Abstract
Recent studies report that, in the absence of heart failure and renal failure, plasma B-type natriuretic peptide (BNP) has prognostic value for mortality. We sought to confirm and extend these previous studies to assess BNP, measured by 3 distinct assays, as a biomarker for mortality in a strategy to enhance efforts at primary prevention and to better understand the clinical phenotype of such subjects at risk. We used a community-based cohort of 2042 subjects from Olmsted County, Minn, and individuals with heart or renal failure were excluded. BNP was assessed using 3 assays including Biosite and Shionogi for mature, biologically active BNP and the Roche assay for apparently nonbiologically active amino-terminal pro-BNP (NT-proBNP). Thorough echocardiographic and clinical data were recorded for all of the participants. Median follow-up for mortality was 5.6 years. BNP by all 3 of the assays was predictive of mortality. NT-proBNP and Biosite assays remained significant even after adjustment for traditional clinical risk factors and echocardiographic abnormalities including left ventricular hypertrophy and diastolic dysfunction. Echocardiography documented widespread structural changes in those with increasing BNP levels yet below levels observed in heart failure. We report in a large, well-characterized community-based cohort, free of heart failure, the first study to compare 3 distinct BNP assays as biomarkers for mortality in the same cohort. Our findings confirm the potential use of NT-proBNP and BNP biomarkers for future events and underscore that these peptides may also serve as biomarkers for underlying cardiac remodeling secondary to diverse cardiovascular disease entities.
Keywords: natriuretic peptide, hypertrophy
The cardiac hormone B-type natriuretic peptide (BNP) has proved useful in the diagnosis of human heart failure (HF).1–3 Recently, studies have provided compelling data that plasma BNP, even in the absence of HF, has prognostic value for future cardiovascular events.4–6 Specifically, Wang et al4 from the Framingham Heart Study reported in a prospective investigation in the general population without HF or renal failure that with each 1 SD increase in log BNP levels, there were significant increases in the risk of death, HF, atrial fibrillation, stroke or TIA, and first cardiovascular event over 5.2 mean years of follow-up. The reported plasma values associated with increased risk were well below the HF diagnosis threshold of 100 pg/mL. These important results suggest that, in the absence of HF, before the development of overt cardiac disease, modest elevations in plasma BNP are meaningful. Whereas this previous study provided significant information regarding the prognostic implications of BNP values below those seen in HF, the Framingham Heart Study4 and others5,7 have asked for validation of these findings in additional large, community-based studies.
The current study was, therefore, designed to validate, as well as extend these recent seminal reports. We used the Prevalence of Asymptomatic Ventricular Dysfunction (PAVD), a cohort of the Rochester Epidemiology Project, composed of 2042 individuals from Olmsted County, Minn, who underwent thorough echocardiographic examinations, including evaluation for systolic and diastolic dysfunction.8 We sought to confirm the prognostic utility of BNP for mortality in the general population without HF or renal failure, using 3 distinct and widely used BNP assays. Assays include Biosite and Shionogi assays for the mature, biologically active BNP and the Roche assay for apparently nonbiologically active amino-terminal proBNP (NT-proBNP). In addition, we sought to provide a descriptive analysis of the clinical and echocardiographic phenotype of individuals who are at greater risk of death as determined by elevated plasma BNP.
In the current study, we hypothesized that plasma BNP would have prognostic value for death in the general population without HF or renal failure. We also predicted that NT-proBNP may be a superior biomarker for mortality because of its prolonged half-life in comparison to BNP and its use in identifying a reduced ejection fraction in the general population.9 In addition, we hypothesized that those individuals who have BNP values that predict increased mortality risk would have an increased prevalence of clinical and echocardiographic phenotypes that may predispose these individuals to greater myocardial release of BNP.
Methods
This study was approved by the Mayo Foundation Institutional Review Board.
Study Population
Using the resources of the Rochester Epidemiology Project, a random sample of Olmsted County residents age ≥ 45 years was identified from the PAVD study cohort, which is a substudy of the Rochester Epidemiology Project. We used the PAVD study cohort of 2042 participants for the current study. The design and selection criteria of the PAVD study, as well as the characteristics of the Olmsted County population, have been described previously.8–10 Analysis of the medical records of 500 nonparticipants revealed no clinical significant differences between participants and nonparticipants.11 Subjects gave written consent and underwent echocardiography and phlebotomy. Of the 2042 total participants, 45 were excluded because of validated HF by Framingham criteria,12 and, consistent with previous reports,4,5 6 were excluded because of renal failure identified by serum creatine > 2.0 mg/dL. The remaining 1991 participants were used for all of the analyses in this study.
