flow), tidal volume (VT), and arterial oxygen saturation (SpO2) indicate where respiratory events occur and the desaturation associated with the events.
Stable Stage 2 Versus Cyclical Stage 2 Sleep (n = 10)
Four subjects did not have any periods of stable breathing without respiratory events in any sleep stage and another subject only had respiratory events during REM sleep. Thus, the comparison of stable to cyclical stage 2 sleep is limited to 10 subjects (n = 9 for EMGGG and n = 8 for EMGTP). An average of 55 ± 12 respiratory events and 15 ± 2 min of stable breathing were analyzed in each subject.
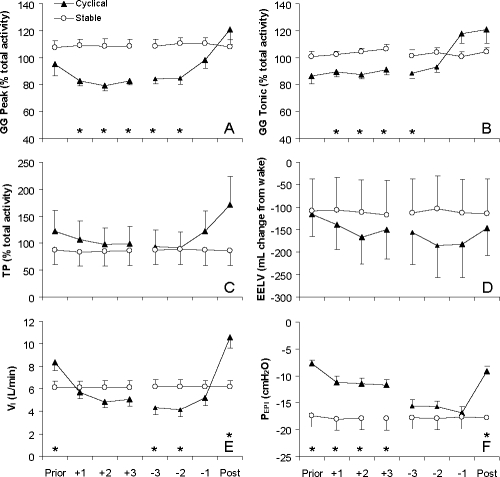
Both Peak and Tonic EMGGG were higher during periods of stable breathing than during respiratory events in stage 2 sleep (Figure 2A, B) although this difference no longer persisted by the termination of the respiratory event (effort −1). EMGTP and EELV were more variable and were not significantly different between conditions (Figure 2C, D). VI (Figure 2E) and VT/TI both fell rapidly at the onset of the respiratory event and continued to decline throughout the respiratory event before increasing above the stable level on termination of the respiratory event. PEPI decreased significantly during respiratory events and these changes were reversed on the first post-event breath (Figure 2F) while PEPI was consistently low during stable breathing periods indicating high respiratory drive and flow limitation. The slope and intercept of the relationship between Peak EMGGG and PEPI were not significantly different between stable (−0.25 ± 0.1 % max/cm H2O and 9.6 ± 3.3 % max) and cyclical (−0.31 ± 0.1 % max/cm H2O and 7.9 ± 2.6 % max) breathing.
Figure 2.
Peak inspiratory (Peak) and expiratory tonic (Tonic) activity of the genioglossus (GG), tensor palatini (TP), end-expiratory lung volume (EELV), inspired minute ventilation (VI) and epiglottic pressure (PEPI) on the breath before (Prior) respiratory events, first (+1 to +3) and last (−3 to −1) 3 respiratory efforts during events and breath following (Post) respiratory events in stage 2 sleep and corresponding time periods (8 breath averages) of stable stage 2 sleep. Muscle activity is expressed as a percent of the average activity across both states in each subject (% total activity) due to high variability in the absolute level of muscle activity between subjects (range 3.9 to 38.2 % max). Mean (SEM) presented, n = 10. *indicates P < 0.05 between cyclical and stable conditions on these breaths/efforts by post hoc testing.
Cyclical Stage 2 Versus Cyclical REM Sleep (n = 12)
Two subjects did not have > 3 min periods of REM sleep and one subject did not have any sleep disordered breathing in NREM. Thus, this analysis is limited to 12 subjects. An average of 18 ± 3 respiratory events in REM sleep were compared to an average of 50 ± 10 events in stage 2 sleep.
