Abstract
Study Objectives:
Arousal parasomnias are expressions of sleep/wake state dissociations in which wakefulness and NREM sleep seem to coexist. We describe the results of a neurophysiological (intracerebral EEG) investigation that captured an episode of confusional arousal.
Design:
Observational analysis.
Setting:
Tertiary sleep center.
Subject:
A 20-year-old male with refractory focal epilepsy.
Measurements and Results:
The intracerebral EEG findings documented the presence of a local arousal of the motor and cingulate cortices associated with increased delta activity in the frontoparietal associative cortices; these findings were noted preceding the onset and persisting throughout the episode.
Conclusions:
The presence of dissociated sleep/wake states in confusional arousals is the expression not of a global phenomenon, but rather of the coexistence of different local states of being: arousal of the motor and cingulate cortices and inhibition of the associative ones. Whether this is an exclusive feature of NREM parasomnias, or a common substrate on which other triggering elements act, needs to be clarified.
Citation:
Terzaghi M; Sartori I; Tassi L; Didato G; Rustioni V; LoRusso G; Manni R; Nobili L. Evidence of dissociated arousal states during NREM parasomnia from an intracerebral neurophysiological study. SLEEP 2009;32(3):409–412.
Keywords: Local arousal, NREM parasomnia, state dissociation, confusional arousal, stereo-EEG
CONFUSIONAL AROUSALS, SLEEP TERRORS, AND SLEEPWALKING BELONG TO A FAMILY OF DISORDERS SHARING COMMON FEATURES, CLASSIFIED AS arousal parasomnias. Their hallmarks are onset during NREM slow wave sleep (although episodes arising from stage 2 NREM sleep are documented) and a lack of conscious awareness or memory of the event; the event is characterized by the presence of automatic behaviors.1
A breakdown of boundaries between wakefulness and NREM sleep, resulting in the coexistence of these 2 states, is considered to be the basis of arousal parasomnias.2 Indeed, routine scalp EEG during these episodes often shows the presence of high-amplitude slow waves with superimposed wake-like activity (α and β activity).3–5 This could be the expression of a global phenomenon in which sleep and wake activity involve the whole brain simultaneously; conversely, it could be the result of coexistence of different local behaviors: local “awakenings” and local sleep. A SPECT study during sleepwalking found an increase in regional blood flow in the posterior cingulate cortex, while blood flow in the frontoparietal associative cortices was decreased.6
Here we describe the results of a neurophysiological investigation that captured a confusional arousal in a subject affected by drug-resistant epilepsy who was undergoing an intracerebral EEG investigation (stereo-EEG [S-EEG]) during presurgical assessment. The S-EEG findings showed the presence of local arousal of the motor and cingulate cortices associated with increased delta activity in the frontoparietal associative cortices.
SUBJECT AND METHODS
A 20-year-old male was admitted to the “C. Munari Epilepsy and Parkinson's Disease Surgery Centre”, Niguarda Hospital, Milan, for presurgical evaluation of refractory focal epilepsy. The patient had suffered meningitis with multiple right parietal abscesses at the age of 3 years; 4 years later, he began to experience epileptic seizures.
Anamnestic data, confirmed by video-EEG investigation, revealed that the seizures occurred only during wakefulness and consisted of an uncomfortable sensation in the left arm, followed by dystonic posturing and involuntary movements (jerks of the arm and fingers). Consciousness was preserved throughout seizures, and lucid recall was always present. The patient was able to apprise watchers of the occurrence of his seizures, as well as speak coherently during the fits.
At 9 years of age, after a seizure-free period of 2 years, the patient began experiencing fits once or twice a week; these lasted from seconds to 1 to 2 min. A variety of antiepileptic agents, in monotherapy or polytherapy, had been administered, but no drug regimen had resulted in satisfactory seizure control.
Analysis of video-EEG recorded seizures suggested a right centroparietal origin of the episodes. Magnetic resonance imaging (MRI) evidence of a right parietal porencephalic cavity was consistent with this finding.