Medical Record Review
All of the Olmsted County healthcare providers have maintained a unified medical record, which is indexed by the Rochester Epidemiology Project. Trained nurse abstractors reviewed each subject’s medical record. Each subject completed medication questionnaires. Mortality data on Olmsted County residents is routinely collected by reviewing community medical records, death certificates, and obituary notices as part of the Rochester Epidemiology Project. Participants were followed up until death or November 1, 2004, at which time they were censored. This provided a mean 5.6 person-years of follow-up, with a median (25th, 75th percentile) of 5.6 (5.0, 6.3) person-years. Hypertension was defined as the presence of clinical diagnosis in the medical record and record of pharmacological treatment. Cardiomegaly was defined by chest x-ray criteria in the absence of congestive heart failure. Coronary artery disease was defined as a clinical diagnosis in the medical record with confirmation by exercise treadmill test, angiogram, or echocardiogram. Diabetes was defined as the presence of clinical diagnosis in the medical record.
Doppler Echocardiography
All of the echocardiograms were performed by 1 of 3 registered diagnostic cardiac sonographers with the same echocardiographic instrument (HP-2500) and were interpreted by a single echocardiologist. 2D and color Doppler imaging were performed to screen for valvular stenosis and regurgitation. In each subject, ejection fraction was measured by M-mode using the modified Quinones formula, quantitative 2D (biplane Simpson), and semiquantitative 2D (visual estimate) as described previously.8,13 Pulsed-wave Doppler examination of mitral (before and with Valsalva maneuver) and pulmonary venous inflow, as well as Doppler tissue imaging of the mitral annulus, was performed in each subject. Diastolic function was categorized as normal, mildly impaired (defined as impaired relaxation without evidence of increase filling pressures), moderately impaired (defined a impaired relaxation associated with moderate elevation of filling pressures or pseudonormal filling), and severely impaired (defined as advanced reduction in compliance).8,14 Diastolic dysfunction was defined as moderate or severe dysfunction. Left ventricular mass was calculated according to the Devereux formula15 and indexed to body surface area. The presence of left ventricular hypertrophy (LVH) was defined on the basis of left ventricular mass index > 130 g/m2 for men and > 100g/m2 for women.16 The presence of left atrial (LA) enlargement was defined as LA volume index > 33 mL/m2 in men and > 30 mL/m2 in women.17
BNP Analysis
All 1991 participants underwent BNP measurement using 3 distinct assays. Plasma BNP was determined by immunoradiometric assay using antibody to human BNP (Shionogi Co Ltd) and by fluorescence immunoassay (Biosite Diagnostics) as described previously.18 Plasma NT-proBNP concentrations were measured with the Elecsys pro-BNP electrochemiluminescencse immunoassay (Roche Diagnostics) as described previously.9,19 Blood was collected in EDTA vacutainers, placed on ice, centrifuged within 2 hours (most times within 20 minutes), and separated into multiple aliquots. Aliquots were placed in a freezer at −80°F. A new, never-thawed aliquot was used for each assay. Interassay and intra-assay coefficients of variation were 7.2% and 8.0%, respectively, for Shionogi BNP, 8.8% and 9.9%, respectively, for Biosite BNP, and 3.1% and 2.5%, respectively, for Roche NT-proBNP.
Statistical Analysis
Continuous variables are summarized as a mean± 1 SD, and comparison between groups were based on the Wilcoxon rank-sum test. Categorical variables are summarized as a percentage of the group total, and comparisons between groups were based on the χ2 test. Survival from entry into the study was estimated using the Kaplan-Meier method. The association of all-cause mortality with clinical, echocardiographic, and BNP assays was assessed using proportional hazards regression. To assess the relative predictive power of each BNP assay to each other, sequential proportional hazards models were evaluated. For a particular assay, the change in overall model fit (log likelihood statistic) relative to the addition of 1 or both of the remaining assays was calculated to determine whether there was incremental value in performing an additional assay.
