Abstract
Study Objectives:
There is debate in dream research as to whether ponto-geniculo-occipital (PGO) waves or cortical arousal during sleep underlie the biological mechanisms of dreaming. This study comprised 2 experiments. As eye movements (EMs) are currently considered the best noninvasive indicator of PGO burst activity in humans, the aim of the first experiment was to investigate the effect of low-intensity repeated auditory stimulation on EMs (and inferred PGO burst activity) during REM sleep. It was predicted that such auditory stimuli during REM sleep would have a suppressive effect on EMs. The aim of the second experiment was to examine the effects of this auditory stimulation on subsequent dream reporting on awakening.
Design:
Repeated measures design with counterbalanced order of experimental and control conditions across participants.
Setting:
Sleep laboratory based polysomnography (PSG)
Participants:
Experiment 1: 5 males and 10 females aged 18-35 years (M = 20.8, SD = 5.4). Experiment 2: 7 males and 13 females aged 18-35 years (M = 23.3, SD = 5.5).
Interventions:
Below-waking threshold tone presentations during REM sleep compared to control REM sleep conditions without tone presentations.
Measurements and Results:
PSG records were manually scored for sleep stages, EEG arousals, and EMs. Auditory stimulation during REM sleep was related to: (a) an increase in EEG arousal, (b) a decrease in the amplitude and frequency of EMs, and (c) a decrease in the frequency of visual imagery reports on awakening.
Conclusions:
The results of this study provide phenomenological support for PGO-based theories of dream reporting on awakening from sleep in humans.
Citation:
Stuart K; Conduit R. Auditory inhibition of rapid eye movements and dream recall from REM sleep. SLEEP 2009;32(3):399–408.
Keywords: Dream recall, auditory stimulation, rapid eye movements, ponto-geniculo-occipital (PGO) waves, Attention
THERE ARE CURRENTLY TWO BROAD CLASSES OF BIOLOGICAL THEORY WITHIN THE AREA OF DREAM RESEARCH: CORTICAL AROUSAL-BASED THEORIES OF dreaming1–6 and brainstem PGO-based theories of dreaming.7–9 Although there is considerable support for each view, there are methodological and theoretical limitations to both that remain to be resolved before it will be clear which process (if indeed either or both) underlies dream reporting on awakening from sleep.
Cortical arousal during sleep has been identified by the appearance of α frequency (8-12 Hz) EEG activity and has been noted by a number of researchers to be associated with mentation reporting.1–6,10–13 α Activity is high at sleep onset when hypnopompic images occur.5 Similarly, although sleep terrors occur during slow wave sleep (SWS), EEG indicates increased arousal to that of a wake pattern.10 Light sleepers with increased α activity across all sleep stages show an equal frequency of mentation reporting from REM and NREM sleep.1 Tyson et al11 proposed dreaming to be a function of cerebral activation. They noted that during REM periods of higher α activity preceding awakening, mentation reports were more vivid and bizarre than those with lower α activity, as judged by an independent rater.
Antrobus compared NREM and REM mentation and found that differences in mentation were a function of report length.2 He concluded that increased cortical activation characteristic of REM sleep resulted in lengthy mentation reports. In further investigating the role of cortical arousal in dreaming, Antrobus et al.12 found an increase in visual mentation reports associated with increased arousal characteristic of both REM sleep and the rising phase of the diurnal sleep cycle. They noted the diurnal rise in arousal accounted for 30% of the difference in cortical arousal between REM and NREM sleep. In a subsequent study by Fosse et al,13 229 REM and 165 NREM reports collected at home were scored for the presence of hallucinations and directed thinking. These data showed that as the night progressed, NREM showed an increase in hallucinations; this was so pronounced that late night NREM reports were described as indistinguishable from early REM reports. This led these researchers to conclude that “…as the night progresses, NREM approaches the neurocognitive characteristics of REM (p. 302).” These findings support the notion of dreaming as a function of cerebral activation as the night progresses.
The “REM-on dream-on” model proposed by Solms6 is consistent with cortical arousal-based theories of dreaming. Using evidence from lesion14–17 and pharmacological18,19 studies, Solms proposed dreaming was a result of arousal of dopaminergic forebrain processes dissociable from the REM sleep stage. More specifically he identified the activation of the mesocortical/mesolimbic dopaminergic system and cortical back projection as responsible for the generation of dreaming.6
The relationship between increased cortical arousal and dream recall has recently been disputed by a number of researchers, who have noted decreased cortical activation is conducive to higher dream recall.20–22 Recall from stage 2 and REM sleep has been found to be associated with lower α power;22 and the accepted α suppression of occipital brain regions during the engagement in visual imagery has been noted during phasic REM sleep.20,21 Thus, research investigating the relationship between cortical arousal and dream recall has produced conflicting results.
Alternative to cortical arousal theories of dreaming are brainstem ponto-geniculo-occipital (PGO) based hypotheses. The identification of PGO waves by Jouvet and Michel23 arose from electrophysiological studies in the cat. These studies identified phasic neuronal activity (now known as PGO waves) originating from the pontine brainstem that could be concurrently recorded from the lateral geniculate nucleus (LGN) of the thalamus and the occipital cortex.
Given the predominance of PGO waves during REM sleep, PGO activity was suggested as a correlate of dreaming.7,8,24 Support for this association arose from lesion and microstimulation studies demonstrating the primary role of the brainstem in the generation of both PGO waves and the REM sleep state, in addition to findings that PGO waves were measurable from the LGN and occipital cortex of cats (which is consistent with the visual nature of dreaming).25,26
The activation synthesis (AS) model put forth by Hobson and McCarley8 proposed that PGO activity is a fundamental neural component underlying dreaming, and that it accounts for the internal signal generation and sensory input characteristic of REM sleep. Based on evidence from microelectrode studies in the cat, Hobson and McCarley proposed that the brainstem was the central generator of dreaming, assigning the forebrain a secondary passive role. They proposed that the forebrain receives tonic and phasic signals from the midbrain reticular formation (MRF) via the thalamus, and phasic eye movement signals from the pontine reticular formation (PRF) via the lateral geniculate nucleus (LGN).8 The forebrain was then thought to compare the internally generated pseudosensory information from the brainstem with previously stored sensorimotor information, resulting in the generation of dreaming.
In light of new neuroimaging and lesion studies, the AS model was revised into the more comprehensive Activation, Input, Modulation (AIM) model.9 Contrary to prior research, neuroimaging studies by Braun et al27 and Maquet et al,28 utilizing positron emission tomography (PET), indicated that the primary visual areas were not involved in dreaming. They identified the extrastriate areas of the visual cortex, limbic, and paralimbic areas as active during dreaming. Lesion studies further supported the involvement of these structures, as they indicated damage to V1 had no impact on dreaming, whereas damage to extrastriate visual areas resulted in abolition of the visual aspects of dreaming.17 Therefore, the AIM model was put forth, proposing that PGO waves stimulated cortical association and limbic areas in humans, rather than primary sensory areas as proposed in the AS hypothesis.
