Abstract
This review summarizes information regarding the rare population of very small embryonic-like stem cells (VSELs) that has been identified in adult tissues, emphasizing both their unique morphological features and potential biological significance. We focus on their pluripotent nature and expression of markers characteristic for embryonic stem cells (ESCs), epiblast (EP)SCs, and primordial germ cells (PGCs). Furthermore, we will discuss their rank in the developmental hierarchy of the SC compartment as well as their relationship to other bone marrow-derived, primitive, non-hematopoietic SCs including: i) endothelial progenitor cells (EPCs); ii) mesenchymal (M)SCs; iii) multipotent adult progenitor cells (MAPCs); iv) marrow-isolated adult multilineage inducible (MIAMIs) cells; v) multipotent adult (MA)SCs; and vi) OmniCytes. We will also present different populations of very “small SCs” that have been recently described in the literature (e.g., spore-like cells and Lin-/ALDHhigh long-term repopulating hematopoietic SCs).
Introduction
Beginning the journey into the world of small SCs
Very small embryonic-like stem cells (VSELs) were initially identified by our group in adult murine bone marrow (BM) as a rare population of stem cells (SCs) with embryonic characteristics. Our journey towards their identification and isolation started in 2001, provoked by increasing enthusiasm at that time concerning the “SCs plasticity theory”. We hypothesized that the existence of an unidentified, non-hematopoietic population of BM cells could alternatively explain the regenerative capacity claimed to be the result of trans-dedifferentiation or plasticity of hematopoietic (H)SCs (1-3). We suspected that such cells would reside in adult tissues and display broad differentiation potential. Thus, our first challenge was to identify multipotent or even pluripotent adult SCs in adult BM that would be endowed with a broad differentiation potential to various non-hematopoietic tissues. We assumed that one of the markers on the surface of these cells would be a G-protein-coupled seven trans-membrane span CXCR4 receptor that binds α-chemokine stromal derived factor-1 (SDF-1). Since no good working antibody for murine CXCR4 was available at that time, we performed chemotactic assays on BM nucleated cells (BMNCs) to SDF-1 gradient. We learned that BM contains very rare, primitive CXCR4+ SCs that respond by chemotaxis to SDF-1 and that these cells are different from classical HSCs (3,4).
Developmental hierarchy of SCs
SCs are a pool of unique cells that possess the capability of self-renewal and the ability to differentiate into cell lines that are committed to particular developmental pathways (5-7). From the first stages of embryonic development up to adulthood, the pool of SCs residing in the body is hierarchically organized according to the cells’ differentiation capacities (7,8). The most primitive SCs exist in the zygote, which results from the fusion of two gametes during the process of fertilization. The totipotent SCs of the zygote have the capability to give rise not only to all tissues of an embryo, but also to extra-embryonic placental tissues. Subsequently, the cells in the morula (embryo at <32-cell stage) have already lost their totipotency, but preserve their ability to differentiate into cells from all three embryonic germ layers, i.e., the ecto-, endo-, and mesoderms. Such cells are defined as pluripotent (P)SCs and can contribute to all the tissues of the developing embryo. PSCs are also identified in the next stages of embryonic development in the inner cell mass (ICM) of the blastocyst (9-11) and as cells of the proximal primitive ectoderm (epiblast) in the developing gastrula (12,13). Subsequent stages of gastrulation lead to the development of three distinct germ layers (ecto-, endo-, and mesoderms), which contain three different SC types specific for each of them. These germ layer-specific SCs are called multipotent and may give rise to monopotent SCs that are specific for tissues and organs developing from this particular germ layer. For example, the multipotent mesodermal SCs give rise to monopotent hematopoietic, skeletal muscle, heart, endothelial, and mesenchymal tissue-committed SCs, the multipotent endodermal SCs give rise to monopotent liver, pancreas, and gut epithelium, while the ectodermal multipotent SCs give rise to monopotent brain, peripheral ganglions and nerves, eye, epidermis, and skin tissues (14).
Generally, while the monopotent and tissue-committed SCs were described in adult tissues, SCs with pluripotent/multipotent capacity were thought to be restricted to the early embryonic stages. However, recent evidence challenged this idea by confirming the presence of pluripotent/multipotent SCs in adult tissues and organs (15-19). Several groups postulated that the BM is enriched for this population.
The hunt for PSCs in the BM
The BM is a major organ harboring HSCs that maintain adult hematopoiesis (20). As mentioned above, the population of BM-derived HSCs has been postulated to be the source of hematopoietic- and multiple non-hematopoietic lineages (21). This assumption established a basis for the SC plasticity hypothesis and was supported by several promising results where HSCs were employed for regeneration of injured organs in animal models, e.g., after myocardial infarct (22), stroke (23), spinal cord injury (24), and liver damage (25). The “plasticity theory” raised much hope that HSCs harvested from BM, mobilized peripheral blood (mPB), or cord blood (CB) could become a universal source of SCs for tissue and organ repair.
However, despite these initial promising results, the role of HSC in the repair of non-hematopoietic tissues became controversial (26-28). Further experiments with highly purified populations of HSCs showed no effects in myocardial or central nervous system regeneration (29,30). Following these unexpected results, the scientific community became polarized on the phenomenon of “plasticity”. The alternative theory that BM may also contain some non-HSCs, which could explain BM plasticity, has been adopted by several investigators including our group (3,27,28). Thus, from beginning, we believed the adult BM, CB, or mPB contained unidentified, rare, primitive SC populations that were responsible for some of the positive results that demonstrated SC plasticity when HSC “contaminated” by these other SCs were employed.