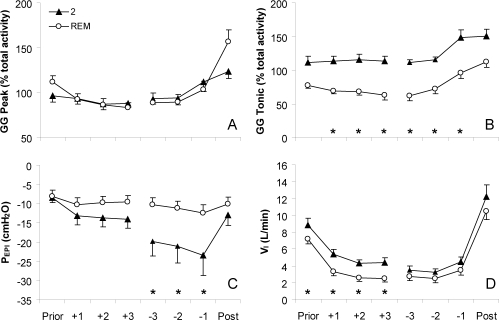
Peak EMGGG (Figure 3A), EMGTP, and EELV were not significantly different between respiratory events in stage 2 or REM sleep. However, Tonic EMGGG was significantly lower during events in REM sleep than in stage 2 (Figure 3B). PEPI reached significantly lower levels during respiratory events in stage 2 than REM sleep (Figure 3E), and VT and VI (Figure 3F) were lower in REM than stage 2. The slope and intercept of the Peak EMGGG versus PEPI relationship was not different between stage 2 and REM sleep (slope: −0.33 ± 0.1 and −0.21 ± 0.1 % max/cm H2O, respectively; intercept: 8.4 ± 2.4 and 9.5 ± 2.3 % max, respectively).
Figure 3.
Peak inspiratory (Peak) and expiratory tonic (Tonic) activity of the genioglossus (GG), epiglottic pressure (PEPI) and inspired minute ventilation (VI) on the breath before (Prior) respiratory events, first (+1 to +3) and last ( −3 to −1) 3 respiratory efforts during events and breath following (Post) respiratory events in stage 2 and REM sleep. Muscle activity is expressed as a percent of the average activity across both states in each subject due to high variability in the absolute level of muscle activity between subjects (range 3.7 to 32.8 % max). Mean (SEM) presented, n = 12. *indicates P < 0.05 between sleep stages on these breaths/efforts by post hoc testing.
Stage 2 Versus Stable SWS (n = 8)
Only 8 subjects reached SWS during the study. Therefore this analysis is limited to these 8 subjects. An average of 18.5 ± 3.9 min of data in stable stage 2 sleep was compared to 22.7 ± 5.3 min in SWS.
Respiratory and muscle activity data are presented in Table 2. Neither EMGGG nor EMGTP differed between stage 2 or SWS when the patients were able to spontaneously overcome their airway obstruction (stable breathing). Stable breathing periods were accompanied by flow limitation and/or snoring in both sleep stages. There were small differences in PEPI, VT and VT/TI between SWS and stable stage 2. However, FB and VI were not different between stages. The slope and intercepts of the relationship between Peak EMGGG and PEPI were not significantly different between stable stage 2 and SWS (slope: −0.21 ± 0.1 and −0.36 ± 0.2 % max/cm H2O respectively; intercept: 8.1 ± 4.1 and 5.0 ± 1.1 % max, respectively).
Table 2.
Muscle Activity, Lung Volume, and Ventilatory Parameters During Stable Stage 2, Stable SWS, and in All Stage 2 Sleep (Cyclical and Stable Breathing Combined)
| Stable Stage 2 | Slow Wave Sleep | All stage 2 | |
|---|---|---|---|
| EMGGG Peak (% total activity) | 99 ± 3.2 | 109.7 ± 4.1 | 91.4 ± 1.9* |
| EMGGG Tonic (% total activity) | 98.5 ± 3.4 | 105.5 ± 6.2 | 96.1 ± 3.1 |
| EMGTP (% total activity) | 86.9 ± 6.9 | 92.0 ± 17.1 | 121.1 ± 19.2 |
| EELV (mL change from wake) | −24.2 ± 144.2 | −161.6 ± 105.3 | −82.9 ± 87.6 |
| VT (L) | 0.40 ± 0.06* | 0.38 ± 0.06 | 0.36 ± 0.06 |
| FB (breaths/min) | 16.4 ± 0.7 | 16.9 ± 0.7 | 16.3 ± 0.7 |
| VI (L/min) | 6.2 ± 0.8 | 6.1 ± 0.7 | 5.7 ± 0.8 |
| VT/TI (L/sec) | 0.24 ± 0.03* | 0.22 ± 0.03 | 0.21 ± 0.04 |
| PEPI (cm H2O) | −18.1 ± 2.5* | −21.8 ± 2.9 | −17.2 ± 1.7* |
| PETCO2 (mm Hg) | 42.5 ± 1.5 | 42.4 ± 1.7 | n/a |
Definitions of abbreviations: EMGGG = genioglossus muscle activity; EMGTP = tensor palatini muscle activity (both expressed as a % of the average level across all conditions); EELV = end-expiratory lung volume; VT = tidal volume; FB = Frequency of breathing; VI = inspired minute ventilation; VT/TI = mean inspiratory flow; PEPI = minimum epiglottic pressure during inspiration; PETCO2 = end tidal partial pressure of carbon dioxide. PETCO2 is not given in all stage 2 sleep because it can not accurately be measured during apneas/hypopneas. Means ± SEM,
P < 0.05 compared to SWS.