Testing a PGO model of dreaming has posed a problem for many researchers, as dreaming and PGO activity cannot be studied concurrently. PGO waves have been investigated extensively in animals using indwelling electrode recordings of the pons, LGN, and cortex.8,25,26 However, observing and quantifying animal “dreams” is not possible with current research methods. Conversely, mentation reports are easily obtained from humans, but indwelling electrode recording procedures are invasive and unethical to perform; thus the presence of PGO waves in humans is yet to be confirmed.30,31 A noninvasive method of measuring proposed PGO activity in humans is yet to be developed, mainly because the spatial and temporal resolution of current neuroimaging techniques is inadequate to accurately measure and track such activity.9
Eye movements are currently the most reliable indicator of eruptions of PGO activity. The relationship between eye movements and PGO burst activity has been confirmed by a number of studies in animals,8,25,29 and EMs have shown results consistent with what would be expected from PGO burst activity in humans.30,32,33
Human studies employing external stimuli to induce eye movements (and inferred PGO burst activity) have enabled the indirect investigation of the phenomenology of PGO activity related to dream reporting. However, these studies to date have been confounded by concurrent cortical arousal induced by the external stimulus.32,33
Conduit et al.32 and Fedyszyn and Conduit33 investigated eye movements as an analogue of PGO burst activity and the effect of their manipulation on dreaming. They presented light and tone stimuli32 and tone stimuli33 below waking threshold during stage 2 and REM sleep to induce EMs. When at least one concurrent eye movement was observed in response to stimulus presentation, subjects were awoken and mentation reports were collected. Mentation reports following induction of EMs and inferred PGO burst activity in stage 2 sleep contained a higher frequency of imagery and were rated as more vivid than control mentation reports. However, stimulus presentation during stage 2 sleep also resulted in an increase in EEG arousal. This arousal was found to significantly correlate with imagery ratings during stage 2. Thus, the increase in imagery following induction of eye movements during stage 2 sleep, may have been a result of increased cortical arousal.2 Hence, neither a PGO hypothesis nor an arousal hypothesis of dream recall was supported by these experiments. Fedyszyn and Conduit33 concluded that the induction of ocular activity during sleep via external stimulation was confounded by cortical arousal, and such PGO indicators and cortical arousal seemed to be inseparable.
Recent experiments from our laboratory have anecdotally reported the suppression of eye movements (EMs) in response to the presentation of a repeated tone at low intensity below waking threshold.34 Such EM suppression during REM sleep has also been observed in response to white noise35 and photic stimulation.36 In a more recent fMRI study,37 it was also observed that external tones presented during REM sleep produced a significant decrease in thalamic blood oxygen level dependent (BOLD) response and suppression of EMs.
The aim of the current study was to investigate the suppressive effect of low-intensity repeated auditory stimulation on eye movements and examine the effect of such decreased EM activity on EEG arousal and dreaming. It was hypothesized that low-intensity repeated tone presentation during REM sleep would result in significant reduction in the frequency and amplitude of rapid eye movements, while producing an increase in cortical EEG arousal. It was further hypothesized that the percentage of imagery reports and the mean vividness rating of dreams would be significantly lower in awakenings following tone presentation than when no tone was presented.
EXPERIMENT 1
METHOD
Participants
Participants were 5 males and 10 females, aged 18 to 35 years (M = 20.82, SD = 5.40). Prior to participating in this study, each volunteer was required to complete a screening questionnaire; this was done to eliminate individuals with abnormal sleep patterns, chronic illnesses, alcohol or drug dependence, or use of medications known to affect sleep patterns. Individuals unsuitable for participation were debriefed and did not participate any further in the study.
Recruitment was via posters displayed around the University campus and through an Undergraduate Psychology Participant Pool, where students gained credit as part of their undergraduate program. Participants were informed of procedures, which were approved by the University Human Ethics Committee (Approval number 2007/0134).
Design
The experiment was a simple repeated measures design. The independent variable was the stimulation condition, which consisted of 3 levels: pre-stimulation, stimulation, and post-stimulation. The dependent variables were the frequency and amplitude (µV) of eye movements.
Apparatus
The study was conducted in a sound-attenuated sleep laboratory with 2 identical bedrooms and a single control room, equipped with a 2-way intercom system for monitoring participants. Sleep was monitored via an S-Series 16-channel polygraph with W-Series Sleep/Replay display and analysis software (Compumedics Pty, Ltd. Melbourne, Australia). The recording montage consisted of electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), and stimulus channels. Prior to electrode attachment, each site was prepared using 70% v/v isopropyl alcohol swabs, and EEG sites were abraded using trace preparation tape. Electrodes were attached to the head using conductive electrode paste and gauze and attached to the face using conductive electrode paste and surgical tape. For EEG recording, Grass gold cup disk electrodes were affixed to the scalp at C4/A1 and C3/A2 according to the international 10-20 placement system.39 For EOG recording, electrodes were placed at the inner and outer canthi of each eye to monitor horizontal eye movements, and above and below the midline of one eye to monitor vertical eye movements. EMG recording electrodes were placed at the chin muscles (mentalis).
Calibration
Participants were asked to clench their jaw, to look from side to side, and to look up and down to check the integrity of the EMG, horizontal EOG, and the vertical EOG recordings, respectively. The tone was then switched on to check sound level recordings and to prepare participants for what to expect. EEG channels were displayed such that 50 µV represented 1 cm of pen deflection and at a rate of 1 cm/sec. All EEG placements were checked to ensure impedances were maintained below 5 kΩ?.
Auditory Stimulus
A custom-made electronic device was used to produce the auditory stimulus (1000 Hz, 50 dB, 500 msec, repeating at 1-sec intervals). During the night, the stimulus was presented via a speaker placed against the wall directly facing participants, approximately 4 meters away from the head of the bed. The volume of the tones was measured and calibrated before the experiment using a sound meter (Brüel & Kjaer Type 2203, Naerum, Denmark) placed on the pillow at the head of the bed with ambient background noise of approximately 35 dB(A).
Procedure
Participants were required to arrive one hour prior to normal bedtime to maintain regular sleep patterns. They were asked to read the explanatory statement and sign a consent form. After preparing for bed, recording equipment was applied and the experimental procedure was again explained. During the night, sleep monitoring and the experimental protocol were carried out in an adjacent room. There was no visual contact between the experimenter and participant, and communication was via an intercom.
Once participants entered REM sleep, the experimenter waited for 1 min of well-defined REM sleep, then presented the auditory stimulus for 1 min. After presentation, eye movements were then observed for a further minute. If REM sleep was maintained, it was allowed to continue for 5 min before the experimental protocol commenced again. If a subject showed microarousals ( > 3 sec α frequency or higher on EEG with concurrent rise in EMG40) during stimulus presentation, the procedure was continued; however, data from this trial were not included in the analysis. Some subjects were found to have lower arousal thresholds; therefore, if tone presentation at 50 dB woke the subject, the stimulus volume was lowered to 40 dB for the next presentation. Subsequently, if 3 successive rapid eye movements appeared, the volume was increased by 2-dB increments until eye movements were suppressed.
