Abstract
Study Objective:
The present study aimed to analyze season of birth effects on preferred sleep-wake cycle timing as assessed by Morningness-Eveningness Questionnaire (MEQ).
Participants and Measurements:
The MEQ was administered to a sample of 5,720 university students (3,851 Italians and 1,869 Spaniards; 3,877 female and 1,843 male; mean age 22.23 ± 2.98 years).
Results:
Females preferred to go to bed significantly earlier and sleep longer than males, regardless of season of birth and nationality. Subjects born in spring and summer went to bed and reached midpoint of sleep later than subjects born in fall and winter. Nationality significantly affected all the sleep parameters considered except duration.
Conclusion:
Overall, the effect of the season of birth on sleep preference timing was significant but quantitatively small. We suggest an evolutionary context for the different contributions of genetic and environmental factors in modulating sleep-wake cycles in humans.
Citation:
Natale V; Adan A; Fabbri M. Season of birth, gender, and social-cultural effects on sleep timing preferences in humans. SLEEP 2009;32(3):423–426.
Keywords: Season of birth, circadian rhythm, morningness-eveningness, gender differences, sleep timing
THE PART OF THE BRAIN THAT ORCHESTRATES RHYTHMICITY IS THE SUPRACHIASMATIC NUCLEUS (SCN) IN THE ANTERIOR HYPOTHALAMUS. THE NEURAL INPUT and output pathways for SCN develop in the fetus and continue to mature during the early neonatal period.1 It has been shown that human SCN grows in cell number and size around the time of birth and during the first few months after birth.2 As a consequence, it has been posited that the environmental variables during pregnancy and the perinatal period could have a decisive effect on the developing circadian system of the fetus. A series of studies have shown that season of birth is an important proxy for such environmental factors vis-à-vis the development of various individual differences and pathologies in adults: handedness,3 personality,4 mental disorders,5 and sleep disorders.6 Etiology of the season of birth effect however is still not completely understood. Neurodevelopmental damage caused by prenatal or postnatal viral infection was one of the first hypotheses7 put forward, though in the last year, the issue of photoperiod has come to the fore. It seems likely that circannual variations of daylight in the late fetal period and the early stages of development influence the maturing neurohormonal system in hypothalamic nuclei.8
Surveys conducted in different countries have demonstrated a relationship between season of birth and circadian typology.9 These studies, in which more evening types were found among people born during spring and summer, have posited the photoperiod around birth as a crucial factor.10,11 As already demonstrated in animals,12 exposure to increasing photoperiod at birth seems to elicit phase delay (eveningness) of the human circadian system. According to this hypothesis, people born in spring (increasing photoperiod) or summer (long photoperiod) would have internal clocks with longer days than people born in autumn (decreasing photoperiod) or winter (short photoperiod). This setting “error” might reflect the fact that the newborn's pineal gland has had only 9 months to develop.13 The melatonin timing mechanism, which codes for day length, is initiated prenatally by the maternal pineal gland and continued postnatally by the infant's pineal gland. Seasonal variation on day length requires 12 months of development (as opposed to the 9-month gestation period). Following this line of thought, it might be expected that people born in the summer season tend to present delayed sleep onset in the first months of their life, and vice versa for people born in autumn.