Results
Baseline Characteristics
Baseline clinical and echocardiographic characteristics of the total study population (n = 1991) are shown in Table 1. Mean (SD) age at baseline was 62.0 (10.4) years. Participants included 1039 (52.2%) women. Characteristics of the 3 assays (NT-proBNP, Biosite, and Shionogi) in the study population are presented in Table 2.
TABLE 1.
Baseline Characteristics of the Total Study Population
| Characteristic | Total Population (n = 1991) |
|---|---|
| Age, mean (SD), y | 62 (10) |
| Women, % | 52 |
| Body mass index, mean (SD) | 28 (5) |
| Serum cholesterol | |
| Total, mean (SD), mg/dL | 204 (35) |
| HDL, mean (SD), mg/dL | 45 (14) |
| Serum creatinine, mean (SD), mg/dL | 1.1 (0.2) |
| Hypertension, % | 29 |
| Diabetes mellitus, % | 7 |
| Coronary artery disease, % | 11 |
| Left atrial enlargement, % | 14 |
| Left ventricular hypertrophy, % | 15 |
| EF < 50%, % | 5 |
| Diastolic dysfunction, % | 6 |
HDL indicates high-density lipoprotein; EF, ejection fraction.
TABLE 2.
Baseline Characteristics of the 3 BNP Assays in the Total Study Population
| Characteristic | NT-proBNP (pg/mL) | Biosite (pg/mL) | Shionogi (pg/mL) |
|---|---|---|---|
| Mean (SD) | 134.4 (230.4) | 46.2 (74.0) | 26.9 (43.2) |
| Median | 66.0 | 23.0 | 14.3 |
| Lowest third | < 36.7 | < 13.4 | < 7.9 |
| Middle third | 36.7–109.0 | 13.4–39.7 | 7.9–23.0 |
| Highest third | > 109.0 | > 39.7 | > 23.0 |
Mortality
There were 106 all-cause deaths recorded among the 1991 participants. Among individuals who died compared with those who were alive at the study completion, baseline median NT-proBNP was 206.5 versus 63.0 pg/mL (P < 0.001); BNP by Biosite assay was 63.3 versus 22.3 pg/mL (P < 0.001); and BNP by Shionogi assay was 33.6 versus 13.9 pg/mL (P < 0.001). Mortality according to tertiles for BNP as determined by all 3 assays is presented in Figure 1. Mortality risk increased with increasing tertiles for all 3 of the assays (P < 0.001) with an absolute increase in risk of death between the highest and lowest tertiles of 13.1%, 9.5%, and 9.1% for the NT-proBNP, Biosite, and Shionogi assays, respectively.
Figure 1.
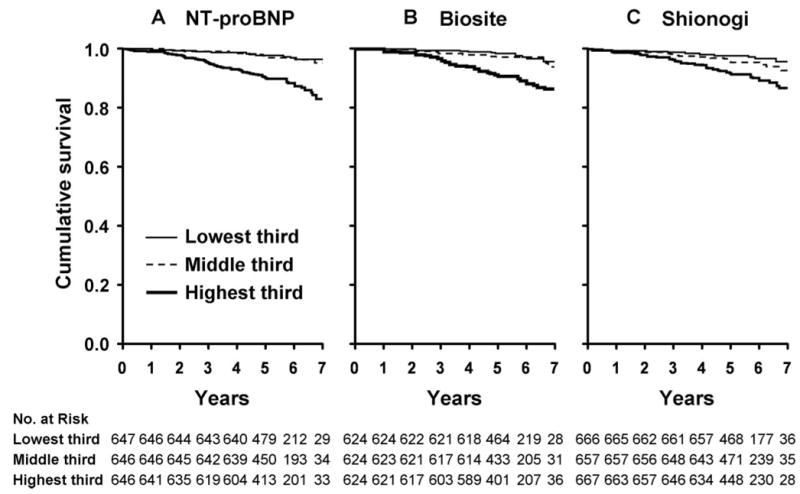
Kaplan-Meier curves for unadjusted cumulative survival in the total population according to tertiles of BNP by NT-proBNP, Biosite, and Shionogi assays. The peptide levels of tertiles 1, 2, and 3 were ≤ 36.7 pg/mL, 36.7 to 109.0 pg/mL, and > 109.0 pg/mL, respectively, for NT-proBNP (A); < 7.9 pg/mL, 7.9 to 23.0 pg/mL, and > 23.0 pg/mL, respectively, Shionogi (B); < 13.4 pg/mL, 13.4 to 39.7 pg/mL, and > 39.7 pg/mL, respectively, for Biosite (C). P for trend across the tertiles was < 0.001 for NT-proBNP, Biosite, and Shionogi assays.
