Abstract
Study Objectives:
The objectives of this study were to (1) characterize cognitive and cerebral correlates of attention and response speed in patients with obstructive sleep apnea (OSA) and (2) assess the association of performance and brain activation with measures of OSA severity.
Design:
Patients with OSA and controls were compared on performance and brain activation during a sustained attention task. The association of reaction time and brain activation with apnea-hypopnea index, nocturnal hypoxia, and arousals was assessed.
Setting:
Functional magnetic resonance imaging was conducted while participants performed a Go–No-Go task. The ‘Go’ trials of the Go–No-Go task were used to index attention processing.
Participants:
Fourteen patients with OSA and 14 normal control subjects with equivalent age, body mass index, blood pressure, and education.
Interventions:
N/A.
Measurements and Results:
Patients with OSA showed decreased brain activation in cingulate, frontal, and parietal regions typically involved in attention tasks, compared with control subjects. Within the patients with OSA, increasing arousal index, but not desaturation index, was associated with slower mean reaction time and with decreased brain activation in areas involved in arousal and attention, response selection, motor response, and decision making. The apnea-hypopnea index, by itself, was not associated with changes in cerebral response.
Conclusions:
Patients with OSA showed decreased brain activation compared with control subjects during an attention task. The association of arousal index (but not hypoxia) with slow reaction times and brain activation suggests that alertness and reaction times show greater correlations with measures of sleep disruption than with measures of hypoxia.
Citation:
Ayalon L; Ancoli-Israel S; Aka AA; McKenna BS; Drummond SPA. Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. SLEEP 2009;32(3):373–381.
Keywords: Obstructive sleep apnea, attention, functional magnetic resonance imaging, hypoxia, arousals
OBSTRUCTIVE SLEEP APNEA (OSA) IS CHARACTERIZED BY REPEATED EPISODES OF UPPER AIRWAY OBSTRUCTION DURING SLEEP, RESULTING IN INTERMITTENT hypoxemia with periodic arousals.1 Prevalence estimates for OSA in adults range from 9% to 28% for at least mild severity and 2% to 14% for at least moderate severity.2 OSA is recognized as a significant public health problem that imposes substantial cardiovascular3 and neurocognitve morbidities.4 Patients with OSA commonly exhibit excessive daytime sleepiness and show vigilance and attention deficits and slowed reaction times (RT).5
Previous studies have shown that sleep-apnea severity (as measured by the number of respiratory events per hour of sleep, the apnea hypopnea index [AHI]) is associated with attention and response speed. Specifically, increased AHI has been associated with prolonged RT (eg6), impaired sustained attention, and monitoring of information.7,8 Cheshire et al9 found that increased AHI was associated with impaired response speed, sustained attention, vigilance, distractibility, and processing capacity and that the number of arousals was related to performance on a simple RT task. In addition, findings from event-related potential studies of increased P300 latency in OSA (eg10) support the behavioral findings of attention deficits in OSA. Importantly, impaired attention and slowed RTs in patients with OSA are related to a diminished driving ability and risk for road accidents.11,12
Although there is discrepancy across studies with respect to the relative importance of nocturnal hypoxia versus arousals in the pathogenesis of the cognitive deficits in OSA, disturbances in executive function seem to show stronger correlations with measures of hypoxia, whereas alterations in vigilance, alertness, and processing speed correlate more with measures of sleep disruption and arousals.9,13,14
Despite the considerable data about impaired performance and prolonged P300 latency in OSA, much less is known about the changes in the brain substrates underlying these impairments. Although a variety of studies have shown morphologic changes and changes in cerebral blood flow and cerebral metabolites in patients with OSA (for a review see15), only a few studies have assessed brain function in OSA and its association with changes in cognitive functioning.16–18
Using functional magnetic resonance imaging (fMRI) Thomas et al18 reported slower working memory speed on a 2-back task in individuals with OSA, compared with control subjects. This impaired performance was accompanied by reduced activation within anterior cingulate, dorsolateral prefrontal cortex, and posterior parietal cortex of the OSA group compared to the control group. They also reported that hypoxic patients showed slightly reduced responses in 3 regions relative to nonhypoxic patients but not within their primary regions of interest. Our group17 found higher false positive rates in patients with OSA accompanied by impaired cerebral activation during a response inhibition task relative to control subjects. In particular, the OSA group showed diminished response in regions involved with conflict monitoring, attention, motor function, and decision making. Given the obvious attention demands in the tasks utilized in these previous studies, those findings are consistent with (although not conclusive of) the notion of impairment in attention-related brain regions in OSA.
Since very little is known about the functional cerebral correlates of nocturnal hypoxia and arousals, the current study aimed to examine the cerebral substrates underlying attention and RT in individuals with OSA and their association with OSA severity. Using a sustained-attention task, patients with OSA and control subjects were compared on measures of performance and brain activation. In addition, the association of AHI, nocturnal hypoxia, and arousals with RT and fMRI measures of brain function during the attention task were assessed. We hypothesized that patients with OSA would show prolonged RTs and decreased cerebral activation compared with control subjects; that prolonged RTs would be related to increased OSA severity, particularly the number of nocturnal arousals; and, given that relationship, a negative relationship between nocturnal arousals and cerebral activation in task-related brain regions.