Previous work would seem to point to a season of birth effect on sleep timing. But is this effect equally distributed between onset and offset of sleep? Is a season of birth effect on sleep duration possible? The aim of the present survey was to examine season of birth effect on the sleep-wake cycle in young adult humans. To our knowledge, there are no published scientific studies on this issue. We found only one work on season of birth and schizophrenia, in which an unexpected relationship was found between hours of reported sleep and season of birth: fall births were associated with increased hours of sleep.14 In a recent longitudinal survey on 1,112 children, sleep-wake schedules were not associated with birth season.15
Taking into account the masking effect of social schedules we decided to investigate preferred sleep-wake cycle timing, using the Morningness-Eveningness Questionnaire (MEQ).16 The MEQ, which was the first tool developed to assess circadian typology, consists of 19 mixed-format questions regarding the time individuals get up and go to bed, preferred times for physical and mental activity, and subjective alertness. Although it has been criticized for not just collecting information on morningness, but on other domains as well,17,18 it has proven to be a valid and reliable tool using physiological and psychological parameters.19,20 The MEQ has been translated into many languages and is still the most frequently used self-evaluation tool. Above all, the MEQ is the only circadian questionnaire with open questions, in which subjects can put their preferred time without the limitations of using ordinal scales; it was for this reason we decided to use it in this study. To better estimate the effect of season of birth on sleep timing preference, we ran analyses on the answers to items 1 (preferred arising time) and 2 (preferred going-to-bed time). Using the preferred sleep onset (item 2) and awakening (item 1) times, we also indirectly calculated preferences regarding sleep need21 (or, more properly, time in bed) and midpoint of sleep.22
To better evaluate the relationship between season of birth and sleep timing preference, we collected data from 2 different countries: Spain and Italy. Despite similar latitudes, Spanish social life is organized more towards eveningness than Italian life is.23 In this way, we thought we could simultaneously evaluate biological (gender), social-cultural (nationality), and birth environment (photoperiod) factors in modulating sleep timing preference in humans.
METHODS
All participants answered the Morningness-Eveningness Questionnaire (MEQ).16 They were tested in groups ranging in size from 10 to 100 students; their participation was voluntary, and they were not paid.
The final sample consisted of 5,720 university students with an age range of 18-34 years (mean, 22.23 ± 2.98 years; median, 21; mode, 20; Table 1).
Table 1.
Sample Characteristics
| Male | Female | Total | |
|---|---|---|---|
| Italian | N = 1,321 | N = 2,530 | N = 3,851 |
| Age 23.66 ± 3.14 | Age 22.52 ± 2.81 | Age 22.92 ± 2.97 | |
| Spanish | N = 522 | N = 1,347 | N = 1,869 |
| Age 21.39 ± 2.75 | Age 20.60 ± 2.26 | Age 20.82 ± 2.43 | |
| Total | N = 1,843 | N = 3,877 | N = 5,720 |
| Age 23.02 ± 3.20 | Age 21.85 ± 2.79 | Age 22.23 ± 2.98 |
N = number of subjects; Age = mean ± SD. Spanish subjects were younger than Italian subjects (t5718 = 26.46; P < 0.00001). Female subjects were younger than male subjects in the whole sample (t5718 = 14.09; P < 0.00001) and in the nationality sub-samples: Italian (t3849 = 11.48; P < 0.00001) and Spanish (t1867 = 6.42; P < 0.00001).
To estimate the season effect we subdivided the subjects in 4 season groups according to birth date: autumn (23 September to 21 December; n = 1,294; 22.62%); winter (22 December to 20 March; n = 1,380; 24.12%); spring (21 March to 21 June; n = 1,567; 27.40%); summer (22 June to 22 September; n = 1,479; 25.86%). As expected,24 significantly more subjects were born in spring and summer than autumn (χ2 = 34.74, P < 0.00001 and χ2 = 16.29, P < 0.0001, respectively) and winter (χ2 = 15.98, P < 0.0001 and χ2 = 4.57, P < 0.05, respectively). The ratios of males to females (female percentage ranged between 65.84% and 70.51%) and Spaniards to Italians (Italian percentage ranged between 64.07% and 69.86%) were stable among subjects born during each season.
To understand which of the 3 independent variables considered in this study (gender, nationality, and season of birth) could be the best predictor of sleep timing, we performed a multiple linear stepwise regression test for each dependent variable collected: preferred time of sleep onset and awakening, preferred hours of sleep, and midpoint of sleep. For each dependent variable an analysis of covariance (ANCOVA) was performed using the 3 independent variables, gender, nationality, and month of birth (12 levels), with age as the covariate.
In view of the large size of the sample, we decided to consider only the results that were significant in both analyses, setting the cut-off value for significant results at P < 0.0005.25 All statistical analyses were performed by SPSS 14.0 (SPSS, Inc. Chicago, IL).
This study was approved by the ethics committees of the Barcelona and Bologna Universities and carried out in accordance with the Declaration of Helsinki; all participants gave informed consent prior to inclusion in the study.