Digging in the extended lymph gate: novel criteria for isolating VSELs
We began our search for VSELs in the suspension of BMNCs by employing a novel fluorescence activated cell sorting (FACS)-based isolation strategy (3,31,32). Encouraged by our initial chemotactic isolation experiments, we assumed that the most primitive SCs would express CXCR4 receptor (3,4). We also assumed that they would express some of the pan-SC markers such as Sca-1 (in mice) and CD133/CD34 (in humans) (2,31,33). Furthermore, we hypothesized that both murine and human VSELs would belong to the non-hematopoietic compartment of the BM, and as such would lack the expression of CD45 (pan-hematopoietic marker) as well as other hematopoietic lineage markers (Lin-). Moreover, the cellular size became one of the major criteria for VSELs’ isolation (31,32). The morphological characteristics of primitive PSCs present in the epiblast of the pre-implantation blastocyst as well as our chemotactic isolation experiments (3,4), which that revealed a presence of some very rare, unusually small SDF-1-responsive SCs in a population of BMNCs, led us to believe that that these cells will be included in the extended “lymphgate.” This gate includes lymphocytes based on their forward- (FSCs) and side scatter characteristics (SSCs) of cytogram (FSCs vs. SSCs flow cytometric dot-plot). Table 1 summarizes major preparatory steps to isolate murine and human VSELs.
Table 1.
Major steps for isolation of murine and human VSELs
| Cells | Murine VSELs | Human VSELs |
|---|---|---|
| Source | Bone marrow (BM) | Cord blood (CB) |
| Major isolation steps |
|
|
| Antibody for flow cytometry |
|
|
| Key points | Depletion of red blood cells on Ficoll-Paque gradient should be avoided, since some fraction of VSELs can be lost during such isolation. Lysis in hypotonic solution is recommended. | Depletion of red blood cells on Ficoll-Paque gradient should be avoided, since some fraction of VSELs can be lost during such isolation. Lysis in hypotonic solution is recommended. |
Depletion of Lin+ cells is an optional, however recommended step; it decreases the time of FACS and increases recovery of cells.
Figure 1 demonstrates our overall FACS-based strategy for VSELs isolation. As mentioned above, based on the predicted very small size of these cells, we employed an innovative sorting strategy by extending the typical lymphgate into the area of a dot-plot typically thought to contain mostly “cellular debris” (31,32) (Figure 1A). For further analysis and sorting, as shown on Panel A, we employed a mixture of predefined sized synthetic beads (1, 2, 4, 6, 10, and 15μm in diameter) to zoom on all objects between 2-10 μm in size (32) (Figure 1A). This region mostly contains cell debris, but also some rare nucleated cellular events. Further analysis of this expanded gate identified a very rare fraction of non-hematopoietic murine cells (0.030 ± 0.008% of total BM cells), which expressed SC antigen 1 (Lin-/CD45-/Sca-1+) (31,32).
Figure 1. Sorting strategy for isolation of murine BM- derived VSELs by FACS.
BM-derived VSELs were sorted by MoFlo cell sorter (Dako) following immunofluorescence staining for Sca-1, CD45, and hematopoietic lineage markers (Lin). Panel A shows distribution of six predefined sized beads populations according to their FSCs vs. SSCs. Gate R1 includes objects between 2 - 10 μm in size after comparison with bead particles with standard sizes of 1, 2, 4, 6, 10, and 15 μm (Flow Cytometry Size beads, Invitrogen; Molecular Probes, Carlsbad, Ca, USA). Panel B presents BMNCs visualized on dot plots showing their FSC and SSC signals related to the size and granularity/complexity of the cell, respectively. Small, agranular cells included in Region R1 are further visualized based on the expression of Sca-1 and Lin markers (Panel D). Region R2 includes only Sca-1+/Lin-, which are subsequently sorted based on CD45 marker expression into CD45- and CD45+ subpopulations visualized on histogram (Panel C). Sca-1+/Lin-/CD45- cells (VSELs) are sorted as events enclosed in a logical gate including Regions R1, R2, and R3, while Sca-1+/Lin-/CD45+ cells (HSCs) from gate include Regions R1, R2, and R4. Approximate percent contents of each cellular subpopulation are indicated on the plots.
A similar strategy has been employed for the isolation of human CB-derived VSELs (CB-VSELs) (Figure 2). Lin-/CD45-/CD133+/CD34+/CXCR4+ CB-VSELs have been identified when all objects larger than 2 μm were included into analysis and further sorting by FACS (32-34).
Figure 2. Sorting strategy for isolation of human CB- derived VSELs by FACS.
CB-derived VSELs were isolated by FACS (MoFlo; Dako) following immunofluorescence staining for CD133, CD45, and hematopoietic lineage markers (Lin). Panel A shows distribution of predefined sized beads according to their FSCs vs. SSCs signals, presented in logarithmic scale. Region R1 includes objects larger than 2μm in size as compared with beads with standard sizes of 1, 2, 4, 6, 10, and 15 μm (Flow Cytometry Size beads, Invitrogen; Molecular Probes). Panel B presents CB nucleated cells visualized based on their FSCs and SSCs. Region R1 includes all cellular objects as well as a fraction of very small events containing VSELs. Panel D presents a histogram showing expression of lineage markers by objects from Region R1. Lin- events included in Region R2 are subsequently visualized based on expression of CD45 and CD133 (Panel C). CD133-1+/Lin-/CD45- cells (VSELs) are sorted as events enclosed in a logical gate including Regions R1, R2, and R3, while CD133+/Lin-/CD45+ cells (HSCs) from gate include Regions R1, R2, and R4. Approximate percent contents of each cellular subpopulation are indicated on the plots.
VSELs: “Missed pearls” that are smaller than erythrocytes but larger than platelets
We employed a multidimensional methodological approach for further morphological and molecular characterization of murine and human VSELs. Transmission electron microscopic (TEM) analysis supported our initial observation that both murine and human VSELs are very small in size (31,33). Accordingly, murine BM-derived VSELs were found to be ~2-4 μm in diameter, while purified murine HSCs (Lin-/CD45+/Sca-1+) were much larger (~6-8 μm). Similar measurements were obtained by confocal microscopic analysis of murine BM-derived VSELs and HSCs (32). Furthermore, when detailed flow cytometric analyses of sorted VSELs and HSCs were performed, we observed that 95.24 ± 0.94% of Sca-1+/Lin-/CD45- (VSELs) were located in the gate, including objects within the 2–6 μm size range, while 85.82 ± 1.28% of Sca-1+/Lin-/CD45+ (HSCs) were found in the 6–10 μm size range (32). Since most sorting protocols exclude events smaller than 6 μm in diameter because of the assumption that they contain cell debris, erythrocytes and platelets, it is conceivable that the very small size of VSELs precluded the identification of these “missed pearls” for many years (32,35).