METHODS
Participants
Twenty-eight participants (14 treatment-naïve patients with OSA and 14 matched healthy control subjects) were studied (13 men, 1 woman in each group). There were no significant differences between the patients and control subjects on any demographic variable (Table 1). For inclusion, patients with OSA needed an AHI of at least 10 and control subjects needed an AHI less than 5. All participants were right-handed, healthy, and free of current and past psychiatric and medical disorders, as determined by history and physical exam, the Composite International Diagnostic Interview for mental disorders, routine lab work, and urine toxicology screens. We excluded individuals with hypertension ( > 180/110), diabetes, body weight over 300 pounds, sleep disorders other than OSA, and patients who were taking medications that affect the central nervous system. All participants reported regular sleep-wake schedules and, based on daily sleep diaries, reported obtaining an average of 7.7 ± 0.9 hours of sleep per night for the 3 nights preceding the study. The study was approved by the University of California San Diego Human Research Protection Program, and all participants provided written informed consent.
Table 1.
Sample Characteristics
| OSA (n = 14) Mean (SD) | Control (n = 14) Mean (SD) | P value | |
|---|---|---|---|
| Age, y | 45.6 (11.7) | 43.6 (8.6) | 0.6 |
| BMI, kg/m2 | 30.6 (5.7) | 28.7 (3.5) | 0.3 |
| Blood pressure, mm Hg | |||
| Systolic | 125.6 (14.4) | 119.1 (12.2) | 0.2 |
| Diastolic | 76.3 (10.6) | 74.1 (10.2) | 0.6 |
| Education, y | 15.9 (2.7) | 16.0 (3.1) | 0.9 |
OSA refers to obstructive sleep apnea; BMI, body mass index.
P-Value is for independent samples t-test.
Experiment Protocol
Patients with a known diagnosis of OSA were referred from the UCSD Medical Center or from their private physicians, and patients who did not have a previous diagnosis of OSA were recruited from the general San Diego community by the UCSD Database of Clinical Research and through advertisements in local media. Interested individuals were screened via telephone for major inclusion and exclusion criteria, and those deemed eligible were scheduled for an appointment. After signing informed consent, participants had medical and psychiatric screening and completed sleep questionnaires. Five to 14 days after screening, participants reported to the UCSD General Clinical Research Center's J. Christian Gillin Laboratory for Sleep and Chronobiology for an overnight polysomnogram. Polysomnography was used to confirm OSA diagnosis and rule out sleep disorders other than OSA. Sleep schedules in the laboratory were based on self-reported habitual sleep schedules. Two to 3 hours after waking on the morning after the polysomnogram, participants underwent a fMRI session.
Polysomnography
A Grass Heritage Digital polysomnography system (Grass, West Warwick, RI) was used for recording sleep. Standard electroencephalograms (C3, C4, O1, and O2 derivations), electrooculogram (left outer canthus and right outer canthus derivations), chin electromyogram, electrocardiogram, airflow, thoracic and abdominal excursions, oximetry, and tibialis electromyogram were recorded. Apnea was defined as any 10-second or longer drop of at least 80% in the respiratory amplitude. Hypopnea was defined as any 10-second or longer drop of at least 30% in the respiratory amplitude plus at least a 3% desaturation or an arousal. AHI was calculated as the number of apnea and hypopnea events per hour of sleep. A desaturation was defined as any 10-second or longer drop of at least 3% in the oxygen saturation and a minimum interval of 4 seconds. An arousal was defined as any increase in electroencephalographic frequency lasting at least 3 seconds during non-rapid eye movement sleep with concurrent increases in submental electromyographic amplitude during rapid eye movement sleep.
fMRI session
Cognitive Task
During the fMRI session, participants performed a Go–No-Go task. The task alternated between active blocks and resting blocks. Five of the active blocks were 30 seconds long and 8 were 15 seconds long. Resting periods were 3 to 15 seconds long (mean = 8.8 sec, SD = 4.7 sec). There were 181 stimuli (68.5% Go stimuli). During resting periods, participants viewed a fixation cross in the center of the screen. During active blocks, a large square, small square, large pentagon, and small pentagon were presented 1 at a time in the center of the screen. Stimuli appeared for 200 ms every 1500 ms. Participants were instructed to press a button as fast as possible every time they saw a shape (Go stimuli) but to withhold a response when they saw the small pentagon (the No-Go stimulus). The task lasted 6 minutes 24 seconds. Stimuli were presented visually via a video projector onto a screen placed at the foot of the MRI bed that participants viewed through a mirror fitted to the head coil. Two of the subjects with OSA used a fiberoptic goggle system (Avotec, Stuart, FL) mounted to the head coil due to the fact that their girth made viewing the screen at the foot of the bed impossible.
At the conclusion of the task, while still in the scanner, participants were administered the Stanford Sleepiness Scale19 and Karolinska Sleepiness Scale20 and 10-point Likert scales assessing the following subjective factors: task difficulty, ability to concentrate, and motivation to perform the task well.
fMRI Data Acquisition
The fMRI session took place on a General Electric 3T scanner (GE Medical Systems, Milwaukee, WI). Functional images consisted of 126 gradient-echo echo-planar images (repetition time: 3 second, echo time: 30 ms, field of view: 256 mm, 4 mm x 4 mm in-plane resolution) of 32 four-mm axial slices covering the whole brain and measuring the blood oxygenation level dependent (BOLD) signal. Functional data were aligned with high-resolution anatomic images (fast spoiled gradient-recalled echo: 1-mm3 resolution).