Hazard ratios for mortality during 7 years of follow-up according to baseline plasma BNP for all 3 of the assays as a continuous variable are presented in Table 3. Increasing levels of BNP by all 3 of the assays were independently associated with increased mortality even after adjustment for clinical phenotypes and echocardiographic abnormalities with the exception of the Shionogi assay after adjustment for echocardiographic abnormalities.
TABLE 3.
Hazard Ratios (HR) for Mortality During 7 Years of Follow-Up According to Baseline BNP
| BNP Assay | HR (95% CI) per 1-SD Increase in Log Variable of BNP Assay | P Value |
|---|---|---|
| NT-proBNP | ||
| Unadjusted model | 2.98 (2.39 to 3.72) | < 0.001 |
| Age- and sex-adjusted | 1.75 (1.36 to 2.25) | < 0.001 |
| Multivariable model 1* | 1.63 (1.25 to 2.13) | < 0.001 |
| Multivariable model 2† | 1.44 (1.08 to 1.94) | 0.014 |
| Biosite | ||
| Unadjusted model | 2.65 (2.11 to 3.33) | < 0.001 |
| Age- and sex-adjusted | 1.61 (1.25 to 2.06) | < 0.001 |
| Multivariable model 1* | 1.50 (1.15 to 1.95) | 0.003 |
| Multivariable model 2† | 1.34 (1.01 to 1.77) | 0.045 |
| Shionogi | ||
| Unadjusted model | 2.26 (1.87 to 2.75) | < 0.001 |
| Age- and sex-adjusted | 1.45 (1.17 to 1.80) | < 0.001 |
| Multivariable model 1* | 1.39 (1.10 to 1.74) | 0.005 |
| Multivariable model 2† | 1.26 (0.99 to 1.60) | 0.052 |
CI indicates confidence interval.
Adjustment for age, sex, total cholesterol, and serum creatinine; presence of diabetes mellitus, hypertension, and coronary artery disease.
Adjustment for variables in model 1 in addition to echocardiographic abnormalities including the presence of ejection fraction < 50%, diastolic dysfunction, valvular dysfunction, left ventricular hypertrophy, left atrial enlargement, and regional wall motion abnormalities.
Specifically, when analyzed as a continuous variable, NT-proBNP was associated with a 63% increase in mortality per 1-SD increment in log peptide values after the adjustment for clinical phenotypes (model 1; P < 0.001). Additional adjustment for echocardiographic abnormalities (model 2), including LVH and diastolic dysfunction, only modestly attenuated the risk of death with a 44% increase in mortality (P = 0.014). BNP by Biosite assay was associated with a 50% increased risk of death per 1-SD increment in log peptide values after adjustment for clinical phenotypes (P = 0.003) and 34% increase in mortality after additional adjustment for echocardiographic abnormalities (P = 0.045). Similar to the NT-proBNP and Biosite assays, BNP by Shionogi assay was associated with increased mortality after adjustment for clinical phenotypes (P = 0.005). However, the inclusion of echocardiographic abnormalities in the model attenuated the prognostic value of the Shionogi assay as a biomarker for mortality (P = 0.052).
Figure 2 illustrates age- and gender-adjusted sequential modeling to determine the incremental value of each assay over the remaining 2 assays as a predictor of mortality. Results for models adjusting for clinical and echocardiographic phenotypes are not shown but are consistent with the age- and gender-adjusted models (Figure 2). For the Shionogi assay, both the Biosite (P = 0.041) and NT-ProBNP (P = 0.003) assays added significant predictive value beyond that of Shionogi alone. For the Biosite assay, only NT-ProBNP (P = 0.022) added significant predictive value beyond that of Biosite alone. In contrast, for NT-proBNP, neither the Shionogi (P = 0.317) nor Biosite (P = 0.572) assays added significant predictive value. Moreover, NT-ProBNP added significant mortality predictive value when added to the model that included a combination of both the Shionogi and Biosite assays (change in model χ2 = 4.45; P = 0.034).