Sleep and Arousal Scoring
Sleep was scored in 30-sec epochs according to Rechtschaffen and Kales criteria.41 EEG records for 1 min before, during, and after tone presentation were visually scored for time in α (8-12 Hz) and β (12-30 Hz) frequency EEG activity and for microarousals.40 All scoring was conducted by a polysomnographic technician with over 10 years experience, who had no knowledge of the details of the experiment and was blind to experimental conditions.
Eye Movement Analysis
The frequency of eye movements during REM sleep before, during, and after tone presentation was determined by calculating the average number of eye movements in each of the 3 conditions across the number of trials. The amplitude of eye movements in each of the 3 conditions was determined by measuring the amplitude of each rapid eye movement (defined as ≥ 50 µV increase or decrease over 500 ms) in each condition. The amplitude of eye movements for each condition was then averaged across the number of trials. Data were collated and analyzed using SPSS for Windows, version 15.0.
RESULTS
Of the 5 males and 10 females who took part in this study, 3 males and 1 female were unable to maintain sleep in the experimental conditions, as EMs could not be suppressed without inducing full arousal41 or microarousals.40 Hence, the sample size was reduced to 11 participants. Of the 11 participants from whom data was obtained, 4 participants showed substantial EEG arousal to the 50-dB tone; therefore a 40-dB tone (stepped in 2-dB increments to 50 dB) was presented in order to achieve EM suppression without full awakening41 or microarousal.40 Average total sleep time of participants in Experiment 1 was 401 min (SD = 69.2 min) with an average of 341 min in NREM (SD = 59.7 min) and 60 min in REM sleep (SD = 20.4 min). The average time into REM sleep stimulus presentations occurred (as scored by the independent sleep scorer) was 1 min 57 sec (SD = 49 sec).
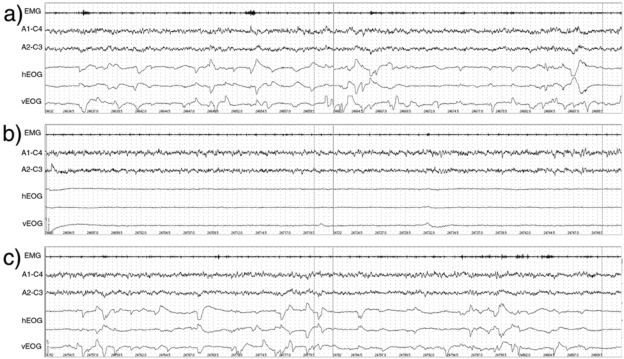
Figure 1 shows an extract from a typical polygraph record showing EEG and EOG activity for 1 min pre-stimulation, during stimulation, and post-stimulation. The mean frequency and amplitude of eye movements in each REM sleep condition are summarized in Figures 2 and 3.
Figure 1.
Example polysomnographic (PSG) record showing REM eye movements (a) 1 minute prior to auditory stimulation, (b) 1 minute during auditory stimulation, and (c) 1 minute following auditory stimulus presentation. Key: EMG: Electromyograph; A1-C4, A2-C3: Electroencephalograph recordings; hEOG: horizontal electroculogram (2 channels); vEOG: vertical electroculogram.
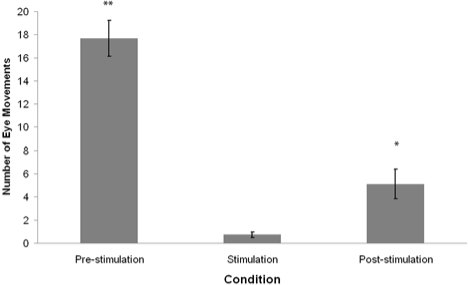
Figure 2.
Mean frequency of REM sleep eye movements 1 min preceding tone presentation (pre-stimulation), during 1 min tone presentation (stimulation,) and 1 min following tone presentation (post-stimulation). Error bars denote standard error of the mean. **P < 0.01, *P < 0.05 - Wilcoxon signed-rank test comparison to the stimulation condition.
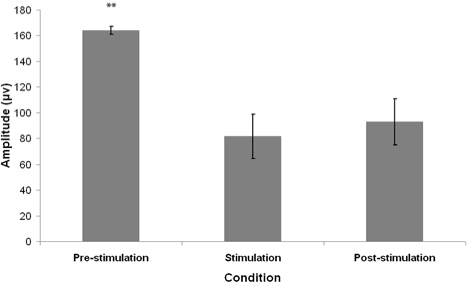
Figure 3.
Mean amplitude of eye movements 1 min preceding tone presentation (pre-stimulation), during 1 min tone presentation (stimulation) and 1 min following tone presentation (post-stimulation). Error bars denote standard error of the mean. **P < 0.01- Wilcoxon signed rank test comparison to the stimulation condition.
The mean EM frequency and amplitude distributions failed to pass analyses for violations of homogeneity of variance (Levene F2,20 = 7.36, P < 0.01; Levene F2,20 = 7.42, P < 0.01) and were significantly different from a normal distribution using the Shapiro-Wilk W statistic (P < 0.05). Hence, Friedman χ2 nonparametric statistical analyses were adopted.
There was a significant difference in the frequency of eye movements between the pre-stimulation, stimulation, and post-stimulation conditions (Friedman χ2 = 18.56, P < 0.01). Post hoc analysis of data using Wilcoxon signed rank test revealed that the frequency of eye movements was significantly lower during stimulation than pre-stimulation (Wilcoxon z = 2.94, P < 0.01), and during post-stimulation compared to pre-stimulation (Wilcoxon z = 2.93, P < 0.01). Furthermore, EM frequency during stimulation was found to be significantly lower than during post-stimulation (Wilcoxon z = 2.50, P < 0.05).
Friedman χ2 revealed a significant difference in EM amplitude between the 3 conditions (Friedman χ2 = 12.05, P < 0.01). Post hoc analyses using Wilcoxon signed-rank test demonstrated that the EM amplitudes in the stimulation condition were significantly lower than that of the pre-stimulation condition (Wilcoxon z = 2.76, P < 0.01). The EM amplitudes of the post-stimulation condition were also found to be significantly lower than the pre-stimulation condition (Wilcoxon z = 2.85, P < 0.01); however there was no significant difference between the stimulation and post-stimulation conditions (Wilcoxon z = 1.07, P > 0.05).
The average amount of visually scored α and β frequency EEG arousal (in seconds) preceding tone presentation (pre-stimulation), during tone presentation (stimulation) and after tone presentation (post-stimulation) is presented in Table 1.
Table 1.