When all stage 2 sleep was considered (average of stable and cyclical breathing while in stage 2 sleep) the Peak EMGGG was found to be lower than in SWS (Table 2). Correspondingly, PEPI was significantly more negative during SWS than all stage 2 sleep. However, no other variables differed significantly.
Exploratory Analysis: Patients Who Failed to Develop Stable Breathing
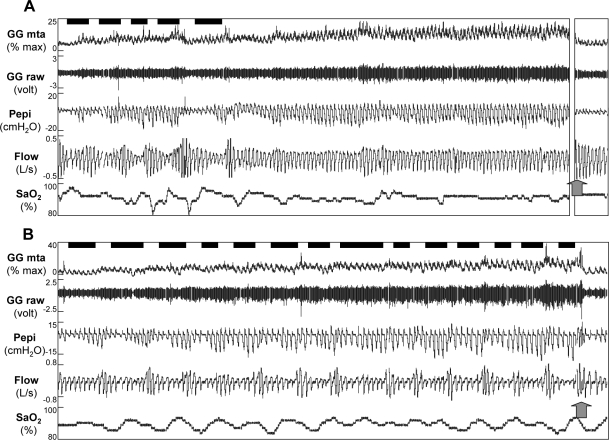
Four patients did not have any > 3 min periods of stable breathing in any sleep stage, despite having documented periods of stable sleep on their clinical sleep studies. These four patients were all male, were similarly aged to the subjects who could achieve stable breathing (44 ± 7 and 42 ± 3 years, P = 0.8 respectively), but had lower body mass indices (30.7 ± 1.4 and 38.5 ± 2.6 kg/m2, P = 0.02 respectively). As expected, they had more severe OSA on the night in the laboratory (70.1 ± 5.8 and 39.5 ± 6.3 events/h, P = 0.005) despite having similar AHIs when in the supine position during the clinical study (37.9 ± 5.3 and 33.3 ± 2.9 events/h, P = 0.5, respectively). EMGGG increased similarly across respiratory events in these patients and the relationship between PEPI-EMGGG was similar to those subjects who were able to achieve stable breathing periods (slope: −0.39 ± 0.22 and −0.28 ± 0.08 % max/cm H2O, respectively, P = 0.7; intercept: 8.3 ± 3.9 and 8.1 ± 2.7 % max, respectively, P = 0.9). An example of the activation of the genioglossus across respiratory events in 2 patients, one with, and one without stable breathing is shown in Figure 4.
Figure 4.
Raw data for 10 min after sleep onset in a 45-year-old man with OSA (AHI = 57.1 events/h) who developed stable breathing (A) and a 49 year old male patient (AHI = 69.7 events/h) who did not develop stable breathing (B). Arterial oxygen saturation (SpO2), airflow (Flow), epiglottic pressure (PEPI), and both the raw and moving time averaged (MTA) genioglossus (GG) electromyogram are shown. Respiratory events marked by the technician are shown by black bars above the GG signal. Note that both individuals had increased GG activity as sleep progressed. However, only subject A was able to stabilize breathing (although still flow limited). Following full awakening (as shown by the arrow), genioglossus activity instantly fell to baseline in both subjects. In Subject A, 6.5 min of data are omitted prior to full awakening (broken axis), such that 10 min of data are shown for both individuals.