Figure 2.
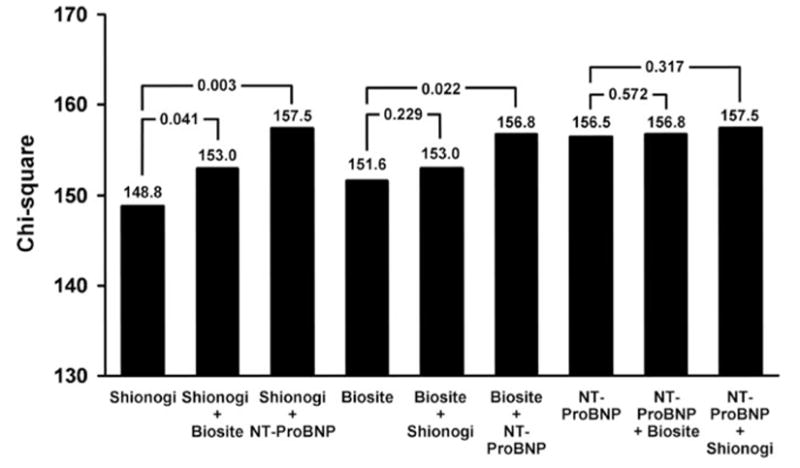
Age- and gender-adjusted sequential modeling to determine the incremental value of each assay over the remaining 2 assays as a predictor of mortality. For a particular assay, the change in overall model fit (log likelihood statistic) relative to the addition of 1 or both of the remaining assays was calculated to determine whether there was incremental value in performing an additional assay. P values represent the statistical significance of the difference between models.
Phenotype of Individuals With Elevated BNP
The prevalence of specific clinical phenotypes and echocardiographic abnormalities according to NT-proBNP tertiles are presented in Figure 3A and 3B. Results adjusted for age and gender did not significantly differ from those shown in Figure 3A and 3B. Results for the Biosite and Shionogi assays are not shown but are parallel to the NT-proBNP assay. There was an incremental and significant increase in the prevalence of all of the clinical phenotypes examined across BNP tertiles (P < 0.001 for all phenotypes except diabetes mellitus; P= 0.030; Figure 3A). This included coronary artery disease, atrial fibrillation, chronic obstructive pulmonary disease, hypertension, cardiomyopathy, and previous myocardial infarction. The same trend held true for echocardiographic characteristics as the prevalence of all of the recorded echocardiographic abnormalities (Figure 3B) including ejection fraction < 50%, valvular dysfunction, regional wall motion abnormality, diastolic dysfunction, LA enlargement, and LVH increased across the tertiles (P < 0.001).
Figure 3.
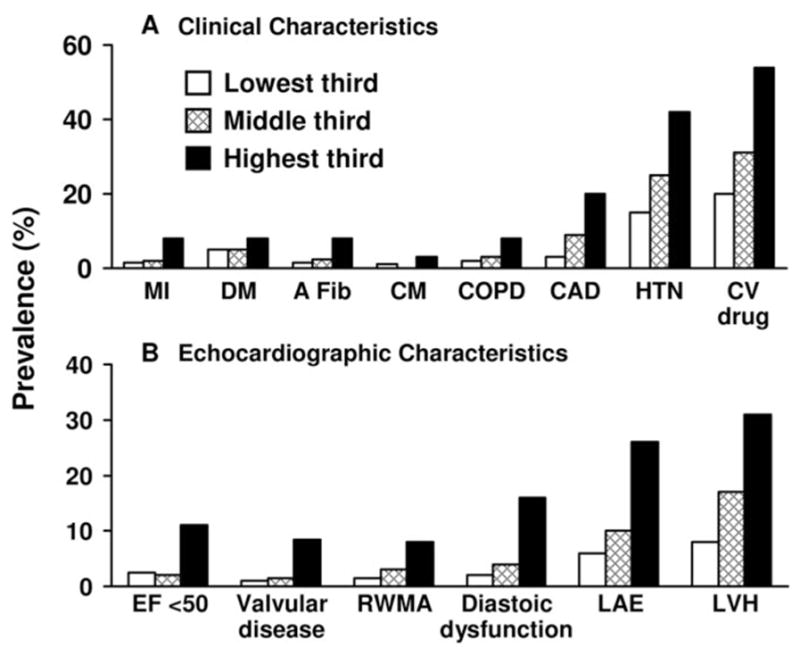
Percentage prevalence of clinical characteristics and echocardiographic abnormalities according to tertiles of NT-proBNP. The lowest, middle, and highest tertiles for NT-proBNP were < 36.7 pg/mL, 36.7 to 109.0 pg/mL, and > 109.0 pg/mL, respectively. The prevalence of all clinical and echocardiographic phenotypes was significantly increased (P < 0.001) across the tertiles with the exception of diabetes mellitus (P = 0.030). A Fib indicates atrial fibrillation; CAD, coronary artery disease; CM, cardiomyopathy; COPD, chronic obstructive pulmonary disease; CV drug, cardiovascular drug use; DM, diabetes mellitus; EF, ejection fraction; HTN, hypertension; LAE, left atrial enlargement; LVH, left ventricular enlargement; MI, myocardial infarction; and RWMA, regional wall motion abnormality.