Average Amount of Visually Scored α and β Frequency EEG Activity (in sec), Prior to, During, and Following 1-min Auditory Stimulation.
| Condition | N | Mean | Standard Deviation |
|---|---|---|---|
| Pre-stimulation | 11 | 3.1 | 1.87 |
| Stimulation | 11 | 17.6 | 10.66 |
| Post-stimulation | 11 | 4.1 | 3.04 |
Using one-way repeated measures ANOVA, there was a significant difference in the average amount of α and β frequency EEG activity between the 3 conditions (F2,20 = 25.97, P < 0.001). Student-Newman-Keuls post hoc analyses showed that there was a significantly higher average amount of α and β frequency EEG activity during auditory stimulation compared to the pre-stimulation (P < 0.05) and post-stimulation (P < 0.05) conditions. The difference between the pre-stimulation and post-stimulation conditions however was not significant.
EXPERIMENT 2
METHOD
Participants
Participants consisted of 7 males and 13 females, aged 18 to 35 years (M = 23.3, SD = 5.5). Participants were recruited and screened as in Experiment 1.
Design
The experiment was a simple repeated measures design. The independent variable was the stimulation condition, consisting of 2 levels: with auditory stimulation and without auditory stimulation. The dependent variable was dream characteristics, which were measured by each participant's own imagery ratings and the percentage of imagery reports occurring in each condition (as rated by an independent judge, blind to experimental conditions). The order of condition presentation was counterbalanced across participants.
Apparatus
Polysomnographic (PSG) recording was the same as Experiment 1. An intercom was used to wake participants during the experimental procedure and to present a set of pre-recorded questions used for mentation report collection. The questions were as follows: “Could you please describe any thoughts or images that were going through your mind just before I woke you?”, “Is there anything else you can remember?”, and “Could you please rate the vividness of what you can remember, from one least vivid to ten most vivid?” An audiotape recorder was used to record mentation reports.
Procedure
Participant arrival and preparation for bed was as in Experiment 1. In the experimental condition, the experimenter waited for 1 min after REM sleep onset. The auditory stimulus was then presented for 1 min during ongoing REM sleep. Participants were then awoken by the experimenter calling their name through the intercom system. After indicating they were awake, participants were required to respond to the set of prerecorded questions. Responses were tape recorded. Participants were then allowed to return to sleep. If the participant woke during tone presentation, the tone was switched off and the trial ceased. They were then allowed to return to sleep for a minimum of 5 min before the next trial could commence. Some subjects were found to have lower arousal thresholds, as in Experiment 1; if participants woke when the tone was switched on at 50 dB, the volume was then lowered to 40 dB for subsequent trials. If, however, at this intensity rapid eye movements were not suppressed, the volume was increased by 2-dB increments until suppression was seen as in Experiment 1.
In the control condition, the experimenter waited for 2 min after REM sleep onset. Participants were then awoken by the experimenter calling their name through the intercom system. After indicating they were awake, participants were required to respond to a set of prerecorded questions. Responses were tape recorded. Participants were then allowed to return to sleep.
Sleep and Arousal Scoring
Sleep was scored in 30-sec epochs according to the criteria of Rechtschaffen and Kales.41 EEG records for 1 min before, during, and after tone presentation were visually scored for time in α (8-12 Hz) and β (12-30 Hz) frequency EEG activity and for microarousals.40 All scoring was conducted by a polysomnographic technician with over 10 years experience, who had no knowledge of the details of the experiment and was blind to experimental conditions.
Eye Movement Analysis
The frequency and amplitude of eye movements during REM sleep 1 min before awakenings were determined by measuring the amplitude of each rapid eye movement (defined as ≥ 50 µV increase or decrease > 500 ms) in each condition. The number and amplitude of eye movements for each condition was then averaged across the number of trials for each participant.
Dream Report Scoring
All audiotaped recordings of mentation reports were transcribed. Mentation reports were scored as containing imagery when at least one visualizable noun (an object that can be seen in the waking state)42 was reported. An independent judge, experienced with dream report scoring, was given the transcripts with no information regarding the experimental conditions. Written instructions included: “Please decide whether each report contains any visual imagery. In other words, did the report contain direct reference to any ‘visualizable nouns.’ Visualizable nouns are defined as nouns that can be seen or can be visualized. For example, ‘car’ is a visualizable noun. However nouns like ‘time’ or ‘luck’ are not.” The percentage imagery was then calculated by dividing the number of mentation reports containing imagery by the number of awakenings for each participant and then converting this value to a percentage. The mean vividness rating for the 2 conditions was obtained by calculating the average rating for each condition across all participants. Collated data were analyzed using SPSS for Windows, version 15.0.
RESULTS
Of the 7 males and 13 females who took part in this study, 4 females and 3 males were unable to maintain sleep in the experimental conditions, as EMs could not be suppressed without inducing full arousal41 or microarousals.40 Hence, the sample size was reduced to 13 participants (mean age = 23.3 years, SD = 5.54). The average total sleep time of participants in Experiment 2 was 320 min (84.6), with 285 min in NREM (72.3) and 35 min in REM sleep (20.0).
Forty paired dream report awakenings (20 with and 20 without auditory stimulation) were successfully sampled across the 13 participants. All PSG records from the dream report awakenings were judged by the independent sleep scorer as occurring from REM sleep. All awakenings were scored by the independent judge as occurring 2 min into a REM sleep period, except for 4 awakenings from each condition which were scored as occurring 2.5 min into REM sleep. Thus the average time into REM sleep for both conditions was identical (M = 126 sec, SD = 8.5 sec). Table 2 shows the average time of night; number and amplitude of EMs 1 min before awakening; and amount of manually scored α and β frequency EEG activity 1 min before awakening for each condition (auditory stimulation and no stimulation dream report). There was no statistical difference between the 2 conditions for the average time of night awakenings occurred. The average number and amplitude of EMs was significantly less in the auditory stimulus condition than the no-stimulus control. The mean amount of α and β frequency EEG arousal in the stimulus condition was significantly higher than the control condition.
Table 2.
Time, Number and Amplitude of Eye Movements, and Amount α and β Frequency EEG Activity 1 min Before Awakening in Different Conditions
| Average | Auditory Stimulation | No Auditory Stimulation | t-test, P-value |
|---|---|---|---|
| Time of night | 05:27 (1.14 h) | 04:59 (1.15 h) | t12 = 1.08, P = 0.30 |
| Number of EMs ( > 50 µV) | 3.4 (1.66) | 17.6 (5.87) | t12 = 10.40, P < 0.001 |
| Amplitude of EMs ( > 50 µV) | 100.6 µV (21.93 µV) | 122.7 µV (12.41 µV) | t12 = 4.94, P < 0.001 |
| α and β frequency EEG (sec) | 13.8 sec (9.71 sec) | 4.9 sec (3.07 sec) | t12 = 4.07, P = 0.002 |
Results of repeated measures t-tests comparing the 2 conditions on each measure are shown in the final column. Results shown as mean (SD). N = 13.