Data Analysis
Demographic variables, performance, and postscan questionnaire data were analyzed with student t-tests (equal variances not assumed) comparing the OSA and the control groups. The Go trials of the Go–No-Go task were used to index sustained attentional processing, since they represent a simple reaction time task. Mean RT to the Go shapes was calculated for each participant.
Partial correlations were used to calculate the associations between performance and OSA severity (AHI, desaturation index, number of minutes below 90% SpO2, and arousal index) and to assess the relationship between performance and subjective sleepiness, task difficulty, ability to concentrate, and motivation. Because OSA severity is often correlated with age,21 we controlled for age in both the performance and the fMRI activation analyses (described below).
fMRI data were processed and analyzed with Analysis of Functional NeuroImaging software22 in a 2-step procedure: individual timecourse analysis followed by group statistical analysis. After motion coregistration, individual time-course BOLD-signal data were fit to a design matrix using the general linear model.23 Parameters estimated from the design matrix represented the constant, linear-drift, 6-motion correction parameters derived from the motion coregistration step (3 relational and 3 translational movement directions) and 2 reference functions: 1 comparing Go stimuli with rest blocks and 1 comparing No-Go stimuli to rest blocks, each convolved with an idealized hemodynamic response function.24 The parameter used in group analyses was the regression coefficient associated with the Go reference function in the general linear model. Prior to group analyses, data sets were transformed to standard atlas coordinates.25
We determined differences in cerebral response to the Go trials versus fixation in each group separately using 1-sample t-tests. Group differences in the cerebral response to the Go trials were assessed using independent samples t-tests. To assess the correlation between brain activation and OSA severity, BOLD-response data were regressed onto AHI, desaturation index, minutes below 90% SpO2, and arousal index. The first regression analysis included AHI as a global measure of disease severity and controlled for age. The second included the desaturation index and arousal index (while controlling for age) to test the relative contribution of each more-specific severity measure statistically separated from the effect of the other.
Group analyses used a search-region-of-interest strategy.26,27 Two search regions were defined. The first included the cortex and was conducted after spatially smoothing the individual data with a 4-mm full-width-half-maximum Gaussian kernel. The second exclusively examined the subcortical regions of thalamus, basal ganglia, putamen, globus pallidus, and surrounding structures. Analysis of this search region was conducted without any spatial smoothing of the data to better discriminate the various closely spaced nuclei in this region. To protect against overall Type I error, we used a cluster threshold method.28 For the group comparison, this required any given voxel to be statistically significant at the P < .05 level and to be part of a cluster of at least 4 contiguous activated voxels (256 mm3) for the subcortical analysis or 12 contiguous voxels (768 mm3) for the cortical analysis, each individually significantly activated. Hence, the clusters we report are equal to or larger than the single largest cluster of activation expected by chance at α = 0.05. To be more conservative for the regression analyses, we protected the overall α at P = 0.01, requiring any given voxel to be statistically significant at the P < .01 level and to be part of a cluster of at least 4 contiguous activated voxels (256 mm3) or 9 contiguous voxels (576 mm3) for the cortical analysis, each individually significantly activated.
RESULTS
Brain activation data were available for all 28 participants. Performance data for 1 participant with OSA was missing due to computer failure.
The OSA group had a mean AHI of 34 (SD = 21, range: 10-68); mean desaturation index of 30 (SD = 18, range: 6-59), and a mean arousal index of 37 (SD = 22, range: 7-87). The 2 severity indexes (desaturation index and arousal index) were correlated (r = .63, P = 0.02), confirming the importance of removing the variance shared by these 2 measures when examining the unique contribution of each to performance or activation measures. There was no significant correlation between arousal index and minutes below 90%.
Performance in Patients with OSA and Control Subjects
The OSA and control groups showed similar mean RT during the Go trials (OSA: mean = 859 msec, SD = 112; Control: mean = 804 msec, SD = 91; t25 = 1.37, P = 0.18) (Cohen d = 0.53, medium effect size). Hit rate during Go trials was 99% for both groups (P = 0.8), and there was also no significant difference between the standard deviation of RT (108 msec for the controls and 114 msec for the OSA, P = 0.35). Both groups showed similar motivation, perceived task difficulty, and ability to concentrate during the task. No differences between the groups were found using the state sleepiness measures of Stanford Sleepiness Scale or the Karolinska Sleepiness Scale.
Brain Activation in Patients with OSA and Control Subjects
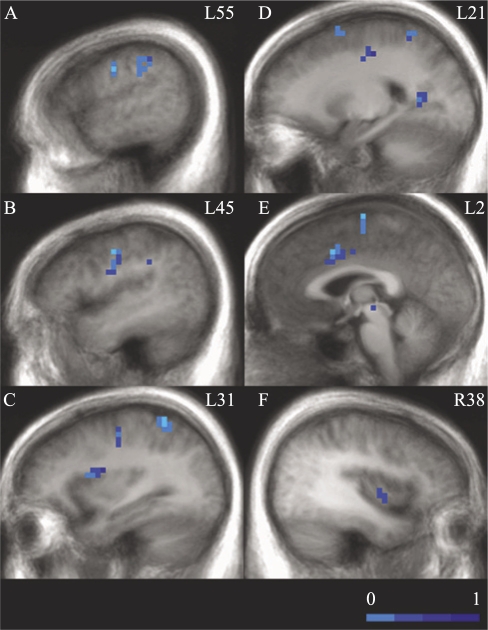
Both groups showed mainly right-hemisphere activation for the Go trials. The activation patterns for the Go trials versus fixation for the control and OSA groups are shown in Tables 2 and 3, respectively. The OSA group showed decreased cerebral activation in several brain regions, compared with control subjects, during the Go trials. These regions included left middle and medial prefrontal cortex, left precentral gyrus, left superior and inferior parietal lobule, and left anterior and posterior cingulate. See Table 4 and Figure 1.