To better support the existence of such small eukaryotic cells in BM, we focused on determining the exact size and morphological characteristics of VSELs. We employed ImageStream system (ISS) to precisely calculate the size of VSELs and compared them to other cell types found in sorted BMNC including erythrocytes, platelets, and leukocytes (32,35).
The ISS technology was developed as a novel method for multi-parameter cell analyses and as a supportive tool for flow cytometry. The ISS integrates the features of flow cytometry and fluorescent microscopy by analyzing a multitude of objects in suspension and collecting their acquired images for offline digital image analyses (36-38). Several applications on the system have been already developed on top of its unique capabilities (37,39-42). Recently introduced multi-laser upgrade of the instrument allows employing of wide range of fluorochromes/ dyes and multicolor analyzes, while the presence of new EDF (Extended Depth of Field) technology increases resolution and improves precision of analysis enabling many new applications of the system including counting FISH spots in suspension (42-44).
Based on direct visualization of acquired objects by ISS, we confirmed that some of the isolated very small Sca-1+/Lin-/CD45- objects contain nuclei and posses cellular features characteristic for VSELs. ISS analysis allowed the precise calculation of the VSELs’ size, which was found to be 3.63 ± 0.14 μm in diameter, and confirmed the larger diameter of Sca-1+/Lin-/CD45+ HSCs (6.54 ± 0.17 μm) (32). Similarly, we calculated the average size of human CB-VSELs as 6.58 ± 1.09 μm (unpublished). When the inter-species differences in cellular sizes are considered, this corresponds to the size of murine VSELs.
Moreover, direct comparison of different cell type images in the same scale combined with digital image analysis confirmed that VSELs are larger than PB platelets but smaller than erythrocytes (Figure 3). This is the first report of the existence of cells smaller than PB erythrocytes in adult tissues of murine (Figure 3B) as well as human (Figure 3C) (8,32,35).
Figure 3. Morphology of murine and human VSELs compared with erythrocytes and platelets by ISS.
Panel A shows images of bead particles with predefined diameter that have been used as standards of size. Panel B presents murine BM-derived VSELs stained for Sca-1 (FITC, green), Lin (PE, orange), and CD45 (PE-Cy5, magenta) compared to murine blood-derived erythrocytes stained for Ter119 (PE, orange) and platelets stained for CD41 (FITC, green). Panel C shows human CB-derived VSELs stained for Lin and CD45 (FITC, green), CD133 (PE; orange), and CD34 (PE-Cy5; yellow). CB-VSELs are compared to CB-derived erythrocytes stained for CD235a (FITC, green) and platelets stained for CD41 (FITC, green). Nuclei of cells presented in all panels are visualized with staining with 7-aminoactinomycin D (7-AAD) following fixation of all populations. Erythrocytes and platelets do not possess nuclei, while VSELs show a cellular structure containing nuclei. The average size of each population is shown (Mean ± SEM). Scale bars show 10μm.
VSELs: “Are they the almighty PSCs?”
Initially, direct electron microscopic analyses of both murine and human VSELs revealed their very small size and several characteristics typical for ESCs. These included a large nucleus surrounded by a narrow rim of cytoplasm, an open-type chromatin (euchromatin) (31,33), and a high nuclear-to-cytoplasmic (N/C) ratio. This high N/C ratio is typically considered to be a feature of developmentally primitive SCs (32).
Further analysis established that VSELs express markers of PSCs identified in the embryonic stages on both the mRNA and protein levels as determined by real time RT-PCR and immunocytochemistry, respectively (31,32). Accordingly, we detected the presence of SSEA-1, Oct-4, Nanog, Rex-1, Dppa3, and Rif-1 in mice cells (31,32) and SSEA-4, Oct-4, and Nanog in human cells (33). Of note, we employed Oct-4-specific primers that do not amplify Oct-4 pseudogenes as well as Oct-4-specific antibodies (31,33).
Moreover, we established that VSELs express several markers of PGCs, which are a mobile population of epiblast cells giving rise to gametes after migration into the genital ridges of the developing embryo. Murine VSELs express the placental form of alkaline phosphatase (PLAP), Stella, Fragilis, Nobox, and Hdac6 as well as CXCR4, which indicates their close relation to a population of epiblast-derived PGCs (8,14). In conclusion, both the primitive features of these cells as well as their expression of several embryonic markers justify the name we gave to these cells (31).
In addition, the presence of CXCR4, as well as other chemoattractants’ receptors such as c-met and LIF-R, indicates the high migratory capability of VSELs towards the gradients of SDF-1, hepatocyte growth factor (HGF), and leukemia inhibitory factor (LIF), respectively (4,31). The motility of VSELs is crucial not only for their mobilization into PB after tissue injury (45,46) or pharmacological mobilization (47), but also supports our hypothesis about their deposition in various organs as a consequence of their migration during embryonic development (14).
Despite their small size, VSELs possess diploid number of chromosomes. They not only express multiple markers of pluripotency, but also exhibit the potential of PSCs in vitro (31,48,49). Accordingly, when cultured in pre-defined differentiation media, VSELs exhibit the potential to give rise into cells from all three germ layers such as ectodermal neural cells, endodermal pancreatic cells, and mesodermal cardiomyocytes (31). Furthermore, freshly isolated VSELs have been shown to improve the function of ischemic heart tissue when injected directly into an injured organ, as shown in the model of murine acute myocardial infarction (48).