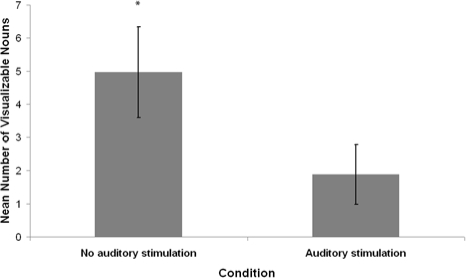
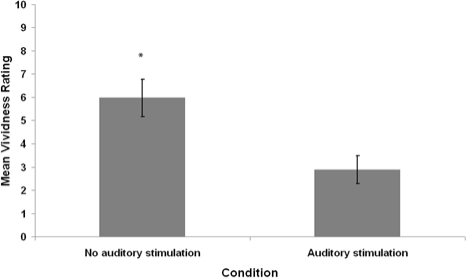
Seven of 20 auditory stimulation awakenings and 15 of 20 control awakenings contained imagery as judged by the independent rater. The average percentage of imagery reports, mean number of visualizable nouns, and mean vividness ratings for participants in each condition are summarized in Figures 4–6.
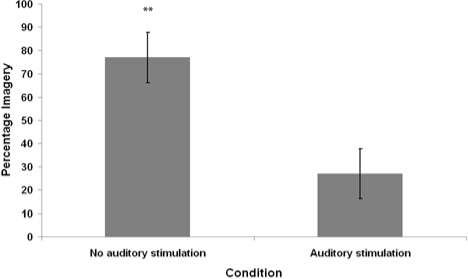
Figure 4.
Average percentage of mentation reports containing visual imagery, as judged by an independent rater (blind to conditions), from REM sleep waking conditions with and without auditory stimulation. Error bars denote standard error of the mean. **P < 0.01 - Wilcoxon signed-rank test comparison to the Stimulation Condition.
Figure 5.
Average number of visualizable nouns (nouns of objects that can be seen as in waking life) as judged by an independent rater (blind to conditions), in mentation reports from REM sleep waking conditions with and without auditory stimulation. Error bars denote standard error of the mean. *P < 0.05 - Wilcoxon signed-rank test comparison to stimulation condition.
Figure 6.
Average imagery ratings of participants' own mentation reports from REM sleep periods with auditory stimulation and without auditory stimulation. Ratings are scores out of 10. Error bars denote standard error of the mean. *P < 0.05 – Wilcoxon signed-rank test comparison to the stimulation condition.
The mean distributions for percentage of visual imagery, visualizable nouns, and imagery ratings in both conditions were analyzed for violations of normality. All differed significantly from normal distribution according to the Shapiro-Wilk W statistic (P < 0.05). Therefore, nonparametric Wilcoxon signed-rank tests were used for pairwise statistical comparisons. The imagery percentage, visualizable nouns, and imagery ratings of the auditory stimulation condition were significantly lower than that of the no-stimulation condition (Wilcoxon z = 2.53, P = 0.01; Wilcoxon z = 2.05, P = 0.04; Wilcoxon z = 2.40, P = 0.02, respectively).
Only 5 of 13 participants provided at least one report judged as containing imagery from the auditory stimulation condition, compared with 11 of 13 participants from the no-stimulation control condition. Therefore, it is possible that the average vividness ratings and noun count scores were confounded by the frequency of imagery reports. For the 5 participants who provided at least one imagery report from both conditions, Wilcoxon signed rank tests were used for pairwise statistical comparisons of noun count (auditory stimulation M = 4.9, SD = 3.62; Control M = 5.1, SD = 5.02) and imagery ratings (auditory stimulation M = 4.9, SD = 1.39; Control M = 5.5, SD = 2.06). No significant differences were observed (Wilcoxon z = 0.14, P = 0.89; Wilcoxon z = 0.73, P = 0.47; respectively). As this analysis was based on 5 participants, the lack of statistical significance of these results could be the result of inadequate sample size rather than reflecting any true state of these relationships.
DISCUSSION
The aim of this experiment was to investigate the suppressive effect of low-intensity repeated tone presentation on eye movements anecdotally reported in past research34–37 and to investigate the subsequent effect on dream report characteristics. Total sleep time and the percentage of REM and NREM sleep of the participants indicated that they experienced normal sleep architecture and sufficient sleep within the laboratory. Therefore, it is reasonable to assume that the data obtained from this study are representative of responses that would typically occur under normal sleep conditions.
Experiment 1
As hypothesized, low-intensity repeated tone presentation during REM sleep resulted in a significant reduction in the frequency and amplitude of rapid eye movements. This result was consistent with the findings of Sammut and Conduit,34 Sockeel et al.,35 Okuma et al.,36 and Wehrle et al.37
The findings of the current study could be considered inconsistent with previous research by Conduit, Bruck, and Coleman,32 and Fedyszyn and Conduit,33 which used auditory stimuli to induce EMs during NREM sleep. This discrepancy in findings could be attributed to differences in the presentation of the auditory stimuli employed. The different characteristics of the stimuli appear to have differing effects on eye movements, arousal, and possibly attention processes; with short loud 1000-Hz tones inducing EMs during NREM sleep, and low-intensity, continuous, repetitive 1000-Hz tones inhibiting EMs during REM sleep.
PGO waves occur spontaneously during sleep;23 however, bursts of PGO activity can be induced during wakefulness, REM and NREM sleep by unexpected presentation of an orienting stimulus.29,43,44 Previous studies by Conduit et al.32 and Fedyszyn and Conduit33 employing external auditory stimuli to phenomenologically study EMs and inferred PGO burst activity have employed single tones of increasing intensities (up to 110 dB) during stage 2 and REM sleep to induce eye movements and PGO activity. The induction of PGO burst activity in these experiments could therefore be interpreted as part of a orienting response, evidenced by the increase in arousal during stimulus presentation and the induction of what could be considered an orienting saccade.
The current study however employed a low-intensity repeated tone ( ≤ 50 dB), which was not related to an orienting response associated with abrupt stimulus presentation. We believe that it is reasonable to assume that the low-intensity repeated stimulus presented during the current study engaged both attentional and arousal mechanisms, leading to a shift from internal cognitive processing to the outward processing of the external stimulus; which in turn resulted in the disengagement of PGO and sleep processes and the engagement of the waking processes of arousal.
Snyder45 proposed that although sleep is necessary for restoration and repair, it leaves the organism vulnerable to predation. Therefore the REM sleep state functions to enable periodic brain activation allowing the organism to monitor the external environment and prepare for fight or flight without significantly disrupting sleep. Thus in the context of the current study it could be inferred that during tone presentation, attention is drawn away from internal cognitive processes to the external environment to determine if the impinging stimulus is a threat. This diversion of attention is indicated by a decrease in REMs and an increase in EEG arousal during stimulus presentation. Given the intimate relationship between eye movements, attention, and arousal processes in awake participants46,47 such a relationship between these seems plausible during sleep.