Table 2.
Brain Regions Showing Activation During the Go Trials (Compared to Fixation) in the Control Group
| Anatomic location | Region (Brodmann area) | Volume (mm3) | Center |
Max Eta-Sq | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Frontal gyrus, precentral and postcentral gyrus, and cingulate gyrus | ||||||
| R inferior frontal gyrus (9) | 3648 | −47 | 6 | 28 | 0.64 | |
| B superior, middle and medial frontal | ||||||
| gyrus (6), precentral and postcentral | ||||||
| gyrus, cingulate gyrus (32/24) | 37376 | 18 | −11 | 53 | 0.86 | |
| R precentral gyrus (6) | 2048 | −35 | −8 | 50 | 0.68 | |
| Posterior cingulate and precuneus | B (23) and precuneus, L31 | 23040 | 0 | −54 | 21 | 0.83 |
| Insula | L (13) (+IFG/9) | 5504 | 40 | 7 | 15 | 0.59 |
| R (13) (+IFG/45) | 4160 | −40 | 18 | 7 | 0.74 | |
| Inferior parietal lobule | R (40) | 3840 | −40 | −50 | 40 | 0.68 |
| Superior temporal gyrus | R (22) | 896 | −53 | −5 | −6 | 0.75 |
| Middle temporal gyrus | R (39) | 576 | −47 | −63 | 23 | 0.65 |
| Middle and inferior occipital gyrus | L (19,37) | 2368 | 39 | −66 | −5 | 0.69 |
| Angular gyrus | R (39) | 1792 | 39 | −73 | 30 | 0.65 |
| Basal ganglia and thalamus | R Thalamus | 4096 | 18 | −8 | 10 | 0.77 |
| R lentiform nucleus and putamen | 2368 | −25 | 3 | 10 | 0.59 | |
| R thalamus-ventral lateral nucleus | 640 | −16 | −13 | 11 | 0.62 | |
| Cerbellum | R culmen, pyramis, declive | 3264 | −23 | −57 | −19 | 0.64 |
Regions of decreased activation are in italics. R refers to right; L, left; B, bilateral.
Table 3.
Brain Regions Showing Activation During the Go Trials (Compared to Fixation) in the OSA Group
| Anatomic location | Region (Brodmann area) | Volume (mm3) | Center |
Max Eta-Sq | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Superior frontal gyrus | B (9) | 8000 | 4 | 52 | 25 | 0.67 |
| L (8) | 1728 | 24 | 25 | 49 | 0.69 | |
| Medial frontal gyrus | B (6) + R (8) | 7140 | 0 | 1 | 57 | 0.81 |
| Middle frontal gyrus | R (6) | 2240 | −37 | −2 | 51 | 0.78 |
| Inferior frontal gyrus | R (9) | 2122 | −45 | 6 | 31 | 0.75 |
| L (6) + precentral gyrus | 1216 | 42 | 2 | 30 | 0.70 | |
| Precentral and postcentral gyrus | L (3, 4) | 14272 | 38 | −20 | 52 | 0.85 |
| L (6) | 576 | 45 | −11 | 31 | 0.61 | |
| Posterior cingulate | B (23/31) | 33216 | 0 | −50 | 23 | 0.82 |
| insula | R (13) | 1600 | −38 | 17 | 5 | 0.66 |
| R | 832 | −37 | −17 | 17 | 0.76 | |
| Inferior parietal lobule | R (40) | 5760 | −37 | −50 | 42 | 0.79 |
| L | 896 | 46 | −26 | 28 | 0.63 | |
| Precuneus | L | 2176 | 25 | −58 | 42 | 0.70 |
| Superior temporal gyrus | R (22) | 832 | −59 | −37 | 16 | 0.60 |
| Middle temporal gyrus | L (39) | 3584 | 41 | −73 | 27 | 0.75 |
| Middle occipital gyrus | L (37) | 1664 | 43 | −67 | 3 | 0.77 |
| R (37) | 704 | −34 | −67 | 8 | 0.74 | |
| Cerebellum | R culmen | 5760 | −19 | −55 | −17 | 0.78 |
| R pyramis | 1536 | −5 | −74 | −28 | 0.74 | |
| L declive | 1216 | 27 | −64 | −21 | 0.57 | |
Regions of decreased activation are in italics. OSA refers to obstructive sleep apnea; R, right; L, left; B, bilateral.
Table 4.