Moreover, when cultured in direct contact over a feeder layer of C2C12 murine sarcoma cells, murine BM-derived VSELs are able to form cellular clusters and spheres resembling embryoid bodies [VSEL-derived spheres (VSEL-DS)] (31). Cells derived from such clusters preserve the pluripotent capacity of VSELs and differentiate into cells from all three germ layers. Culture of VSELs in the presence of C2C12 cells allows their expansion for more efficient, potentially regenerative therapeutic applications in vivo. Our experience in the treatment of ischemic heart tissues with expanded and ex vivo pre-differentiated VSELs (those committed into cardiac lineage) indicate their higher beneficial effects after ex vivo preparation when compared with freshly isolated, quiescent VSELs (49).
Moreover, our recent experiments revealed that VSELs isolated initially as non-HSCs from the BM may finally differentiate if cultured in a permissive environment into HSCs. This observation additionally supports their pluripotent nature (8). Accordingly, we found that VSELs freshly isolated from BM do not reconstitute hematopoiesis in lethally irradiated mice (31,50). When cultured in the presence of an OP-9 feeder layer (cell line that supports hematopoietic differentiation of established ESC lines), these cells grow cobblestone areas that contain HSCs developing de novo from VSELs. More importantly, HSCs that are expanded from VSELs grow hematopoietic colonies in vitro and reconstitute hematopoiesis in vivo after transplantation in lethally irradiated mice. Thus, VSELs possess properties of long repopulating HSCs (8). Their role in the potential turn over of HSCs in murine BM both in steady state and stress conditions is currently investigated in our laboratory.
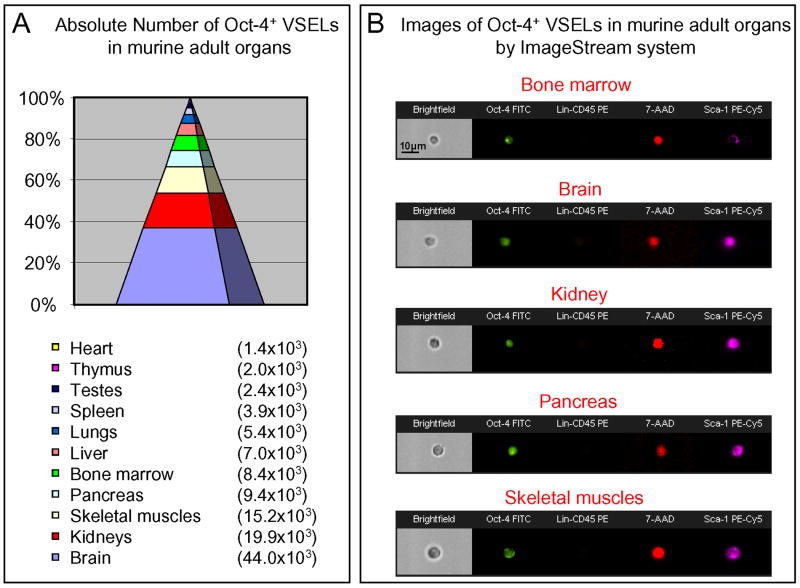
Of considerable note, we identified VSELs not only in BM (31,32), mPB (46,47), and CB (33), but our recent research revealed the presence of VSELs in other adult tissues as well (35,51). The Oct-4+/Lin-/CD45-/Sca-1+ VSELs were identified in the major organs of murine adults, predominantly in brain, kidneys, pancreas, and skeletal muscles (Figure 4). Additionally, preliminary in vitro data suggest that VSELs sorted from spleen, thymus, and fetal liver form VSEL-DS similarly to those isolated from BM (35,51). However, the pluripotent nature of Oct-4+/Lin-/CD45-/Sca-1+ VSELs derived from non-hematopoietic organs requires further investigation. In particular since brain contains a highest number of VSELs, further studies are needed to compare these cells with neural stem cells that reside in hippocampus and subventricular region (52-54), as well as to asses the potential role of brain-derived VSEL in brain regeneration.
Figure 4. Oct-4+ VSELs present in adult murine tissues.
Panel A shows absolute numbers of Oct-4+ VSELs identified in each analyzed organ (Mean ± SEM). Panel B presents representative images of small Oct-4+ VSELs identified in all analyzed organs by ISS. VSELs are stained for Oct-4 expression (FITC, green). They are negative for Lin markers and CD45 (PE, orange) and express Sca-1 (PE-Cy5, magenta). Scale bars show 10μm.
The embryonic heritage of VSELs: A hypothesis about their embryonic origin
Accumulating evidence demonstrates the presence of stem cells that express markers of very early developmental stages in multiple adult tissues. The presence of Oct-4+ cells that are markers of pluripotency has been described in adult BM (15,18,31), epidermis (16,19), bronchial epithelium (17), myocardium (55), pancreas (56), and testes (57,58). In agreement with these observations, we discovered Oct-4+ VSELs in multiple adult tissues (35,51). Based on the pluripotent nature of VSELs, their mobile character, and phenotypical similarities with PGCs, we hypothesize that VSELs may originate from the migrating epiblast-derived pluripotent (EP)SCs.
To support this notion, the epiblast has been described as a source of PSCs for all three germ layers and PGCs during gastrulation. We hypothesize that migrating EPSCs can be deposited in the developing peripheral tissues and organs and that they may serve as a reserve pool of quiescent PSCs (14). These cells may participate in cellular turnover and the rebuilding pool of the tissue specific progenitors, especially under urgent circumstances such as tissue injury (14). On the other hand, such primitive SCs may potentially initiate carcinogenesis when their potential becomes unleashed under conditions that are still poorly understood. Development of multiple types of primitive tumors such as teratomas, germinomas, and pediatric sarcomas that express several markers characteristic for epiblast/germ line cells supports this theory (8). However, the mechanisms controlling the quiescence of PSCs in adult tissues remain unknown. We are currently examining the EPCS’s origin of VSELs in our laboratory.
BM as a home of versatile primitive SCs: Are VSELs the new kids on the block?