The notion of sublevel processing of external stimuli during REM sleep is further supported by research by Langford, Meddis, and Pearson,48 who investigated response latencies to personally significant and insignificant stimuli in 8 participants. They found that presentation of personally significant stimuli resulted in significantly shorter response latencies than personally insignificant stimuli. The differences in voluntary response latencies were significantly shorter from REM sleep than stage 2 sleep. Furthermore, they noted that α activity onset latencies were shorter for REM than stage 2 sleep, but this difference was not statistically significant. In a subsequent study using a larger sample by Jacobs and Conduit,49 such differences in EEG arousal latency were found to be statistically significant. These results support the proposal that sleeping subjects are able to selectively attend to their environment during REM sleep.
Although the tones presented in the present experiment were not personally significant stimuli, attentional resources would have been diverted to the external environment in order to make appraisal of the stimulus, thus impeding attention related to ongoing internal cognitive processes during the stimulus presentation. We propose that this engagement of outward attentional processing and arousal resulted in the disengagement of REMs, PGO sleep processes, and dream mentation (resulting in poorer subsequent dream recall on awakening in the second experiment).
Such an interpretation is consistent with the findings of an fMRI study by Wehrle et al.,37 showing that external tones presented during REM sleep produced a significant decrease in thalamic BOLD response and suppression of EMs. It was argued that their findings “…support a phasic to tonic transition in response to acoustic stimuli, which can be explained by switching to a higher level of arousal with raised sensitivity to environmental changes…(p. 869).” Wehrle et al37 also compared the fMRI BOLD response in 3 participants during tone presentations in tonic versus phasic REM sleep and found that auditory activation patterns were reduced during tonic REM and almost absent during phasic REM periods, reflecting minimal cortical processing of external information during phasic REM. Combined with an observation of enhanced thalamocortical synchronized activity during phasic REM, they interpreted these findings as an indication that the brain acts as a closed thalamocortical loop during phasic REM sleep, whereas “…tonic periods may be beneficial to detect potential danger (p. 870).”
Experiment 2
An independent judge, blind to experimental conditions scored all awakenings as occurring from REM sleep. The duration of time into REM sleep and time of night were both positively related to increased reported dream quality.12,13 These did not significantly differ between conditions in the current study. EM frequency and amplitude was significantly reduced in the tone condition, as observed in Experiment 1. Statistical analysis of manually scored α and β frequency EEG arousal indicated increased arousal time during tone presentation as seen in Experiment 1.
One result that seemed to be inconsistent across the 2 experiments was that the mean amplitude of EMs was considerably larger in the pre-stimulation REM period in Experiment 1 than the no-stimulation control REM period in Experiment 2 (164 µV vs 123 µV). This difference could have occurred because the average duration into REM sleep for stimulus presentation was approximately 2 min in Experiment 1 and 1 min in Experiment 2. The determination of REM sleep for conditions was done in real time during both experiments. Although the intention was for samples to be 1 min into REM sleep for both experiments, there was a greater emphasis on well-defined REM sleep with clear EMs in the Experiment 1. In Experiment 2, there was much stricter emphasis on adhering to the time into REM sleep. Total sleep time was also lower in the second experiment, but this was likely caused by the procedure of waking participants to collect dream reports.
As predicted, the average percentage of imagery reports, visual noun count and the mean vividness ratings were significantly lower from awakenings following auditory stimulus presentation compared to those from the control condition. However, the results regarding noun count and imagery ratings should be considered carefully. Since imagery reports were infrequent in the auditory stimulation condition, there were only 5 participants who provided at least one imagery report from both the stimulation and control conditions. Therefore, the overall analysis of noun count and imagery ratings may have been confounded by the frequency of imagery reports. Unfortunately, a sample of 5 was not adequate to statistically compare noun count and imagery ratings in participants who provided imagery reports from both conditions.
Although EM activity was externally induced, the findings from the current experiment are consistent with previous studies showing increased dream report characteristics following naturally occurring phasic REM sleep compared to tonic REM sleep. Tonic/phasic differences in dream characteristics were postulated to be due to differences in underlying REM sleep processes such as PGO activity.50
Initially, it may appear that the findings of the current study are inconsistent with past research from this laboratory showing that external auditory stimulation could enhance dream recall.32,33 However, this discrepancy in findings can be attributed to differences in the type of the stimuli employed. The single loud tone employed by Conduit, Bruck, and Coleman32 and Fedyszyn and Conduit,33 is thought to have invoked a orienting response and thus a burst of PGO activity and eye movement; which was then related to an increase in dream recall from stage 2 sleep. As the stimulus used in the current study was a low-intensity repetitive tone, it is presumed that an orienting response was not invoked. Results of the current study show support for a PGO hypothesis of dreaming, as the unique decrease in eye movements and possibly PGO burst activity was paralleled with a decrease in dream recall characteristics and EEG activation.
The aforementioned interpretation of the findings should be considered carefully. Although past research supports our assertions, PGO burst activity was not directly measured during the studies; thus their manipulation by external stimuli can only be assumed. The proposed suppression of PGO burst activity is based on the established relationship between PGO burst activity and eye movements in animals.8,25,26,29 A recent case study using indwelling electrode recordings from a human patient showed only a limited relationship between recorded pontine activity and EMs during REM sleep and even less correspondence during waking.31 Although this was a single case study and the measures taken were not necessarily that of human PGO activity, one must be open to the possibility that PGO activity is not a human phenomenon, or if it does exist in humans, it may be unlike that of other mammals such as rats, cats, and monkeys.23–26 Additionally, differences in the way PGO burst activity and EMs might respond and habituate to such external stimuli must be acknowledged. For example, there is evidence that various physiological components of the alerting response habituate to external stimuli at different rates.51 This discrepancy, noted by Sanford et al.,51 was between centrally and peripherally controlled alerting physiological processes. REMs are centrally controlled by the CNS, as is PGO activity; thus a discrepancy in habituation rates between the two is unlikely. However PGO burst activity was not directly measured in the current study and thus cannot be definitively shown to be suppressed with REMs. Ideally, the same paradigm should be carried out in animals in which direct recordings of PGO activity with REMs can be obtained.
One possible interpretation of these results is that it is merely an investigation of the effect of gradually induced arousal on dream recall. Previous research indicates that mentation reports from gradual awakenings become more thought-like and are less vivid compared to those from abrupt awakenings.52–54 Thus, as the dream characteristics explored in this study included ratings of imagery and vividness, the effect could be interpreted as indicating a decrease in visual dream characteristics to more thought-like mentation. Upon further consideration of the collected mentation reports, it is evident that this is not the case. In the present experiment, the awakening procedure required that REM sleep be maintained before awakening participants for collection of all dream reports. Of the 20 experimental awakenings, “no imagery” (as judged by an independent rater) was recorded for a total of 13 (65%) of these awakenings. Thus, it seems likely that the total lack of imagery in the stimulus condition may have confounded the differences observed in the 2 measures of imagery quality. Hence if the results of the current study were due to gradual awakening, one would expect that gross measures of the presence of imagery would be less sensitive than the more subtle measures of imagery quality, such as imagery ratings and visualizable noun count. This was not the case. In fact it seems (based on a very small sample) that the stimulus presentation appears to result in a total lack of imagery recall rather than a diluting of the quality of the imagery report.