Brain Regions Showing Group Differences (OSA vs Control) During the Go trials (All Regions Showed Decreased Activation in OSA Compared to Controls)
| Anatomic location | Region (Brodmann area) | Volume (mm3) | Center |
Max Eta-Sq | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Medial frontal gyrus | L(6) | 1536 | −7 | −17 | 62 | 0.41 |
| Middle frontal gyrus | L | 960 | −17 | 7 | 62 | 0.34 |
| Precentral gyrus | L(6) | 2048 | −45 | −11 | 33 | 0.39 |
| Insula | L | 1088 | −29 | 9 | 17 | 0.36 |
| R | 832 | 36 | −4 | 2 | 0.30 | |
| Superior parietal lobule | L(7) | 1600 | −24 | −52 | 57 | 0.47 |
| Inferior parietal lobule | L(40) | 1536 | −55 | −32 | 35 | 0.28 |
| Anterior cingulate | L(32) | 2112 | −1 | 5 | 42 | 0.34 |
| Posterior cingulate | L(18/30) | 1088 | −17 | −56 | 8 | 0.33 |
| Red nucleus | L | 1024 | −8 | −22 | −3 | 0.26 |
Regions of decreased activation are in italics. OSA refers to obstructive sleep apnea; R, right; L, left; B, bilateral.
Figure 1.
Brain regions showing significant group differences (obstructive sleep apnea [OSA] minus control) in activation during the Go trials (See Table 4). In all clusters showing group differences, the OSA group exhibited decreased responses (light blue, smallest differences; dark blue, largest). The panel shows sagittal slices from left to right. Numbers correspond to Talairach and Tournoux coordinates of the sagittal slices (L: left, R: right). For all images, clusters surviving our cluster threshold method are overlaid in color on top of the group average anatomic image. The color corresponds to the effect-size eta2, corresponding to the amount of variance accounted for by group membership. A: left precentral gyrus, inferior parietal; B: Left precentral gyrus; C: Left insula, precentral gyrus, inferior parietal lobe; D: left middle frontal gyrus, superior parietal, posterior cingulate; E: left medial frontal gyrus, cingulate gyrus; F: right insula.
Association Between OSA Severity and Performance
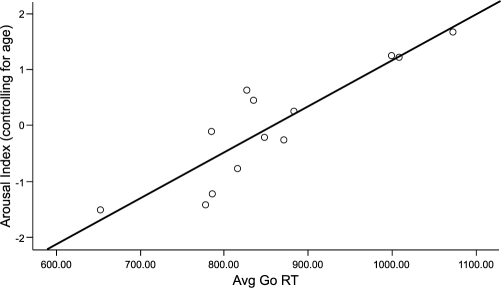
Longer mean RT was associated with a higher arousal index (r = .66, P = 0.028) Figure 2. Patients with OSA with an arousal index less than 30 performed very similarly to the control subjects, whereas those with a higher arousal index showed slower RT (RT = 802 msec in low arousal OSA, 948 msec in high arousal, t11 = 2.89, P = 0.015). AHI, desaturation index, average nocturnal oxygen saturation, and number of minutes below 90% SpO2 were not associated with mean RT (all P > 0.1). Increased subjective sleepiness, as measured by the Stanford Sleepiness Scale and Karolinska Sleepiness Scale, was associated with prolonged RT (r = .60, P = 0.04 and r = .59, P = 0.04, respectively). No association was found between RT and perceived task difficulty, motivation, and ability to concentrate.
Figure 2.
Relationship between arousal index and mean reaction time (Avg Go RT) in the obstructive sleep apnea (OSA) group, after removing the influence of age. The x-axis shows each subject's mean RT, and the y-axis shows the residual of the arousal index (partialing out the effect of age).
Association Between OSA Severity and Cerebral Activation
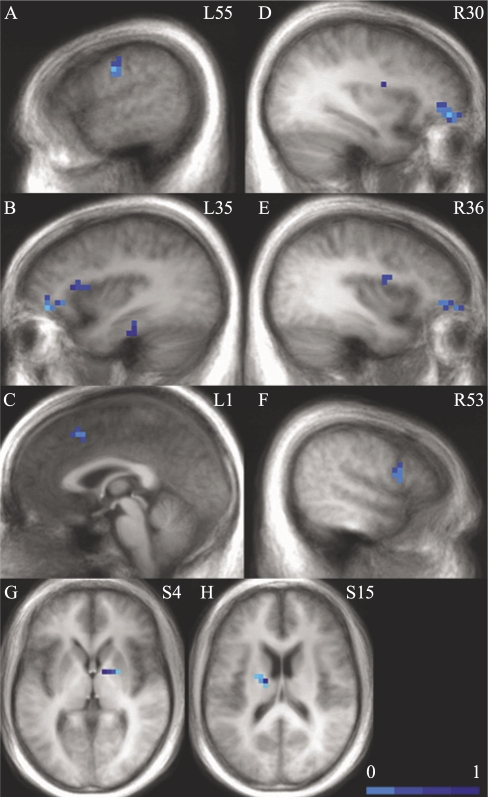
AHI was not associated with brain activation in the OSA group. In the regression analysis assessing the relative contribution of oxygen desaturations and arousals to brain activation, arousals were associated with decreased brain activation during the Go trials in bilateral superior and middle frontal gyri, right inferior frontal gyrus, bilateral insula, left parahippocampal gyrus, left thalamus, left precentral gyrus, and right lentiform nucleus and lateral globus pallidus. Desaturations were correlated only with increased activation in bilateral superior frontal gyrus and right inferior frontal gyrus (Table 5 and Figure 3). Number of minutes with an SpO2 below 90% was not correlated with brain activation.