Recently, several populations of non-hematopoietic primitive SCs have been described in the BM and other adult tissues (Table 2) including: i) mesenchymal (M)SCs; ii) multipotent adult progenitor cells (MAPCs); iii) marrow-isolated adult multilineage inducible (MIAMI) cells; iv) multipotent adult (MA)SCs; and v) endothelial progenitor cells (EPCs). It is conceivable that these cells are the same or overlapping populations of SCs that have been described by different investigators and given various names according to circumstance. These cells and their potential relevance to VSELs will be briefly discussed below.
Table 2.
Versatile multipotent stem cells described in adult tissues.
| Stem Cells | Phenotype |
|---|---|
| Endothelial Progenitor cells (EPC) |
Human - CD133+, CD34+, c-kit (CD117)+, VE-cadherin+, VEGFR2+, CD146+, vWF+, CD31+ Mice – Sca-1+, c-Kit (CD117)+, Lin-, VEGFR2+, VE-cadherin+, Tie2+, CD146+, vWF+, CD31+ |
| Mesenchymal Stem Cells (MSC) * |
International Society for Cellular Therapy criteria: CD105+, CD73+, CD90+, CD45-, CD34-, CD14-, CD11b-, CD79a-, CD19-, HLA-DR- Other additional markers: Stro-1+, SB-10+ (CD166), SH-2+ (epitope on CD105), SH-3+ (epitope on CD73), SH-4+ (epitope on CD73), CD44+, CD29+, CD31-, vWF- Markers of most primitive MSC: CXCR4, CD133, CD34 (?), p75LNGFR |
| Multipotent Adult Progenitor Cells (MAPC) * | SSEA-1+, CD13+, Flk-1low, Thy-1low, CD34-, CD44-, CD45-, CD117(c-kit)-, MHC I-, MHC II- |
| Marrow-isolated Adult Multilineage Inducible Cells (MIAMI) * | CD29+, CD63+, CD81+, CD122+, CD164+, c-met+, BMPR1B+, NTRK3+, CD34-, CD36-, CD45-, CD117 (c-kit)-, HLA-DR- |
| Multipotent Adult Stem Cells (MASCs)* | CD13+, CD49b+, CD90+, CD73+, CD44+, CD29+, CD49a+, CD105+, MHC I+, HLA-DR-, CD14-, CD34-, CD45-, CD38-, CD133-, c-kit (CD117)- |
| OmniCytes | CD34+, CD133+, CXCR4+, c-Met+, CD33low, CD38low, MHC-IIlow |
| Very Small Embryonic Like (VSEL) Stem Cells | CXCR4+, AC133+, CD34+, SSEA-1+ (mouse), SSEA-4+ (human), AP+, c-Met+, LIF-R+, CD45-, Lin-, HLA-DR-, MHC I -, CD90-, CD29-, CD105- |
(phenotype of expanded/cultured adherent cells)
Abbreviations: AP – fetal alkaline phosphatase, BMPR1B - bone morphogenetic protein receptor 1B, c-met – receptor for hepatocyte growth factor, LIF-R – receptor for leukemia inhibitory factor, NTRK3 - neurotropic tyrosine kinase receptor 3, vWF – von Willebrand factor.
MSCs have been identified and well characterized as a population of BM-derived adherent cells capable of differentiating into multiple mesodermal lineages (59,60). Our data indicate a robust interaction of VSELs with BM-derived stroma that results in emperipolesis of VSELs inside the stroma-derived fibroblasts (31).
MAPCs have been isolated from a fraction of BMNCs as an adherent fibroblast-shaped population of CD45-GPA-A- cells (61). MAPCs have been depicted as pluripotent cells capable of differentiating into cells from all three germ layers and contributing to all embryonic tissues when injected into a developing blastocyst (61).
MIAMI cells are an adherent human adult BM population that expresses ESC markers Oct-4 and Rex-, while their cultured colonies express several markers characterizing cells from all three germ layers (62). However, their ability to contribute to tissues of the developing blastocyst as well as their further morphological characterization on the single cell level has not been examined.
Similarly, MASCs have been recently identified as adult SCs with pluripotent characteristics and the capability to differentiate into cells from multiple lineages in vitro. They were cloned from human adult BM as well as other tissues including the liver and heart (63). MASCs express typical MSCs markers and expand in MSC-supporting media.
Finally, EPCs identified in both murine and human BM are mobilized in response to tissue injury (64,65). Since VSELs are mobilized during tissue and organ injuries and a high antigenic similarity between VSELs and EPCs exists, they can be potentially co-isolated with EPCs.
The potential relationship between MSCs, MAPCs, MIAMI cells, MASCs, and EPCs is not clear, although it is possible that these are overlapping populations identified by different isolation and expansion strategies. On the other hand, these populations are largely derived from the adherent fraction of BM cells that potentially could contain attached or internalized VSELs by a process of emperipolesis. The appealing potential interaction between VSELs and the aforementioned stem/primitive cell populations is very intriguing and requires further investigation.
Other small SCs described in adult tissues
Although SCs have been traditionally described as “small lymphocytes”, the exact size of SC and primitive SC populations was rarely measured. The vast majority of the available literature describes the morphology and particularly the size of stem/primitive cells in comparison to more mature cells from the same tissue or organ. Therefore, the relatively small size of stem/primitive cells has rarely been assessed with precision.
Based on our observation, the term “small SCs” should be only used to describe cells that are smaller than red blood cells. This is a criterion we use in our work with murine and human VSELs. By employing the ISS, we calculated the size of murine and human VSELs as 3.63 ± 0.14 μm and 6.58 ± 1.09 μm, respectively, while murine and human erythrocytes are 4.94 ± 0.46 μm and 7.87 ± 0.85 μm in diameter, respectively (32,35,66).
Other “small SCs” isolated from murine tissues
The first description of small cells with such primitive morphology in the murine BM was published in the late 1980s. These cells were usually described in the context of subpopulations from the most primitive HSCs.