We have earlier suggested that the external stimulus used in this study may have diverted attentional resources to the external environment, thus impeding attention and recall of ongoing internal cognitive processes, resulting in increased EEG arousal, decreased EMs, and poorer dream recall. Based on this notion, future experimentation could investigate the influence of meaningful vs non-meaningful stimuli presented during REM sleep on EMs and dream recall, with the hypothesis that personally significant stimuli would result in more EEG arousal, fewer EMs, and even poorer dream recall.
General Conclusions
In summary, low-intensity repeated tone presentation resulted in a significant decrease in the amplitude and frequency of rapid eye movements and possibly PGO burst activity. As evident from anecdotal findings of previous studies, eye movement suppression is not exclusive to pure tone auditory stimulation. The suppressive effect can also be brought about by white noise (70-95 dB)35 and photic stimulation (a train of 306 flashes at 1/sec emitted from a stroboscopic bulb).36 Low-intensity repeated tone presentation also resulted in a significant increase in EEG arousal and a decrease in the occurrence of reported dream imagery on awakening. Provided one is willing to assume that the appearance (or non-appearance) of REMs correlate with PGO burst activity in humans, as it does in other mammals (such as cats and monkeys), these results provide phenomenological support for PGO-based hypotheses of dream reporting, including the AIM model.9
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Zimmerman WB. Sleep mentation and auditory awakening thresholds. Psychophysiology. 1970;6:540–9. doi: 10.1111/j.1469-8986.1970.tb02243.x. [DOI] [PubMed] [Google Scholar]
- 2.Antrobus JS. REM and NREM sleep reports: comparison of word frequencies by cognitive classes. Psychophysiology. 1983;20:562–8. doi: 10.1111/j.1469-8986.1983.tb03015.x. [DOI] [PubMed] [Google Scholar]
- 3.Wollman MC, Antrobus JS. Sleep and waking thought, effects of external stimulation. Sleep. 1986;9:438–48. doi: 10.1093/sleep/9.3.438. [DOI] [PubMed] [Google Scholar]
- 4.Wollman MC, Antrobus JS. Cortical arousal and mentation in sleeping and waking subjects. Brain Cogn. 1987;6:334–6. doi: 10.1016/0278-2626(87)90130-8. [DOI] [PubMed] [Google Scholar]
- 5.Vogel GW. Sleep-onset mentation. In: Ellman SJ, Antrobus JS, editors. The mind in sleep: psychology and physiology. 2nd ed. NY: Wiley; 1991. pp. 125–42. [Google Scholar]
- 6.Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci. 2000;23:843–50. doi: 10.1017/s0140525x00003988. [DOI] [PubMed] [Google Scholar]
- 7.Rechtschaffen A. the psychophysiology of mental activity during sleep. In: McGuigan FJ, Schoonover RA, editors. The psychophysiology of thinking. New York: Academic Press; 1973. pp. 153–205. [Google Scholar]
- 8.Hobson JA, McCarley RW. The brain as a dream-state generator: an activation-synthesis hypothesis of the dream process. Am J Psychiatry. 1977;134:1335–48. doi: 10.1176/ajp.134.12.1335. [DOI] [PubMed] [Google Scholar]
- 9.Hobson JA, Pace-Schott EF, Stickgold R. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci. 2000;23:793–842. doi: 10.1017/s0140525x00003976. [DOI] [PubMed] [Google Scholar]
- 10.Broughton RJ. Sleep disorders: Disorders of arousal? Science. 1968;159:1070–8. doi: 10.1126/science.159.3819.1070. [DOI] [PubMed] [Google Scholar]
- 11.Tyson PD, Olgilvie RD, Hunt HT. Lucid, prelucid and nonlucid dreams related to the amount of EEG alpha activity during REM sleep. Psychophysiology. 1984;21:442–51. doi: 10.1111/j.1469-8986.1984.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 12.Antrobus JS, Kondo T, Reinsel R, Fein G. Dreaming in the late morning and summation of REM and diurnal cortical activation. Conscious Cogn. 1995;4:275–99. doi: 10.1006/ccog.1995.1039. [DOI] [PubMed] [Google Scholar]
- 13.Fosse R, Stickgold R, Hobson JA. Thinking and hallucinating: reciprocal changes in sleep. Psychophysiology. 2004;41:298–305. doi: 10.1111/j.1469-8986.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones BE. Elimination of paradoxical sleep by lesions of the pontine gigantocellular tegmental field in the cat. Neurosci Lett. 1979;13:285–93. doi: 10.1016/0304-3940(79)91508-8. [DOI] [PubMed] [Google Scholar]
- 15.Chase T, Moretti L, Prensky A. Clinical and electroencephalographic manifestations of vascular lesions of the pons. Neurology. 1968;18:357–68. doi: 10.1212/wnl.18.4.357. [DOI] [PubMed] [Google Scholar]
- 16.Cummings JL, Greenberg R. Sleep patterns in the ‘locked in’ syndrome. Electroencephalogr Clin Neurophysiol. 1977;43:270–1. doi: 10.1016/0013-4694(77)90134-1. [DOI] [PubMed] [Google Scholar]
- 17.Solms M. Mahwah, NJ: Lawrence Erlbaum; 1997. the neuropsychology of dreams: a clinic-anatomical study. [Google Scholar]
- 18.Nausieda P, Weiner W, Kaplan L, Weber S, Klawans H. Sleep disruption in the course of chronic levodopa therapy. An early feature of the levodopa psychosis. Clin Neuropharmacol. 1982;5:183–4. doi: 10.1097/00002826-198205020-00003. [DOI] [PubMed] [Google Scholar]
- 19.Scharf B, Moskowitz C, Lupton M, Klawans H. Dream phenomena induced by chronic Levodopa therapy. J Neural Transm. 1978;43:143–51. doi: 10.1007/BF01579073. [DOI] [PubMed] [Google Scholar]
- 20.Cantero JL, Atienza M, Salas RM, Gomez C. Alpha power modulation during periods with rapid occulomotor activity in human REM sleep. Cogn Neurosci. 1999;10:1817–20. doi: 10.1097/00001756-199906230-00003. [DOI] [PubMed] [Google Scholar]
- 21.Jouny C, Chapotot F, Merica H. EEG spectral activity during paradoxical sleep: further evidence for cognitive processing. Sleep. 2000;11:3667–71. doi: 10.1097/00001756-200011270-00016. [DOI] [PubMed] [Google Scholar]
- 22.Esposito MJ, Nielsen TA, Paquette T. Reduced alpha power associated with the recall of mentation from stage 2 and stage REM sleep. Psychophysiology. 2004;41:288–97. doi: 10.1111/j.1469-8986.00143.x. [DOI] [PubMed] [Google Scholar]