Table 5.
Brain Regions Showing Correlation with Arousal Index and Desaturation Index
| Anatomic location | Region (Brodmann area) | Volume (mm3) | Center |
Max Eta-Sq | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Arousals | ||||||
| Superior frontal gyrus | B(8) | 896 | −1 | 18 | 48 | 0.65 |
| Middle frontal gyrus | R(10) | 1472 | 30 | 50 | −2 | 0.89 |
| L(47,10) | 1344 | −35 | 40 | −2 | 0.80 | |
| Inferior frontal gyrus | R(44) | 1152 | 53 | 10 | 19 | 0.68 |
| R(13) | 704 | 41 | 23 | 9 | 0.54 | |
| Precentral gyrus | L(4) | 960 | −55 | −11 | 33 | 0.54 |
| Insula | L(13) | 1216 | −37 | 12 | 11 | 0.71 |
| R(13) | 576 | 36 | 0 | 18 | 0.52 | |
| Parahippocampal gyrus | L(36) | 832 | −29 | −24 | −19 | 0.62 |
| Thalamus | L | 320 | −17 | −8 | 16 | 0.48 |
| Lentiform nucleus and lateral globus pallidus | R | 256 | 15 | −1 | 4 | 0.44 |
| Desaturations | ||||||
| Superior frontal gyrus | B(8) | 704 | 0 | 19 | 48 | 0.59 |
| Inferior frontal gyrus | R(44) | 768 | 53 | 10 | 19 | 0.59 |
Regions of decreased activation are in italics. R, right; L, left; B, bilateral.
Figure 3.
Brain regions showing significant correlations with arousal index during the Go trials (See Table 5). In all clusters showing correlations with arousal index, increased arousal index was associated with smaller brain responses. The panel shows sagittal slices and axial slices with numbers corresponding to Talairach and Tournoux coordinates (L: left, R: right, S: superior). For all images, clusters surviving our cluster-threshold method are overlaid in color on top of the group-average anatomic image. The color corresponds to the effect-size eta2, corresponding to the amount of variance accounted for by arousal index. A: left precentral gyrus; B: left middle frontal gyrus, insula, parahippocampal gyrus; C: bilateral superior frontal gyrus; D: right middle frontal gyrus; E: right insula, middle frontal gyrus; F: right inferior frontal gyrus; G: right lentiform nucleus, lateral globus pallidus; H: left thalamus.
DISCUSSION
We used a sustained-attention task to test whether individuals with OSA differ from control subjects in their cerebral response to cognitive challenges and whether this change in cerebral response was modified by OSA severity. Supporting our hypotheses, we found that patients with OSA showed decreased brain activation, compared with control subjects. Also consistent with our hypotheses, among the patients with OSA, increased arousal index, but not desaturation index, was associated with slower RTs and decreased activation in task-related brain areas.
Cerebral Activation in Patients with OSA Compared to Control Subjects
Both groups activated mainly right-hemisphere regions, as expected for this type of task. However, when compared with control subjects, patients with OSA showed decreased brain activation in several brain regions. The decreased activation was seen largely in the left hemisphere in cingulate, frontal, and parietal regions typically involved in attentional tasks. This suggests that control subjects, relative to patients with OSA, either showed a greater spatial extent of activation that extended from the right hemisphere to the left hemisphere (eg, midline regions such as anterior cingulate and medial frontal gyrus) or showed bilateral involvement of brain regions activated only unilaterally in patients with OSA (eg, precentral gyrus, insula). This extends findings of decreased brain activation in patients with OSA, compared with control subjects, during working-memory18 and response inhibition17 tasks. Common to all 3 of these fMRI studies is decreased cerebral responses among patients with OSA, relative to control subjects, in anterior cingulate, various frontal areas, and both cortical and subcortical motor regions. This suggests that these brain regions may be particularly vulnerable to OSA-related impairment, independent of the specific cognitive challenge measured.
OSA Severity and Performance
Increasing arousal index, but not AHI, desaturation index, and minutes spent with an SpO2 below 90%, was associated with slower mean RT. The finding of slowed RT with increase in some measures of OSA severity is consistent with previous findings of an association between OSA severity and attention and RT deficits in individuals with OSA.13 The association between RT and arousal index reported here is also consistent with previous reports of an association between arousals and performance on a simple RT task9 and between sleep quality and processing speed.14 This finding, coupled with a lack of association between the desaturation index and RT, provides further support to the suggestion that vigilance and attention are more related to nocturnal arousals than to hypoxia.13
OSA Severity and Cerebral Activation
The most novel finding of this study is that the number of nocturnal arousals was associated with changes in cerebral response. More specifically, increased arousal index was correlated with decreased activation in task-related brain areas, including areas involved in response selection and attention (insula, superior, middle, and inferior frontal gyri), motor response (precentral gyrus and basal ganglia), and decision making (insula).29 Decreased activation with increasing arousal index was also found in the thalamus, which has a major role in arousal and attention,30 particularly under conditions of sleep loss.31
The finding that increased arousal index, but not hypoxia (at least as measured by desaturation index and time spent with an SpO2 below 90%), was associated with decreased activation in attention and motor areas is consistent with reports suggesting that alterations in vigilance and alertness as well as slowed RTs show greater correlations with measures of sleep disruption than with measures of hypoxia.13 It is also consistent with findings from Thomas et al's paper suggesting that hypoxia may not be a necessary determinant of cognitive dysfunction, and sleep fragmentation may be sufficient.18
Given the impact of arousals on the attention system, the motor findings may reflect a secondary process whereby motor responses are slowed because of impairment in the attention system. Our task design did not allow us to tease out the separate effects on attention and motor systems, although future studies may wish to better make this distinction.