DISCUSSION
The main findings of this study are that when patients with OSA spontaneously overcome their tendency for airway collapse and have stable breathing during sleep, the genioglossus muscle is more active than during disordered breathing events. In contrast end-expiratory lung volume and the activity of the tensor palatini were not different between cyclical and stable breathing. In addition, during respiratory events in REM sleep, the tonic activity of the genioglossus muscle was even further reduced below the level which occurred during respiratory events in stage 2. When patients were able to achieve stable SWS there were no differences in muscle activity or lung volume compared to stable stage 2 sleep. However, genioglossus muscle activity was elevated compared to all of stage 2 sleep (when cyclical and stable breathing during stage 2 were averaged). Finally, despite activation of the genioglossus muscle, not all subjects were able to stabilize their breathing during sleep. It should be noted that the breaths analyzed in the stable breathing condition were invariably flow limited and often accompanied by snoring.
It is important to recognize that this study has only demonstrated associations between sleep stages/breathing conditions and muscle activity and these data do not prove causality. However, electrical activation of the genioglossus muscle or hypoglossal nerve is known to dilate the retroglossal airway12 and reduce the pharyngeal critical closing pressure11 in humans. Thus, it would appear likely that the increased genioglossus muscle activity is playing a causal role in contributing to the sleep stage and time of night differences in the severity of OSA.
From the data collected, we are unable to determine why the genioglossus muscle becomes active at some times of the night. However, prior research has shown that the genioglossus is activated by chemoreceptor stimulation4,5,20 and by reflex activation in response to negative pressure.21,22 There is also evidence to suggest that the genioglossus receives an independent stimulation during wakefulness which is lost at sleep onset and is known as the “wakefulness stimulus”.23–25 Unfortunately PETCO2 could not be accurately determined during apneas and hypopneas because the expired volume is often less than the anatomical dead space and thus the end expiratory gas is not representative of alveolar gas. It is therefore unknown whether the genioglossus received greater chemoreceptor input during stable versus unstable breathing. However, we do recognize that some CO2 accumulation during flow limitation is quite likely. Interestingly, the genioglossus-epiglottic pressure relationship was not different between any of the sleep stages or breathing conditions. This finding implies that genioglossus regulation is not different between sleep stages but raises the interesting possibility that periods of high respiratory drive (large negative epiglottic pressures), which are generally considered to predispose to airway collapse, may in fact, not be detrimental to airway patency if the genioglossus muscle activity is correspondingly high. This concept suggests that a low arousal threshold (arouse at low levels of negative epiglottic pressure) would predispose to OSA as periods of sleep with high negative epiglottic pressures and genioglossal activity would not occur. That a low arousal threshold may be a pathogenic mechanism responsible for OSA has recently been emphasized by Younes et al.26 but clearly requires further testing. We did not systematically measure the arousal threshold in this study. However, the arousal threshold is often measured as the epiglottic pressure on the breath prior to arousal from sleep. To the extent that the last breath during obstruction is often the breath prior to an arousal we can estimate that the arousal threshold in the unstable breathing condition is approximately −17 cm H2O (Figure 2F breath −1). Thus, during stable breathing the subjects are sleeping without arousal at approximately this same level of epiglottic pressure indicating the arousal threshold may indeed be elevated during these stable periods as compared to unstable ones. Clearly this hypothesis requires further testing before firm conclusions can be drawn.