- 23.Jouvet M, Michel F. Correlations electromyographiques du sonuneil chez le chat decortique et mesencephalique chronique. [Electromyographic correlations of sleep in the chronic decorticate & mesencephalic cat] C R Seances Soc Biol Fil. 1959;153:422–5. [PubMed] [Google Scholar]
- 24.Hobson JA, McCarley RW. Cortical unit activity in sleep and waking. Electroencephalogr Clin Neurophysiol. 1971;30:97–112. doi: 10.1016/0013-4694(71)90271-9. [DOI] [PubMed] [Google Scholar]
- 25.Callaway CW, Lydic R, Baghdoyan HA, Hobson JA. Pontogeniculooccipital waves: spontaneous visual system activity during rapid eye movement sleep. Cell Mol Neurobiol. 1987;7:105–49. doi: 10.1007/BF00711551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell Mol Neurobiol. 1997;17:341–65. doi: 10.1023/A:1026398402985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. an H2(15)O PET study. Brain. 1997;120:1137–97. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 28.Maquet P, Peters J, Aerts J, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–6. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 29.Bowker RM, Morrison AR. the startle reflex and PGO spikes. Brain Res. 1976;102:185–90. doi: 10.1016/0006-8993(76)90586-2. [DOI] [PubMed] [Google Scholar]
- 30.McCarley RW, Winkleman JW, Duffy FH. Human cerebral potentials associated with REM sleep rapid eye movements: links to PGO waves and waking potentials. Brain Res. 1983;274:359–64. doi: 10.1016/0006-8993(83)90719-9. [DOI] [PubMed] [Google Scholar]
- 31.Lim AS, Lozano AM, Moro E, et al. Characterization of REM-sleep associated ponto-geniculo-occipital waves in the human pons. Sleep. 2007;20:823–7. doi: 10.1093/sleep/30.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conduit R, Bruck D, Coleman G. Induction of visual imagery during NREM sleep. Sleep. 1997;20:948–56. doi: 10.1093/sleep/20.11.948. [DOI] [PubMed] [Google Scholar]
- 33.Fedyszyn I, Conduit R. Tone induction of ocular activity and dream imagery from stage 2 sleep. Dreaming. 2007;17:35–47. [Google Scholar]
- 34.Sammut E. Melbourne Victoria, Australia: Monash University; 2006. Investigation of auditory contingent cueing during REM sleep on visual perceptual learning. [Google Scholar]
- 35.Sockeel P, Mouze-Amady M, Leconte P. Modification of EEG asymmetry induced by auditory biofeedback loop during REM sleep in man. Int J Psychophysiol. 1987;5:253–60. doi: 10.1016/0167-8760(87)90056-0. [DOI] [PubMed] [Google Scholar]
- 36.Okuma T, Nakamura K, Hayashi a, Fujimori M. Psychophysiological study on the depth of sleep in normal human subjects. Electroencephalogr Clin Neurophysiol. 1966;21:140–7. doi: 10.1016/0013-4694(66)90121-0. [DOI] [PubMed] [Google Scholar]
- 37.Wehrle R, Kaufmann C, Wetter TC, et al. Functional microstates within human REM sleep: first evidence from fMRI of a thalamocortical network specific for phasic REM periods. Eur J Neurosci. 2007;25:863–71. doi: 10.1111/j.1460-9568.2007.05314.x. [DOI] [PubMed] [Google Scholar]
- 38.Llinas R, Pare D. Of dreaming and wakefulness. Neuroscience. 1991;44:521–35. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 39.Jasper HH. The ten-twenty electrode placement system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:418–24. [Google Scholar]
- 40.American Sleep Disorders Association. EEG arousals: Scoring rules and examples. a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 41.Rechtschaffen A, Kales A. Washington, DC: Public Health Services, U.S. Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 42.Antrobus JS, Fein G, Jordan L, Ellman SJ, Arkin AM. Measurement and design in research on sleep reports. In: Ellman SJ, Antrobus JS, editors. The mind in sleep: psychology and physiology. New York: John Wiley & Sons, Inc; 1991. [Google Scholar]
- 43.Wu MF, Mallick BN, Siegel JM. Lateral geniculate spikes, muscle atonia and startle response elicited by auditory stimuli as a function of stimulus parameters and arousal state. Brain Res. 1989;499:7–17. doi: 10.1016/0006-8993(89)91130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu MF, Suzuki SS, Siegel JM. Anatomical distribution and response patterns of reticular neurons active in relation to acoustic startle. Brain Res. 1989;457:399–406. doi: 10.1016/0006-8993(88)90716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder F. Toward an evolutionary theory of dreaming. Am J Psychiatry. 1966;123:121–36. doi: 10.1176/ajp.123.2.121. [DOI] [PubMed] [Google Scholar]
- 46.Fouche JR, Otzenberger H, Gounot D. Where arousal meets attention: a simultaneous fMRI and EEG recording study. Neuroimage. 2004;22:688–97. doi: 10.1016/j.neuroimage.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 47.Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–61. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 48.Langford GW, Meddis R, Pearson AJ. Awakening latency from sleep for meaningful and non-meaningful stimuli. Psychophysiology. 1974;11:1–5. doi: 10.1111/j.1469-8986.1974.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 49.Jacobs D, Conduit R. Name discrimination from stage 2 and REM sleep. Sleep. 2005;28:367. [Google Scholar]
- 50.Pivik RT. Tonic states and phasic events in relation to sleep mentation. In: Ellman SJ, Antrobus JS, editors. the mind in sleep: psychology and physiology. 2nd ed. New York: J. Wiley; 1991. [Google Scholar]
- 51.Sanford LD, Ball WA, Morrison AR, Ross RJ, Mann GL. Peripheral and central components of alerting: Habituation of acoustic startle, orienting responses and elicited waveforms. Behav Neurosci. 1992;106:112–20. doi: 10.1037//0735-7044.106.1.112. [DOI] [PubMed] [Google Scholar]
- 52.Shapiro A, Goodenough DR, Gryler RB. Dream recall as a function of method of awakening. Psychosom Med. 1963;25:174–80. doi: 10.1097/00006842-196303000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Shapiro A, Goodenough DR, Lewis HB, Sleser I. Gradual arousal from sleep: a determinant of thinking reports. Psychosom Med. 1965;27:342–9. doi: 10.1097/00006842-196507000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Goodenough DR, Lewis HB, Shapiro LA, Jaret L, Sleser I. Dream reporting following abrupt and gradual awakenings from different types of sleep. J Pers Soc Psychol. 1965;2:170–9. doi: 10.1037/h0022424. [DOI] [PubMed] [Google Scholar]