RESULTS
Nationality was the best predictor of time of awakening (item 1) (β = 0.15; t5716 = 11.68; P < 0.00001), followed by season of birth (β = 0.05; t5716 = 4.07; P < 0.00005); gender had no significant effect (β = 0.01; t5716 = 2.04; P = 0.041). On ANCOVA, gender and month of birth factors were not significant (respectively: F1,5671 = 4.86; P = 0.027 and F11,5671 = 2.48; P = 0.004). In contrast, nationality proved significant, since the Spaniards (09:43 ± 1 h 09 min) got up later than the Italians (09:21 ± 1 h 16 min) (F1,5671 = 29.41; P < 0.00001). Interaction between factors did not reach significance.
For preferred sleep onset time (item 2), all 3 independent variables had a significant predictive effect: gender (β = 0.19; t5716 = 14.67; P < 0.00001), nationality (β = 0.18; t5716 = 14.08; P < 0.00001), and season of birth (β = 0.06; t5716 = 4.75; P < 0.00001). Men (24:42 ± 1 h 23 min) went to bed later than women (24:13 ± 1 h 13 min) (F1,5671 = 140.62; P < 0.00001), and Spaniards (24:40 h ± 1 h 11 min) later than Italians (24:13 ± 1 h 19 min) (F1,5671 = 124.15; P < 0.00001); month of birth had a significant effect on sleep onset time (F11,5671 = 3.25; P < 0.0005). The post hoc test (Tukey for unequal samples) showed that subjects born in May (24:36 ± 1 h 18 min), June (24:36 ± 1 h 24 min), and August (24:37 ± 1 h 21 min) tend to go to bed later than subjects born in December (24:18 ± 1 h 13 min), January (24:19 ± 1 h 12 min), and February (24:19 ± 1 h 12 min, P < 0.005). No interaction reached significance.
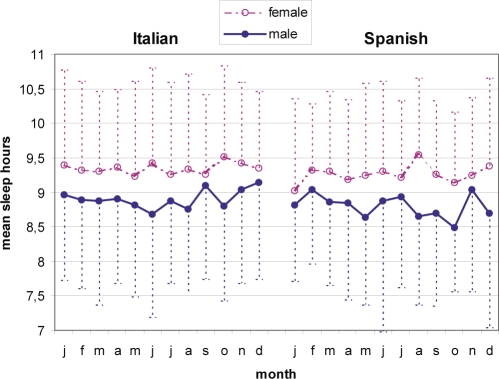
As regards preferred hours of sleep, only gender reached significance (β = 0.16; t5716 = 12.51; P < 0.00001), while season of birth (β = 0.01; t5716 = 0.75; P = 0.45) and nationality (β = 0.03; t5716 = 2.58; P = 0.009) elicited no significant effect. ANCOVA (Figure 1) indicated that men (8 h 49 min ± 1 h 09 min) slept significantly less than women (9 h 18 min ± 1 h 11 min) (F1,5671 = 93.87; P < 0.00001), and Spaniards (9 h 1 min ± 1 h 15 min) significantly less than Italians (9 h 8 min ± 1 h 18 min) (F1,5671 = 34.34; P < 0.00001). Month of birth effect and interaction between factors were not significant.
Figure 1.
Mean values and standard deviations (dotted line) of preferred sleep hours for every month of birth, subdivided by gender (male = line and female = outline) and nationality (Italian left; Spanish right). Women prefer significantly more sleep than men, independent of nationality and season of birth (F1,5671 = 93.87; P < 0.00001).