An alternative explanation for the progressive activation of genioglossal activity and increase in drive (more negative epiglottic pressures) after sleep onset (Figure 4) is that the hypoxemia/mild hypercapnia associated with the respiratory events may be activating long-term facilitation of the diaphragm and genioglossus muscles. Long-term facilitation (LTF) is a form of neural memory which is evoked in the phrenic and hypoglossal nerves of experimental animals by multiple, short episodes of hypoxia.27 In awake humans, LTF has been more difficult to elicit.28,29 However, with mild hypercapnia30 or during snoring/flow limitation during sleep31,32 LTF has been reported. The protocols used to elicit LTF in humans typically involve several min of hypoxia (nadir SpO2 ~85%) repeated 10 times. Thus, whether shorter episodes of hypoxemia, such as those occurring during apneas and hypopneas, are sufficient to elicit LTF in OSA remains to be tested. However, although genioglossal activity clearly increased across respiratory events in the majority of subjects, this activation occurred concurrently with more negative epiglottic pressures and disappeared immediately on full awakening from sleep in every subject (arrows in Figure 4). Thus, it would appear unlikely that the increased genioglossus activity observed in this study is a result of a long-term neural memory such as LTF.
The concept that large negative epiglottic pressures may not be bad for airway function when accompanied by concurrent high activity of the genioglossus relies on the assumption that increasing electrical activity of the genioglossus corresponds to muscle contraction/movement and airway stiffening/dilation. It is possible that the 4 individuals who failed to develop stable breathing during sleep either have muscle physiology or airway anatomy such that significant airway dilation was not achieved with muscle activation. Alternatively, the inability of the genioglossus to cause airway dilation may be related to other factors. For example, although the changes in end-expiratory lung volume were not statistically significant, there was an approximate 100 mL decrease in lung volume across respiratory events which may be physiologically important (at least in some individuals) and result in the genioglossus muscle activity being relatively ineffective at dilating the airway. In summary, we believe that increased genioglossus activity is likely an important mechanism by which patients with OSA can prevent airway collapse, but that there are likely several other factors determining airway collapse (e.g., lung volume, anatomical predisposition), and thus high genioglossus muscle activity alone may not be sufficient to enable stable breathing to occur in all patients with OSA.
An important consideration is that even if high respiratory drive with concurrently high genioglossus muscle activity can allow stable breathing to develop, this may not provide any cardiovascular benefit over repetitive apneas/hypopneas. The markedly negative intrathoracic pressures present during stable flow limited breathing likely increase left ventricular transmural pressure (and therefore afterload) considerably.33,34 This phenomenon may help to explain the cardiovascular consequences of OSA that are observed at low AHI levels or with snoring.3 Whether an individual patient benefits from stable breathing (with concomitant high left ventricular afterload) versus unstable breathing (with associated catecholamine surges from arousals and hypoxemia) is unknown and untested. Thus, strategies to manipulate arousal threshold35,36 to yield stable breathing with flow limitation and high respiratory drive may have risks as well as benefits.
Sleep Stage Effects
The activity of the genioglossus has previously been reported to be higher during SWS than stage 2 sleep in 5 healthy normal subjects.37 In the current study we performed 2 comparisons of stage 2 sleep to SWS. In the first case, only stable stage 2 sleep was compared to SWS, and no difference in genioglossal activity was found. In contrast, when all stage 2 sleep was considered, the genioglossus activity was slightly lower than SWS, consistent with the prior study. However, the relationship between epiglottic pressure and genioglossus activity was similar in all 3 conditions, indicating that the muscle activity is likely increased because respiratory drive and negative pressure are increased. We suggest therefore, that there is nothing fundamentally different about genioglossus control during SWS that protects the airway from collapse, but rather, the high arousal threshold that occurs during deeper stage 2 can allow large genioglossus muscle activity to develop, stabilizing the airway such that sleep can continue and SWS can develop.
Respiratory events are often worse during REM than NREM sleep. In this study we found that the tonic (expiratory) activity of the genioglossus was reduced during respiratory events in REM compared to stage 2 sleep. This reduction in tonic genioglossus activity may contribute to the predisposition to airway collapse that occurs in REM because airway collapse typically occurs at the end of expiration.