BM mononuclear cells- derived progenitor-like cells
By employing electron microscopic analysis, Matsuoka et al. identified the existence of cells in the BM mononuclear cell fraction that were between 4 and 5 μm in size. These small cells exhibited some morphological features characteristic for hematopoietic progenitors (67).
Lin-/Rhdull/Hodull long-term repopulating HSCs
At the same time, Radley et al. described a population of very primitive HSCs isolated from the murine BM that were capable of long-term hematopoietic reconstitution. These cells were characterized by a lack of hematopoietic lineage markers and their ability to exclude both Rhodamine 123 (Rhdull) and Hoechst 33342 (Hodull) dyes (68). The average size of these cells was estimated by TEM analysis as ~4.6 μm. Interestingly, these cells could be separated into in three different subpopulations with one of them being very similar to the murine BM-derived VSEL phenotype (68). We have noticed that VSELs exclude Rhodamine 123 and Hoechst 33342 dyes. As such, it is possible that by sorting HSC with above mentioned characteristics in these experiments, some VSELs were co-isolated as well.
Lin-/ALDHhigh long-term repopulating HSCs
In the 1990s, Jones et al. isolated a population of small long-term repopulating HSCs by employing elutriation followed by FACS based on the high activity of aldehyde dehydrogenase (ALDHhigh) and a lack of hematopoietic lineage markers (69,70). The criteria for elutriation were optimized to obtained the fraction of the smallest lymphocytes and separate them from the more mature hematopoietic progenitors (69-71). Although the size of these cells was not published in the original publication, a recent paper from this group described that these SCs are smaller than 5 μm (72). Unfortunately, these interesting cells were never analyzed for expression of potential ESC markers. It is very likely that they share several characteristics with VSELs.
Spore-like SCs
The presence of cells with a similarly small size as murine VSELs (<5 μm) has been recently postulated by Vacanti et al. These small cells were isolated from adult murine tissues and described as “spore-like SCs” (73). The authors challenged the idea that erythrocytes are the smallest cells in the adult body. Unfortunately, the isolation strategy of spore-like SCs and their exact markers has not been described by the authors. It is likely that these cells are closely related to VSELs.
CD45-Sca-1+c-kit- cells with pluripotent characteristics
By employing flow cytometric analysis of single cell suspensions from murine brain, blood, and intestinal epithelium, Howell et al. unveiled the presence of very small CD45-Sca-1+c-kit- cells that may represent universal PSCs residing in multiple murine tissues (74). However, the authors did not reveal an exact size of these interesting cells.
Other “small SCs” isolated from human tissues
Recently, several studies have indicated the presence of small SCs in human tissues that correspond to our VSELs.
Small round ovarian surface epithelium SCs
Virant-Klun et al. described very small SCs with pluripotent characteristics in the human ovarian surface epithelium. These cells are small round cells (2-4 μm) and express embryonic markers such as SSEA-4, Oct-4, Nanog, Sox-2, and c-kit and can form embryoid body-like structures in vitro (75). It is very likely that these cells correspond to a population of human VSELs.
Small CB-derived SCs
Recently, McGuckin et al. demonstrated the presence of very small SCs in umbilical CB and estimated the size of these cells at 2-3 μm (76). These cells exhibit pluripotent characteristics such as the expression of Oct-4 and Sox2 and have neural differentiation potential. Despite the elegant and detailed isolation and culture protocols, the authors did not provide the information regarding the methodology employed for size calculation and the majority of images presented in the paper do not contain a scale. In our findings, a similar population of cells (human CB-VSELs) is slightly larger in size.
Other small cells
Interestingly, Huang et al., while isolating MSCs on a double layer culture plate containing 3-μm pores that were employed to sieve out the relatively large MSCs, described a population of very small cells residing in the human BM (77). These very small cells were able to migrate through such small pores. However, the authors have never further investigated and characterized these cells.
Similarly, it was also reported that MAPCs may contain a subpopulation of very small cells that express ESC markers (78). Further characterization of these small embryonic-like MAPCs is needed to address their potential similarity to VSELs.
Final conclusions
The existence of versatile, rare populations of primitive SCs has been already been documented in the literature. Furthermore, their small cellular size has been described in the context of their primitive/pluripotent nature. In some cases, the small size of these cells has been successfully applied as important parameter during isolation strategies (e.g., VSELs, Lin-/ALDHhigh). Thus, detailed morphological characterization of primitive cells isolated from adult tissues became a very important issue. Novel technologies such as ISS analysis provide the opportunity for more precise analysis and have become valuable identification tools. ISS analysis also allows these real small cellular events to be distinguished from artifacts and cell fragments
Acknowledgments
Supported by NIH grant R01 CA106281-01 and the Stella & Henry Hoenig endowment to MZR. We also thank Andrew Marsh at the JGBCC for editorial help.