A history of arousal parasomnias starting at the age of 2 years was reported. These episodes were rare and appeared within the first 2-3 hours of sleep. By report, these episodes consisted of either:
(1) confusional arousals: the patient would move around in the bed or sit on the edge of the bed—sometimes accompanied by apparently purposeful actions (as through trying to take hold of something or someone, or move them out of the way), moaning or talking; during these episodes his eyes were open, and he would fail to recognize his parents who tried unsuccessfully to awake him; or
(2) sleepwalking: sitting up in bed and looking around, followed by walking and moving objects in the room.
In both cases, the episodes lasted just a few minutes, and the following morning the patient would have no recall of them. The parasomnias became less frequent with increasing age.
Family history was positive for sleepwalking. The neurological examination was unremarkable.
Neurophysiological study
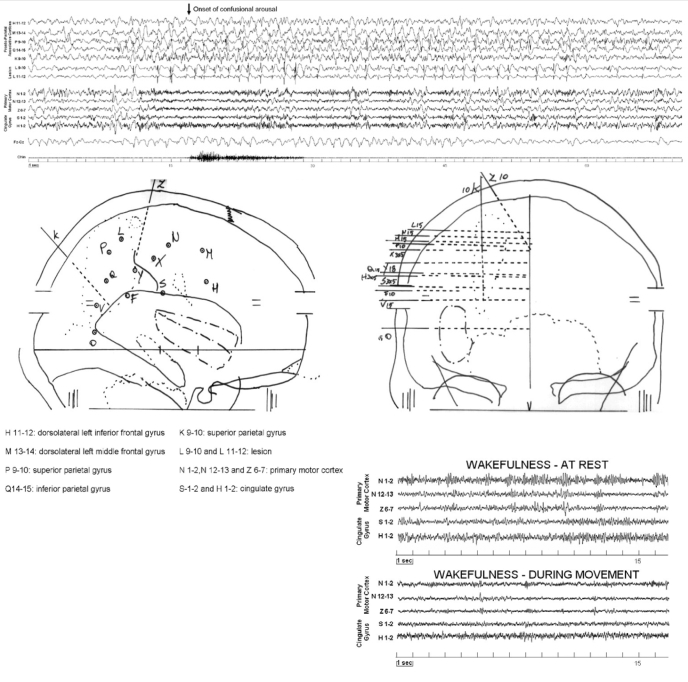
The patient underwent a personalized investigation in which intracerebral implanted electrodes were used to define the epileptogenic area for surgical purposes (S-EEG). Fourteen stereotactically implanted intracerebral multilead electrodes were placed in the primary sensory-motor cortex (central and post-central gyrus), supplementary sensory motor area, superior and inferior parietal lobules, pre-central and central cingulate gyrus, middle and inferior frontal gyrus, and F1-F2 sulcus (Figure 1). The placement of the intracerebral leads was checked by MRI. In addition, submental EMG was recorded.
Figure 1.
Stereo-EEG recording of the confusional arousal: each EEG trace is a bipolar derivation from contiguous contacts. Five derivations record frontoparietal associative cortex (dorsolateral cortex) activity; 2 derivations the epileptic focus (intralesional contacts); 3 derivations record from the primary motor cortex and 2 from central cingulate cortex. One derivation records from the midline of the scalp (Fz-Cz). The arrow indicates the onset of the clinical manifestation: note the absence of ictal epileptic discharges during the episode. Lower part: Right lateral and frontal views of the stereotactic electrode implantation for the stereo-electroencephalogram (S-EEG) investigation. At right: activity recorded from the primary motor cortex and from central cingulate cortex during wakefulness at rest and during movement.
S-EEG confirmed that the ictal epileptic discharge originated from the parietal lobe (128-channel acquisition system, Telefactor Corp. West Conshohocken, PA, USA).
S-EEG allowed the identification of the epileptogenic zone; its surgical removal led to a seizure-free outcome at 2 years' follow up (Engel's class Ia outcome).
RESULTS
Video-S-EEG captured an episode, occurring during slow wave sleep, in which the patient turned in bed with his eyes closed, extended his arms as if to embrace someone, gave a kiss, and uttered a few unintelligible words. When called by the technician, he did not answer but lay down, and continued to sleep in stage 2 NREM sleep. The episode lasted 32 seconds; the patient had no recall of the event the following morning.