Limitations
A major limitation of this study is that we have shown associations between certain sleep states/breathing conditions and muscle activity/lung volume but this does not prove causality. While this was a necessary first step, future studies will be required to assess causality of these relationships. In addition, the patients were relatively heavily instrumented during the study with mask, pneumotachograph, epiglottic catheters, and intramuscular wire electrodes. This equipment may alter sleep and the pathogenesis of apneas such that the night in the laboratory was not representative of what occurs in the home. However, this is of course unavoidable in any detailed physiological study.
Another limitation is that the magnetometer coils are only useful for quantifying changes in end-expiratory lung volume measurements not absolute values. Therefore we made all comparisons to the nearest period of stable wakefulness which was likely temporally further for SWS and REM sleep than stage 2. We doubt however, that the extra time that had elapsed from the reference wakefulness period had an important influence on our results or conclusions because the magnetometers drift very little in the absence of gross body movements. A further limitation is that not all subjects had all sleep stages/breathing conditions and as a result the number of subjects used in each comparison varied slightly. However, when only the subjects with all data were considered the results were near identical, although some statistical differences persisted over fewer breaths. Finally, we selected OSA patients for participation based on observed periods of stable breathing in the supine position. While we are unable to quantify the proportion of our sleep laboratory patients that have stable breathing periods in the supine position (due to the multiple exclusion criteria and our Human Subjects' Consent Procedures, which do not allow us to gather data on non-consenting subjects), we certainly had more difficulty recruiting for this study than other similar studies in our laboratory, suggesting that many OSA patients may not have stable breathing periods in the supine position in our clinical laboratory. In addition, we only studied patients in the supine position. Therefore our findings may not generalize to all sleep apnea patients or while in the lateral or prone positions.
Summary
This study has demonstrated that when patients with obstructive sleep apnea have spontaneously occurring stable breathing, the activity of the genioglossus muscle was elevated. We propose that this relationship is likely causal, and that adequate genioglossus muscle recruitment may be able to stabilize the airway allowing adequate ventilation although flow limitation and snoring may persist. When stable breathing did not occur, we found the genioglossus muscle was still activated to a similar degree for the amount of negative airway pressure present. The failure of increased genioglossus activity to stabilize breathing at some times and in some individuals may be related to anatomical variability, reduced lung volume or a low arousal threshold such that the subjects wake up before a critical level of respiratory drive/muscle activity is reached. During REM sleep, further suppression of the tonic component of the genioglossus activity was observed, potentially contributing to the increased severity of OSA in REM sleep. However, during stable breathing, muscle activation did not differ between stage 2 and slow wave sleep. In summary, we propose that activation of the genioglossus can allow stable flow limited breathing to occur during sleep at least in some individuals with OSA. However, there also appear to be other factors that influence the effectiveness of genioglossus activation at stabilizing the upper airway. Finally, increased tensor palatini activity and end-expiratory lung volume do not appear to contribute to spontaneous breathing stability.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. White is Chief Medical Officer for Respironics and has consulted for Itamar, Aspire, and Pavad. Dr. Wellman has participated in speaking engagements and has consulted for Respironics. Dr. Eikermann has received research support from and has consulted for Organon and Pfizer. Ms. Stevenson has consulted for Sepracor and has participated in speaking engagements for Sanofi-Aventis. Dr. Malhotra has received research support and/or consulted for Pfizer, Respironics, NMT Medical, Apnex, Restore Medical, Inspiration Medical, Itamar, Cephalon, and Sepracor. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the American Heart Association and the National Institutes of Health: P50 HL60292, RO1 HL048531, AG02837 and NCRR GCRC RR01032.
This work was performed at institution 2.