References
- 1.Kucia M, Ratajczak J, Ratajczak MZ. Are bone marrow stem cells plastic or heterogenous--that is the question. Exp Hematol. 2005;33:613–623. doi: 10.1016/j.exphem.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19:1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- 3.Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells ‘hide out’ in the bone marrow. Leukemia. 2004;18:29–40. doi: 10.1038/sj.leu.2403184. [DOI] [PubMed] [Google Scholar]
- 4.Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004;32:52–57. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Young HE, Black AC., Jr Adult stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:75–102. doi: 10.1002/ar.a.10134. [DOI] [PubMed] [Google Scholar]
- 7.Ratajczak MZ, Zuba-Surma EK, Machalinski B, Kucia M. Bone-marrow-derived stem cells - our key to longevity? J Appl Genet. 2007;48:307–319. doi: 10.1007/BF03195227. [DOI] [PubMed] [Google Scholar]
- 8.Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol. 2008;36:742–51. doi: 10.1016/j.exphem.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- 10.Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 11.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 13.McLaren A, Lawson KA. How is the mouse germ-cell lineage established? Differentiation. 2005;73:435–437. doi: 10.1111/j.1432-0436.2005.00049.x. [DOI] [PubMed] [Google Scholar]
- 14.Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4+ stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 15.Anjos-Afonso F, B D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298–1306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- 16.Dyce PW, Zhu H, Craig J, Li J. Stem cells with multilineage potential derived from porcine skin. Biochem Biophys Res Commun. 2004;316:651–658. doi: 10.1016/j.bbrc.2004.02.093. [DOI] [PubMed] [Google Scholar]
- 17.Ling TY, Kuo MD, Li CL, Yu AL, Huang YH, Wu TJ, Lin YC, Chen SH, Yu J. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc Natl Acad Sci U S A. 2006;103:9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pallante BA, Duignan I, Okin D, Chin A, Bressan MC, Mikawa T, Edelberg JM. Bone marrow Oct3/4+ cells differentiate into cardiac myocytes via age-dependent paracrine mechanisms. Circ Res. 2007;100:e1–11. doi: 10.1161/01.RES.0000253487.02398.85. [DOI] [PubMed] [Google Scholar]
- 19.Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879–1888. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godin I, Cumano A. Of birds and mice: hematopoietic stem cell development. Int J Dev Biol. 2005;49:251–257. doi: 10.1387/ijdb.041945ig. [DOI] [PubMed] [Google Scholar]
- 21.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 22.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 23.Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, Masuya M, Fleming PA, Drake CJ, Ogawa M. Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol. 2004;186:134–144. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Corti S, Locatelli F, Donadoni C, Strazzer S, Salani S, Del Bo R, Caccialanza M, Bresolin N, Scarlato G, Comi GP. Neuroectodermal and microglial differentiation of bone marrow cells in the mouse spinal cord and sensory ganglia. J Neurosci Res. 2002;70:721–733. doi: 10.1002/jnr.10455. [DOI] [PubMed] [Google Scholar]
- 25.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 26.McKinney-Freeman SL, Jackson KA, Camargo FD, Ferrari G, Mavilio F, Goodell MA. Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proc Natl Acad Sci U S A. 2002;99:1341–1346. doi: 10.1073/pnas.032438799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orkin SH, Zon LI. Hematopoiesis and stem cells: plasticity versus developmental heterogeneity. Nat Immunol. 2002;3:323–328. doi: 10.1038/ni0402-323. [DOI] [PubMed] [Google Scholar]
- 28.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 29.Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- 30.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 31.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 32.Zuba-Surma EK, Kucia M, Abdel-Latif A, Dawnn B, Hall B, Singh R, Lillard JW, Ratajczak MZ. Morphological characterization of Very Small Embryonic-Like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12:292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4(+) SSEA-4(+) Oct-4(+) very small embryonic-like cells purified from human cord blood - preliminary report. Leukemia. 2007;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- 34.Kucia M, Zuba-Surma EK, Wysoczynski M, Wu W, Ratajczak J, Machalinski B, Ratajczak MZ. Adult marrow-derived very small embryonic-like stem cells and tissue engineering. Expert Opin Biol Ther. 2007;7:1499–1514. doi: 10.1517/14712598.7.10.1499. [DOI] [PubMed] [Google Scholar]
- 35.Ratajczak MZ, Zuba-Surma EK, Shin DM, Ratajczak J, Kucia M. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol. 2008 June 14; doi: 10.1016/j.exger.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basiji DA, Ortyn WE, Liang L, Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clin Lab Med. 2007;27:653–670. doi: 10.1016/j.cll.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuba-Surma EK, Kucia M, Abdel-Latif A, Lillard JJ, Ratajczak MZ. The ImageStream System: a key step to a new era in imaging. Folia Histochem Cytobiol. 2007;45:279–290. [PubMed] [Google Scholar]
- 38.Zuba-Surma EK, Kucia M, Ratajczak MZ. “Decoding of Dot”: The ImageStream System (ISS) as a Supportive Tool for Flow Cytometric Analysis. Cent Eur J Biol. 2008;3:1–10. [Google Scholar]
- 39.Henery S, George T, Hall B, Basiji D, Ortyn W, Morrissey P. Quantitative image based apoptotic index measurement using multispectral imaging flow cytometry: a comparison with standard photometric methods. Apoptosis. 2008;13:1054–1063. doi: 10.1007/s10495-008-0227-4. [DOI] [PubMed] [Google Scholar]
- 40.George TC, Basiji DA, Lynch DH, Ortyn WE, Perry DJ, Seo MJ, Zimmerman CA, Morrissey PJ. Distinguishing Modes of Cell Death Using the ImageStream Multispectral Imaging Flow Cytometer. Cytometry A. 2004;59A:237–245. doi: 10.1002/cyto.a.20048. [DOI] [PubMed] [Google Scholar]
- 41.George TC, Fanning SL, Fitzgeral-Bocarsly P, Medeiros RB, Highfill S, Shimizu Y, Hall BE, Frost K, Basiji D, Ortyn WE, Morrissey PJ, Lynch DH. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J Immunol Methods. 2006;311:117–129. doi: 10.1016/j.jim.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 42.McGrath KE, Bushnell TP, Palis J. Multispectral imaging of hematopoietic cells: where flow meets morphology. J Immunol Methods. 2008;336:91–97. doi: 10.1016/j.jim.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortyn WE, Perry DJ, Venkatachalam V, Liang L, Hall BE, Frost K, Basiji DA. Extended depth of field imaging for high speed cell analysis. Cytometry A. 2007;71:215–231. doi: 10.1002/cyto.a.20370. [DOI] [PubMed] [Google Scholar]