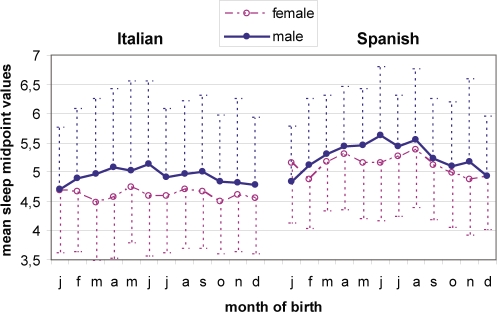
As regards the midpoint of preferred sleep, all 3 independent variables had a significant predictor effect: nationality (β = 0.19; t5716 = 15.01; P < 0.00001), gender (β = 0.12; t5716 = 9.79; P < 0.00001), season of birth (β = 0.07; t5716 = 5.13; P < 0.00001). With ANCOVA (Figure 2), males (05:07 ± 1 h 13 min) reached midpoint later than women (04:52 ± 1 h 01 min) (F1,5671 = 68.08; P < 0.00001), and Spaniards (05:12 ± 1 h) later than Italians (04:47 ± 1 h 07 min) (F1,5671 = 93.50; P < 0.00001). The month of birth factor was also significant (F3,5671 = 3.58; P < 0.00001), with post hoc test (Tukey for unequal samples) indicating that subjects born in June (05:08 ± 1 h 10 min) and August (05:09 ± 1 h 08 min) reach sleep midpoint later than subjects born in October (04:51 ± 1 h 01 min), December (04:51 ± 1 h), January (04:51 ± 1 h 02 min), and February (04:53 ± 1 h 01 min ) (P < 0.001). Interaction between factors did not reach significance.
Figure 2.
Mean hour and SD (dotted line) of the midpoint of preferred sleep for month of birth, subdivided by gender (male = line; female = outline) and nationality (Italian left; and Spanish right). Spanish subjects reach the mean midpoint later than Italian subjects (F1,5671 = 93.50; P < 0.00001). Subjects born in summer reach the midpoint of preferred sleep later than subjects born in autumn and winter (F3,5671 = 3.58; P < 0.00001).
DISCUSSION
On the basis of recent published data, we posited a possible season of birth effect on sleep timing and sleep duration. With regard to sleep timing, the present study found a significant season of birth effect on preferred times for going to bed and sleep midpoint times. In line with previous studies on the effect of season of birth on circadian typology, subjects born in spring and summer preferred going to bed later and reached sleep midpoint later than those born in autumn and winter. This finding might fit the hypothesis that exposure to a long photoperiod elicits a phase delay in human circadian rhythms. The effects of season of birth on sleep-wake cycles are statistically significant but quantitatively small. In our study, the phase-delay linked to season of birth effect reached a maximum of 18 minutes, which is not a great deal if we consider that the mean sleep phase delay displayed by evening types is around 80 minutes versus morning types.19,26
In accordance with previous research23 sleep onset times, hours of sleep and sleep midpoints were different in women and men. Women preferred to go to bed earlier and reached sleep midpoint earlier than men, regardless of nationality and season of birth.
Season of birth seems to affect preferred sleep onset times rather than preferred sleep offset times. An explanation of this might be found in the social factors potentially involved. It may be that in this study, based on subjective answers, the season of birth effect was partially masked by social-cultural factors (nationality), which seems to offer a better explanation of variance in sleep timing preferences. In other words, it may be that people plan their preferred working day not just on the basis of preferred personal rhythms but on social schedules, too. Thus the only factor in our study that significantly modulated awakening time was nationality.
Season of birth had no effect on preferred sleep durations, confirming once more that sleep timing and sleep duration are essentially independent traits.27 The only variable significantly modulating preferred number of sleeping hours in our study was gender: women scored higher than men, confirming both objective28,29 and subjective30,31 reports documenting longer sleep duration in women. Sleep patterns are sexually dimorphic in several species, suggesting that sex hormones influence sleep physiology.32 However, a recent large survey31 reported that females preferred sleeping longer than males irrespective of age, indicating that such sex difference is independent of gonadal function. It has been shown that administration of gonadal hormones to adult animals has minimal effect on sleep and does not accentuate gender differences in regard to sleep patterns. The responsiveness of the hamster's circadian system to estradiol is dependent on androgen-dependent differentiation during early postnatal life.33 Thus, the process of hormone-induced sexual differentiation may affect the circadian system's sensitivity to hormones in the fetus or in early neonatal period. This indicates that there may be a critical period in brain development when androgens have their strongest influence on sleep.33 In view of the fact that sex influences sleep-wake parameters across a wide range of species, we might try to interpret longer sleep durations in women from an evolutionary point of view. Early human societies were small hunter-gatherer units, with a clear division of labor between females and males.34 The male contribution to the family unit was to provide proteins from hunting; a male who needed less sleep might have had a fitness advantage. In contrast, females might draw a fitness advantage from behavior inactivity (sleep), which would allow them to conserve energy and resources required for pregnancy, child-rearing, and birth. Our results contrasted with recent data regarding sleep gender differences in mice (C57BL/6J), in which females presented a longer active phase than males at baseline condition.35 Further comparative studies, including objective physiological measures, should address this issue to more accurately explore the impact of evolutionary factors on the sleep-wake cycle in humans.