REFERENCES
- 1.Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population- based sample of employed adults. Sleep. 1997;20:608–13. doi: 10.1093/sleep/20.8.608. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 4.Stanchina ML, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–9. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 5.Lo Y, Jordan AS, Malhotra A, et al. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep. 2006;29:470–7. doi: 10.1093/sleep/29.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms Used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–7. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 8.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in sleep apnoea patients. Thorax. 2006;61:435–9. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–6. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 10.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–56. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 11.Oliven A, O'Hearn DJ, Boudewyns A, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol. 2003;95:2023–9. doi: 10.1152/japplphysiol.00203.2003. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi I, Perry A, Rhymer J, et al. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol. 1996;80:1595–604. doi: 10.1152/jappl.1996.80.5.1595. [DOI] [PubMed] [Google Scholar]
- 13.Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–7. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan AS, Schory K, Dover L, Fogel RB, Malhotra A, White DP. Genioglossus muscle activity during stable sleep in patients with obstructive sleep apnea [Abstract] Am J Respir Crit Care Med. 2005;2:A610. [Google Scholar]
- 15.Jordan AS, Schory K, Smith S, et al. Upper airway muscle activity and lung volume during NREM and REM sleep in obstructive sleep apnea [Abstract] Am J Resp Crit Care Med. 2007;175:A754. [Google Scholar]
- 16.Jordan AS, McEvoy RD, Edwards JK, et al. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol. 2004;558:993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra A, Pillar G, Fogel RB, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–62. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. UCLA Los Angeles, USA: National Institute of health; 1968. [Google Scholar]
- 19.American Sleep Disorders Association Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 20.Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol. 1981;50:1052–5. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- 21.Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol. 2007;581:1193–205. doi: 10.1113/jphysiol.2007.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–12. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo YL, Jordan AS, Malhotra A, et al. The influence of wakefulness on pharyngeal airway muscle activity. Thorax. 2007;29:470–77. doi: 10.1136/thx.2006.072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillipson EA. Regulation of breathing during sleep. Am Rev Respir Dis. 1977;115:217–24. doi: 10.1164/arrd.1977.115.S.217. [DOI] [PubMed] [Google Scholar]
- 25.Orem J. The nature of the wakefulness stimulus for breathing. Prog Clin Biol Res. 1990;345:23–30. [PubMed] [Google Scholar]
- 26.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;6:6. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell GS, Baker TL, Nanda SA, et al. Invited review: intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–75. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 28.McEvoy RD, Popovic RM, Saunders NA, White DP. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J Appl Physiol. 1996;81:866–75. doi: 10.1152/jappl.1996.81.2.866. [DOI] [PubMed] [Google Scholar]
- 29.Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present in healthy men or women. J Appl Physiol. 2002;93:2129–36. doi: 10.1152/japplphysiol.00135.2002. [DOI] [PubMed] [Google Scholar]
- 30.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1111–9. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- 31.Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–16. [PubMed] [Google Scholar]
- 32.Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol. 2001;91:2751–7. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- 33.Fessler HE. Heart-lung interactions: applications in the critically ill. Eur Respir J. 1997;10:226–37. doi: 10.1183/09031936.97.10010226. [DOI] [PubMed] [Google Scholar]
- 34.Hausknecht MJ, Brin KP, Weisfeldt ML, Permutt S, Yin FC. Effects of left ventricular loading by negative intrathoracic pressure in dogs. Circ Res. 1988;62:620–31. doi: 10.1161/01.res.62.3.620. [DOI] [PubMed] [Google Scholar]
- 35.Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep. 2007;30:478–88. doi: 10.1093/sleep/30.4.478. [DOI] [PubMed] [Google Scholar]
- 36.Heinzer RC, Malhotra A, Jordan AS, et al. Trazodone increases arousal threshold in response to hypercapnia in OSA patients Eur Respir J. 2008;31:1308–12. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basner RC, Ringler J, Schwartzstein RM, Weinberger SE, Weiss JW. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir Physiol. 1991;83:189–200. doi: 10.1016/0034-5687(91)90028-h. [DOI] [PubMed] [Google Scholar]