The finding of decreased activation with increased arousal index in areas subserving attention and RT tasks also extends findings from event-related potential studies reporting an association between increased OSA severity and prolonged P300 latencies.32 Our finding of decreased activation in the cingulate and the thalamus, both of which have been suggested as potential sources of the P300 response,33,34 may help elucidate the brain regions underlying prolonged P300 latencies found in patients with OSA using event-related potential techniques.
Interestingly, AHI, by itself, was not associated with changes in cerebral response. It may be the case that the arousal and desaturation indexes are more sensitive measures that are more related to the cerebral correlates of the cognitive deficits in OSA than is the more global measure of AHI. If true, data reported here argue that sleep fragmentation in OSA has a greater impact on cognitive and brain function during attention tasks than do intermittent oxygen desaturations. On the other hand, anatomic studies have suggested that hypoxia has a variety of neuropathologic and neuropsychological effects.35 Here, we found that increased hypoxia index was related to greater activation in 2 small frontal areas. Although it is possible to interpret such findings as compensatory recruitment, given the lack of behavior effects of hypoxia index here, such speculation cannot be strongly supported by our data. Nonetheless, the behavior and cerebral impact of arousals and hypoxia seem to be distinct, suggesting that these 2 measures of OSA severity impact attention processes differently. This suggests that no 1 single indication of OSA severity can properly anticipate neurocognitive impairment in patients. Clearly, more work needs to be done in this area to fully clarify the differential impact of arousals and hypoxia.
Implications, Limitations, and Future Directions
From an operational or applied perspective, the association between arousals and impaired cerebral functions in regions involved in attention and RT may be relevant to the relationship between disrupted sleep and impaired driving ability, including increases in motor vehicle crashes in this population.11 In determining who is at greatest risk for driving crashes, and thus who may need to be legally prevented from driving prior to treatment, clinicians may wish to focus more on sleep fragmentation and arousals, as well as the expected consequence of excessive daytime sleepiness, than on AHI or desaturations. Future studies may wish to more directly examine the effects of trait, state sleepiness, or a combination of trait and state sleepiness on both performance and cerebral responses to cognitive tasks.
The small sample size, as well as the large variability in performance in the OSA group, may account for the fact that the differences between the groups in performance did not reach statistical significance. Importantly, the arousal index seems to account for much of the variability, for both performance and cerebral responses, within the OSA group. More specifically, patients with OSA with an arousal index less than 30 performed very similarly to the control subjects, whereas those with a higher arousal index showed slower RTs. This effectively increased the variance within the OSA group and decreased the variance between groups, potentially masking group differences in performance. As has been reported elsewhere, our data suggest that fMRI measures may be more sensitive to impairments than are behavior measures, as there were group-level fMRI differences in the patients with OSA. Even there, though, regression analyses revealed the patients with OSA with more arousals also showed greater impairment in task-related regions than did those with fewer arousals. These findings underscore the heterogeneity of patients with OSA and again suggest that severity measures in addition to AHI may provide clinically relevant information.
Studies using larger sample sizes may also be able to apply more complex or sophisticated statistical models to better understand the links among OSA severity, performance, and brain function. In addition, as with any fMRI study, we had to exclude any patient weighing more than 300 pounds. This, and the fact we excluded individuals with hypertension or diabetes, necessarily limits the generalizibility of the findings to the overall OSA population. Despite that exclusion criterion, though, our patients included a wide range of AHI values (10-68 events/hour). Thus, our findings can be considered relevant to patients with even severe OSA. Finally, incorporating additional measures of brain function (including measures of cerebral blood flow and white matter integrity) may shed light on the mechanisms underlying decreased performance in OSA. Although we did not incorporate these measures here, we do not believe the group-level decreases in OSA reported here represent a fundamental alteration of the hemodynamics underlying the BOLD signal, because (1) we excluded patients with conditions that perturb basal blood-flow levels (eg, hypertension, diabetes, etc) and (2) these same patients showed no activation differences in a simple sensorimotor task relative to control subjects.16
Importantly, because treatment with continuous positive airway pressure has been shown to be effective in shortening RTs in individuals with OSA,36 it would be important to determine whether the improved performance following treatment of OSA is associated with reversibility of the cerebral changes reported here.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Ayalon has received research support from Cephalon. Dr. Ancoli-Israel has received research support from Sepracor, Takeda, and Litebook, Inc; has consulted or been an advisory board member for Arena, Acadia, Cephalon, Sanofi-Aventis, Sepracor, Somaxin, and Takeda; and has received discounted equipment from Litebook and Respironics. Dr. Drummond has participated in a study sponsored by Cephalon and is on the advisory board of Actelion. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The work was performed at the Department of Psychiatry, University of California San Diego, and the Veterans Affairs San Diego Healthcare System.
Support: NIH M01 RR00827, NIMH 5 T32 MH18399, NIA AG08415, National Sleep Foundation Pickwick Fellowship (LA).