- 44.Ortyn WE, Hall BE, George TC, Frost K, Basiji DA, Perry DJ, Zimmerman CA, Coder DC, Morrissey PJ. Sensitivity Measurement and Compensation in Spectral Imaging. Cytometry A. 2006;69A:852–862. doi: 10.1002/cyto.a.20306. [DOI] [PubMed] [Google Scholar]
- 45.Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 46.Zuba-Surma EK, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865–873. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kucia M, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence that Very Small Embryonic Like (VSEL) Stem Cells are Mobilized into Peripheral Blood. Stem Cells. 2008;26:2083–2092. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- 48.Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuba-Surma EK, Taher H, Kucia M, Guo Y, SanganalMath SK, Hunt G, Vincent RJ, Abdel-Latif A, Dawn B, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived Very Small Embryonic-Like stem cells (VSELs) improves left ventricular function and remodeling after myocardial infarction. Circulation. 2007;116:204. [Google Scholar]
- 50.Kucia M, Ratajczak J, Ratajczak MZ. Bone marrow as a source of circulating CXCR4+ tissue-committed stem cells. Biol Cell. 2005;97:133–146. doi: 10.1042/BC20040069. [DOI] [PubMed] [Google Scholar]
- 51.Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Wan W, Ratajczak J, Wojakowski W, Kucia M. Hunt for pluripotent stem cell - Regenerative medicine search for almighty cell. J Autoimmun. 2008;30:151–62. doi: 10.1016/j.jaut.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trujillo C, Schwindt T, Martins A, Alves J, Mello L, Ulrich H. Novel perspectives of neural stem cell differentiation: from neurotransmitters to therapeutics. Cytometry A. 2009;(Special Issue) doi: 10.1002/cyto.a.20666. [DOI] [PubMed] [Google Scholar]
- 53.Lu HZ, Wang YX, Li Y, Fu SL, Hang Q, Lu PH. Proliferation and differentiation of oligodendrocyte progenitor cells induced from rat embryonic neural precursor cells followed by flow cytometry. Cytometry A. 2008;73:754–760. doi: 10.1002/cyto.a.20577. [DOI] [PubMed] [Google Scholar]
- 54.Martins AH, Alves JM, Trujillo CA, Schwindt TT, Barnabe GF, Motta FL, Guimaraes AO, Casarini DE, Mello LE, Pesquero JB, Ulrich H. Kinin-B2 receptor expression and activity during differentiation of embryonic rat neurospheres. Cytometry A. 2008;73:361–368. doi: 10.1002/cyto.a.20519. [DOI] [PubMed] [Google Scholar]
- 55.Mendez-Ferrer S, Prat S, Lukic A, Diego A, Badimon JJ, Fuster V, Nadal-Ginard B. ES-like cells in the adult murine heart. Fourth ISSCR Annual Meeting. 2006 [Google Scholar]
- 56.Kruse C, Kajahn J, Petschnik AE, Maass A, Klink E, Rapoport DH, Wedel T. Adult pancreatic stem/progenitor cells spontaneously differentiate in vitro into multiple cell lineages and form teratoma-like structures. Ann Anat. 2006;188:503–517. doi: 10.1016/j.aanat.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 58.Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 60.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 61.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 62.D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 63.Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 64.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 65.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 66.Ratajczak MZ, Zuba-Surma EK, Machalinski B, Ratajczak J, Kucia M. Very Small Embryonic-Like (VSEL) Stem Cells: Purification from Adult Organs, Characterization, and Biological Significance. Stem Cell Rev. 2008;4:89–99. doi: 10.1007/s12015-008-9018-0. [DOI] [PubMed] [Google Scholar]
- 67.Matsuoka T, Tavassoli M. Electron microscopic identification of hemopoietic progenitor cells by exploiting their sugar-recognizing receptors using a newly developed minibead technique. Exp Hematol. 1989;17:326–329. [PubMed] [Google Scholar]
- 68.Radley JM, Ellis S, Palatsides M, Williams B, Bertoncello I. Ultrastructure of primitive hematopoietic stem cells isolated using probes of functional status. Exp Hematol. 1999;27:365–369. doi: 10.1016/s0301-472x(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 69.Jones RJ, Collector MI, Barber JP, Vala MS, Fackler MJ, May WS, Griffin CA, Hawkins AL, Zehnbauer BA, Hilton J, Colvin OM, Sharkis SJ. Characterization of mouse lymphohematopoietic stem cells lacking spleen colony-forming activity. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 70.Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 71.Lanzkron SM, Collector MI, Sharkis SJ. Hematopoietic stem cell tracking in vivo: a comparison of short-term and long-term repopulating cells. Blood. 1999;93:1916–1921. [PubMed] [Google Scholar]
- 72.Krause DS. Bone marrow-derived cells and stem cells in lung repair. Proc Am Thorac Soc. 2008;5:323–327. doi: 10.1513/pats.200712-169DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vacanti MP, Roy A, Cortiella J, Bonassar L, Vacanti CA. Identification and initial characterization of spore-like cells in adult mammals. J Cell Biochem. 2001;80:455–460. [PubMed] [Google Scholar]
- 74.Howell JC, Lee WH, Morrison P, Zhong J, Yoder MC, Srour EF. Pluripotent stem cells identified in multiple murine tissues. Ann N Y Acad Sci. 2003;996:158–173. doi: 10.1111/j.1749-6632.2003.tb03244.x. [DOI] [PubMed] [Google Scholar]
- 75.Virant-Klun I, Rozman P, Cvjeticanin B, Vrtacnik-Bokal E, Novakovic S, Ruelicke T. Parthenogenetic Embryo-Like Structures in the Human Ovarian Surface Epithelium Cell Culture in Postmenopausal Women with No Naturally Present Follicles and Oocytes. Stem Cells Dev. 2008 Jul 7; doi: 10.1089/scd.2007.0238. [DOI] [PubMed] [Google Scholar]
- McGuckin C, Jurga M, Ali H, Strbad M, Forraz N. Culture of embryonic-like stem cells from human umbilical cord blood and onward differentiation to neural cells in vitro. Nat Protoc. 2008;6:1046–1055. doi: 10.1038/nprot.2008.69. [DOI] [PubMed] [Google Scholar]
- 77.Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–258. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- 78.Zeng L, Rahrmann E, Hu Q, Lund T, Sandquist L, Felten M, O’Brien TD, Zhang J, Verfaillie C. Multipotent adult progenitor cells from swine bone marrow. Stem Cells. 2006;24:2355–2366. doi: 10.1634/stemcells.2005-0551. [DOI] [PubMed] [Google Scholar]