In the past 10 years, the basis for cellular rhythmicity has become clearer with the discovery of a series of essential genes.36,37 However, to explain a phenotypic trait as complex as the sleep-wake cycle, we need always to refer to a combination of genetic and environmental factors.38 In sum, our data demonstrate that season of birth (we posit photoperiod at birth) has a slight but significant effect on the timing of sleep: preferred bedtimes and preferred midpoint of sleep. Social-cultural factors (nationality) also modulate the preferred timing of sleep, while sleep duration is more linked to biological factors (gender).
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Kenneway DJ. Programming of the fetal suprachiasmatic nucleus and subsequent adult rhythnicity. Trends Endocrinol Metabol. 2002;13:398–402. doi: 10.1016/s1043-2760(02)00692-6. [DOI] [PubMed] [Google Scholar]
- 2.Swaab DF, Hofman MA, Honnebier MB. Development of vasopressin neurons in the human suprachiasmatic nucleus in relation to birth. Brain Res Dev Brain Res. 1990;52:289–93. doi: 10.1016/0165-3806(90)90247-v. [DOI] [PubMed] [Google Scholar]
- 3.Jones GV, Martin M. Seasonal anisotropy in handedness. Cortex. 2008;44:8–12. doi: 10.1016/j.cortex.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Chotai J, Forsgren T, Nilsson LG, Adolfsson R. Season of birth variations in the temperament and character inventory of personality in a general population. Neuropsychobiology. 2001;44:19–26. doi: 10.1159/000054909. [DOI] [PubMed] [Google Scholar]
- 5.Castrogiovanni P, Iapichino S, Pacchierotti C, Pieraccini F. Season of birth in psychiatry: a review. Neuropsychobiology. 1998;37:175–81. doi: 10.1159/000026499. [DOI] [PubMed] [Google Scholar]
- 6.Picchioni D, Mignot EJ, Harsh JR. The month-of-birth pattern in narcolepsy is moderated by cataplexy severity and may be independent of HLA-DQB1*0602. Sleep. 2004;27:1471–75. doi: 10.1093/sleep/27.8.1471. [DOI] [PubMed] [Google Scholar]
- 7.Sham PC, O'Callaghan E, Takei N, Murray GK, Hare EH, Murray RM. Schizophrenia following pre-natal exposure to influenza epidemics between 1939 and 1960. Br J Psychiatry. 1992;160:461–6. doi: 10.1192/bjp.160.4.461. [DOI] [PubMed] [Google Scholar]
- 8.Sivan Y, Laudon M, Tauman R, Zisapel N. Melatonin production in healthy infants: evidence for season variations. Pediatr Res. 2001;49:63–8. doi: 10.1203/00006450-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Natale V, Adan A. Season of birth modulates the morningness-eveningness preference in humans. Neurosci Lett. 1999;274:139–41. doi: 10.1016/s0304-3940(99)00672-2. [DOI] [PubMed] [Google Scholar]
- 10.Mongrain V, Paquet J, Dumont M. Contribution of the photoperiod at birth to the association between season of birth and diurnal preference. Neurosci Lett. 2006;406:113–16. doi: 10.1016/j.neulet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Natale V, Adan A, Chotai J. Further results on the association between morningness-eveningness preference and the season of birth in human adults. Neuropsychobiology. 2002;46:209–14. doi: 10.1159/000067803. [DOI] [PubMed] [Google Scholar]
- 12.Prichard JR, Fahy JL, Obermeyer WH, Behan M, Benca RM. Sleep responses to light and dark are shaped by early experience. Behav Neurosci. 2004;118:1262–73. doi: 10.1037/0735-7044.118.6.1262. [DOI] [PubMed] [Google Scholar]
- 13.Weissbluth M, Weissbluth L. Colic, sleep inertia, melatonin and circannual rhythms. Med Hypotheses. 1992;38:224–8. doi: 10.1016/0306-9877(92)90099-x. [DOI] [PubMed] [Google Scholar]
- 14.Reid HM, Zborowski MJ. Schizophrenia-proneness, season of birth and sleep: elevated schizotypy scores are associated with spring birth and extremes of sleep. Pers Individ Dif. 2006;41:1185–93. [Google Scholar]