The S-EEG trace showed a delta burst in the frontal and parietal dorsolateral cortices, also detected by the scalp midline (Fz-Cz) channel. An increase in the frequency of interictal epileptiform discharges (spike/polyspike and wave) in the leads exploring the lesional area accompanied the delta burst. In the motor and central cingulate cortices, after a short initial increase in delta activity, the presence of sustained fast activity with a frequency of approximately 25 Hertz was detectable (the same fast activity seen in wakefulness during movements, Figure 1 lower right corner); this fast activity was then interrupted by bursts of delta waves, before receding into the background NREM sleep activity (Figure 1). The clinical episode started 5 seconds after the observed changes in S-EEG activity.
This S-EEG pattern was completely different from the one presented during the epileptic manifestations. Indeed, during S-EEG monitoring, ictal epileptic phenomena were captured: low-voltage fast activity followed by rhythmic high-voltage activity with spike and wave components were seen in the parietal lesional area and spreading to the bordering frontal, parietal, and cingulate cortices. This pattern corresponded to dystonic posturing of the left arm with myoclonic jerks, recognized by the patient as his typical manifestation (Figure 2). During the 8 days of S-EEG monitoring, consistently with the patient's anamnestic data, no manifestations of an epileptic nature were observed during sleep.
Figure 2.
Stereo-EEG recording of a seizure. Montage as in Figure 1. The ictal epileptic discharge consists of low-voltage fast activity followed by rhythmic high-voltage activity, with spike and wave components involving the parietal lesional area and spreading to the bordering frontal, parietal, and cingulate cortices. Black arrow indicates the beginning of the seizure.
DISCUSSION
Arousal parasomnias are reflected in a spectrum of automatic nocturnal behaviors ranging from quiet sitting to ambulation and compulsive, violent actions. It has been proposed that the pathophysiological mechanisms of arousal parasomnias might involve a dysfunction in the maintenance of stable sleep, as markers of unstable slow-wave sleep7,8 have been reported. Arousal parasomnias, occurring predominantly during slow wave sleep, are thought to be the effect of incomplete arousals from sleep, caused by the coexistence of conflicting elements of wakefulness and NREM sleep.2
In this patient, we showed that brain activity can exhibit different local behaviors during arousal parasomnias. The simultaneous presence of different states of being (i.e., wakefulness and NREM sleep) was evidenced as local brain phenomena.
Indeed, during an episode of confusional arousal, we found that sleep instability manifested itself in a dual way: the motor and cingulate cortices were precociously activated and displayed the same fast activity seen during wakefulness,9,10 while the frontoparietal associative cortices displayed an enhancement of delta activity. From this perspective, the awakening of the motor and cingulate cortices is in conflict with the persistent sleep state of the associative ones. These findings constitute the neurophysiological substrate of data obtained with ictal SPECT.6
Our data do not explain the cause of this dissociated arousal behavior. We might hypothesize a different threshold of arousability between the motor/cingulate cortices and frontoparietal associative areas, with the awakening threshold being lower in the motor and cingulate cortices. An initial delta burst did appear but quickly reverted to wake-like activity in these cortices, while in the frontoparietal associative cortices the delta activity persisted and became stronger. The marked increase in delta activity in the frontoparietal associative cortices could be interpreted as an attempt to maintain the continuity of sleep when other brain areas are moving towards arousal. This different susceptibility to arousing stimuli could underlie the dissociated activity of these different brain regions; if this dissociated activity is not interrupted, motor phenomena of the kind observed in arousal parasomnias can occur. In fact, the cingulate gyrus is involved in the genesis of complex motor behaviors11 and is thought to be under the inhibitory control of associative cortices.12 Loss of the inhibitory function of the frontoparietal cortices, together with the activation of the motor and cingulate cortices, could explain the appearance of innate, complex motor patterns.13
It is known that sleep deprivation may facilitate the occurrence of arousal parasomnias.14 The increased homeostatic sleep pressure observed after sleep deprivation,15 enhancing delta power especially over the frontal regions,16 could favor the persistence of slow activity in the presence of a local motor arousal.
We hypothesize that the local, limited arousal in the motor and cingulate cortices is at the root of the motor behaviors peculiar to arousal parasomnias, while the persistence of slow wave activity in associative cortices could explain the specific lack of awareness and insight.
The observed dissociated activity appears before the clinical manifestation; it remains to be clarified if dissociated arousal is an exclusive feature of NREM parasomnias or a common substrate on which other triggering elements act.