REFERENCES
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea—a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Placidi F, Diomedi M, Cupini LM, Bernardi G, Silvestrini M. Impairment of daytime cerebrovascular reactivity in patients with obstructive sleep apnoea syndrome. J Sleep Res. 1998;7:288–92. doi: 10.1046/j.1365-2869.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 4.Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23:102–8. [PubMed] [Google Scholar]
- 5.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta- analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 6.Bedard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol. 1991;13:950–64. doi: 10.1080/01688639108405110. [DOI] [PubMed] [Google Scholar]
- 7.Redline S, Strauss ME, Adams N, et al. Neuropsychological function in mild sleep-disordered breathing. Sleep. 1997;20:160–7. doi: 10.1093/sleep/20.2.160. [DOI] [PubMed] [Google Scholar]
- 8.Roehrs T, Merrion M, Pedrosi B, Stepanski E, Zorick F, Roth T. Neuropsychological function in obstructive sleep apnea syndrome (OSAS) compared to chronic obstructive pulmonary disease (COPD) Sleep. 1995;18:382–8. doi: 10.1093/sleep/18.5.382. [DOI] [PubMed] [Google Scholar]
- 9.Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ. Factors impairing daytime performance in patients with sleep apnea/hypopnea syndrome. Arch Intern Med. 1992;152:538–41. [PubMed] [Google Scholar]
- 10.Kotterba S, Rasche K, Widdig W, et al. Neuropsychological investigations and event-related potentials in obstructive sleep apnea syndrome before and during CPAP-therapy. J Neurol Sci. 1998;159:45–50. doi: 10.1016/s0022-510x(98)00131-2. [DOI] [PubMed] [Google Scholar]
- 11.Kingshott RN, Cowan JO, Jones DR, et al. The role of sleep-disordered breathing, daytime sleepiness, and impaired performance in motor vehicle crashes-a case control study. Sleep Breath. 2004;8:61–72. doi: 10.1007/s11325-004-0061-z. [DOI] [PubMed] [Google Scholar]
- 12.Mazza S, Pepin JL, Naegele B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020–8. doi: 10.1183/09031936.06.00112905. [DOI] [PubMed] [Google Scholar]
- 13.Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249–59. doi: 10.1016/s0272-5231(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 14.Naismith S, Winter V, Gotsopoulos H, Hickie I, Cistulli P. Neurobehavioral functioning in obstructive sleep apnea: differential effects of sleep quality, hypoxemia and subjective sleepiness. J Clin Exp Neuropsychol. 2004;26:43–54. doi: 10.1076/jcen.26.1.43.23929. [DOI] [PubMed] [Google Scholar]
- 15.Ayalon L, Peterson S. Functional central nervous system imaging in the investigation of obstructive sleep apnea. Curr Opin Pulm Med. 2007;13:479–83. doi: 10.1097/MCP.0b013e3282f0e9fb. [DOI] [PubMed] [Google Scholar]
- 16.Ayalon L, Ancoli-Israel S, Klemfuss Z, Shalauta MD, Drummond SPA. Increased brain activation during verbal learning in obstructive sleep apnea. NeuroImage. 2006;31:1817–25. doi: 10.1016/j.neuroimage.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Ayalon L, Ancoli-Israel S, Drummond SPA. Altered brain activation during response inhibition in obstructive sleep apnea. J Sleep Res. 2008 doi: 10.1111/j.1365-2869.2008.00707.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–34. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 19.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness—new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 20.Akerstedt T, Gillberg M. Subjective and Objective Sleepiness in the Active Individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 21.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 22.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 23.Ward BD. Deconvolution Analysis of fMRI Time Series Data. Milwaukee, WI: Biophysics Research Institute, Medical College of Wisconsin; 2002. [Google Scholar]
- 24.Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 25.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York, NY: Thieme Medical; 1988. [Google Scholar]
- 26.Drummond SPA, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 27.Eyler Zorrilla LT, Jeste DV, Paulus M, Brown GG. Functional abnormalities of medial temporal cortex during novel picture learning among patients with chronic schizophrenia. Schizophrenia Res. 2003;59:187–98. doi: 10.1016/s0920-9964(01)00340-1. [DOI] [PubMed] [Google Scholar]
- 28.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic-resonance-imaging (fMRI)—use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 29.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 30.Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18:8979–89. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chee MW, Tan JC, Zheng H, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sangal RB, Sangal JM. Obstructive sleep apnea and abnormal P300 latency topography. Clin Electroencephalogr. 1997;28:16–25. doi: 10.1177/155005949702800104. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki T, Kamijo K, Kenmochi A, et al. Multiple equivalent current dipole source localization of visual event-related potentials during oddball paradigm with motor response. Brain Topogr. 2000;12:159–75. doi: 10.1023/a:1023467806268. [DOI] [PubMed] [Google Scholar]
- 34.Linden DEJ. The P300: Where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11:563–76. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- 35.Gale SD, Hopkins RO. Effects of hypoxia on the brain: neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10:60–71. doi: 10.1017/S1355617704101082. [DOI] [PubMed] [Google Scholar]
- 36.Ovesen J, Nielsen PW, Wildschiodtz G. Shortened reaction-time during nasal CPAP treatment of obstructive sleep-apnea. Acta Oto-Laryngologica. 1992;119:21. doi: 10.3109/00016489209136828. [DOI] [PubMed] [Google Scholar]