- 15.Touchette E, Mongrain V, Petit D, Tremblay RE, Montplaisir JY. Development of sleep-wake schedules during childhood and relationship with sleep duration. Arch Pediatr Adolesc Med. 2008;162:343–49. doi: 10.1001/archpedi.162.4.343. [DOI] [PubMed] [Google Scholar]
- 16.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 17.Adan A, Almirall H. Horne Östberg morningness-eveningness questionnaire: a reduced scale. Pers Individ Dif. 1991;12:241–53. [Google Scholar]
- 18.Neubauer AC. Psychometric comparison of two circadian rhythm questionnaires and their relationship with personality. Pers Individ Dif. 1992;13:125–32. [Google Scholar]
- 19.Kerkhof GA. Inter-individual differences in the human circadian system: a review. Biol Psychol. 1985;20:83–112. doi: 10.1016/0301-0511(85)90019-5. [DOI] [PubMed] [Google Scholar]
- 20.Natale V, Ciciogna PC. Morningness-eveningness dimension: is it really a continuum? Pers Individ Dif. 2002;32:809–16. [Google Scholar]
- 21.Taillard J, Philip P, Bioulac B. Morningness/eveningness and the need for sleep. J Sleep Res. 1999;8:291–5. doi: 10.1046/j.1365-2869.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 22.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotype. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 23.Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19:709–20. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- 24.Wehr TA. Photoperiodism in humans and other primates: evidence and implications. J Biol Rhythms. 2000;16:348–64. doi: 10.1177/074873001129002060. [DOI] [PubMed] [Google Scholar]
- 25.Whitely E, Ball J. Statstics review 3: hypothesis testing and P values. Crit Care. 2002;6:222–5. doi: 10.1186/cc1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tankova I, Adan A, Buela-Casal G. Circadian typology and individual differences. A review. Pers Individ Dif. 1994;16:671–84. [Google Scholar]
- 27.Roenneberg T, Kuhnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–38. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47:921–27. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 29.Redline S, Kirchner LH, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 30.Kronholm E, Harma M, Hublin C, Aro AR, Partonen T. Self-reported sleep duration in Finnish general population. J Sleep Res. 2006;15:276–90. doi: 10.1111/j.1365-2869.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 31.Tonetti L, Fabbri M, Natale V. Sex difference in sleep time preference and sleep need: a cross-sectional survey among Italian pre-adolescents, adolescents, and adults. Chronobiol Int. 2008;25:745–59. doi: 10.1080/07420520802394191. [DOI] [PubMed] [Google Scholar]
- 32.Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–51. [PubMed] [Google Scholar]
- 33.Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of the circadian system in the golden hamster. Am J Physiol. 1980;238:R97–R101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]
- 34.Barash DP. Sociobiology and behavior. Elsevier: New York; 1982. [Google Scholar]
- 35.Paul KN, Dugovic C, Turek FW, Laposky AD. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29:1211–23. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- 36.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 37.Ebisawa T. Circadian rhythms in the CNS and peripheral clock disorders: human sleep disorders and clock genes. J Pharmacol Sci. 2007;103:150–4. doi: 10.1254/jphs.fmj06003x5. [DOI] [PubMed] [Google Scholar]
- 38.Kihlbom M, Johansson SE. Month of birth, socio-economic background, and development. J Biosoc Sci. 2004;36:547–59. [Google Scholar]