It should be remembered that our data derive from an S-EEG investigation in an epileptic patient, thus raising the question of whether our findings can be considered valid for the general population. We remain confident that these findings could represent a general behavior, as we were able to rule out an epileptic origin of the nocturnal manifestation recorded.
Finally, the coexistence of parasomnias and epilepsy, as we have found, is a complex relationship that is still a long way from being fully understood.17,18 Further studies are needed to clarify this association.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr Daniele Marchese for his help in drawing figures.
REFERENCES
- 1.American Academy of Sleep Medicine. diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- 2.Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437:1279–85. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- 3.Schenck CH, Milner DM, Hurwitz TD, Bundlie SR, Mahowald MW. A polysomnographic and clinical report on sleep-related injury in 100, adult patients. Am J Psychiatry. 1989;146:1166–73. doi: 10.1176/ajp.146.9.1166. [DOI] [PubMed] [Google Scholar]
- 4.Zadra A, Pilon M, Joncas S, Rompre S, Montplaisir J. Analysis of postarousal EEG activity during somnambulistic episodes. J Sleep Res. 2004;13:279–84. doi: 10.1111/j.1365-2869.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 5.Schenck CH, Pareja JA, Patterson AL, Mahowald MW. Analysis of polysomnographic events surrounding 252 slow-wave sleep arousals in thirty-eight adults with injurious sleepwalking and sleep terrors. J Clin Neurophysiol. 1998;15:159–66. doi: 10.1097/00004691-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet. 2000;356:484–5. doi: 10.1016/S0140-6736(00)02561-7. [DOI] [PubMed] [Google Scholar]
- 7.Bruni O, Ferri R, Novelli L, Finotti E, Miano S, Guilleminault C. NREM sleep instability in children with sleep terrors: The role of slow wave activity interruptions. Clin Neurophysiol. 2008;119:985–92. doi: 10.1016/j.clinph.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Guilleminault C, Kirisoglu C, da Rosa AC, Lopes C, Chan A. Sleepwalking, a disorder of NREM sleep instability. Sleep Med. 2006;7:163–70. doi: 10.1016/j.sleep.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 2006;32:1281–9. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Kristeva R, Patino L, Omlor W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage. 2007;36:785–92. doi: 10.1016/j.neuroimage.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Talairach J, Bancaud J, Geier S, et al. The cingulate gyrus and human behaviour. Electroencephalogr Clin Neurophysiol. 1973;34:45–52. doi: 10.1016/0013-4694(73)90149-1. [DOI] [PubMed] [Google Scholar]
- 12.Hikosaka O. Neural systems for control of voluntary action-a hypothesis. Adv Biophys. 1998;35:81–102. [PubMed] [Google Scholar]
- 13.Tassinari CA, Rubboli G, Gardella E, et al. Central pattern generators for a common semiology in fronto-limbic seizures and in parasomnias. A neuroethologic approach. Neurol Sci. 2005;26(Suppl 3):S225–32. doi: 10.1007/s10072-005-0492-8. [DOI] [PubMed] [Google Scholar]
- 14.Zadra A, Pilon M, Montplaisir J. Polysomnographic diagnosis of sleepwalking: effects of sleep deprivation. Ann Neurol. 2008;63:513–9. doi: 10.1002/ana.21339. [DOI] [PubMed] [Google Scholar]
- 15.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara M, De Gennaro L, Curcio G, Cristiani R, Bertini M. Regional differences of the human sleep electroencephalogram in response to selective slow-wave sleep deprivation. Cereb Cortex. 2002;12:737–48. doi: 10.1093/cercor/12.7.737. [DOI] [PubMed] [Google Scholar]
- 17.Tinuper P, Provini F, Bisulli F, et al. Movement disorders in sleep: guidelines for differentiating epileptic from non-epileptic motor phenomena arising from sleep. Sleep Med Rev. 2007;11:255–67. doi: 10.1016/j.smrv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Nobili L. Nocturnal frontal lobe epilepsy and non-rapid eye movement sleep parasomnias: differences and similarities. Sleep Med Rev. 2007;11:251–4. doi: 10.1016/j.smrv.2007.03.009. [DOI] [PubMed] [Google Scholar]