Discussion
The current study demonstrates that circulating concentrations of the cardiac peptide BNP in our study population, free of HF (FHS criteria) or renal failure (serum creatine > 2.0 mg/dL), was predictive of increased mortality, thereby confirming earlier studies.4,5 Our studies further extend previous reports with 3 widely used assays for quantifying BNP, all of which predicted increased mortality. Whereas all 3 of the assays were predictive of mortality, the NT-proBNP and Biosite assays had a higher predictive value and remained significant even after the adjustment for both clinical phenotypes and echo-cardiographic abnormalities. We also provide important information on the cardiovascular clinical phenotype of those at greatest risk, as well as key structural and functional cardiac abnormalities as documented by in-depth echocardiographic examination. Thus, this investigation in adult humans in the general community provides new insights into BNP as a biomarker for cardiovascular disease and survival in subjects without HF of renal failure.
The cardiac hormone BNP is currently used in the diagnosis and prognosis of symptomatic HF.1–3,20–22 To date, both active BNP and the apparently nonbiologically active NT-proBNP fragment have emerged as potential biomarkers of preclinical asymptomatic left ventricular dysfunction.9,13,23–26 In this study, we confirm recent reports, which show that BNP may identify those at increased risk even in the absence of HF and with circulating concentrations well below the threshold for HF. We and others7,27,28 have speculated that the association between the natriuretic peptides and mortality may be because of increased filling pressures in the setting of diastolic dysfunction, which was not assessed in the previous large, population-based biomarker studies.4,5 In the current study, diastolic dysfunction was indeed more common among those with higher BNP levels. However, adjustment for diastolic function and other echocardiographic measurements was not found to alter the prognostic significance of the NT-proBNP or Biosite assays. This analysis, including diastolic dysfunction, further confirms the value of BNP as a biomarker for mortality beyond traditional clinical and echocardiographic risk factors. These findings may impact the clinical utility of these widely used assays to unmask individuals with increased risk of mortality during the study period of 7 years.
A significant aspect of our study was the use of 3 widely used assays for BNP to predict mortality in the general population. Importantly, we observed that all 3 of the assays possessed prognostic properties in predicting death. However, 1 assay emerged as more robust, as is illustrated in Figure 2. Here, our models compared the relative predictive value for mortality among the 3 assays and suggested that NT-proBNP may be superior as a biomarker compared with the Biosite and Shionogi assays. Specifically, NT-proBNP adds significant predictive value to both the Shionogi and Biosite assays, whereas the predictive value of NT-proBNP is not improved with the addition of either the Shionogi or Biosite assays. This observation is also consistent with a recent report by Costello-Boerrigter et al9 of a superiority of NT-proBNP compared with Biosite BNP in this same cohort in detecting reduced ventricular systolic function. The superiority of NT-proBNP to BNP is likely secondary to the prolonged half-life of the nonbiologically active NT-proBNP that translates to relatively consistent intraday plasma levels. This is in contrast to the high intraday variability of the biologically active BNP. It is possible that the relationship between BNP and mortality during the study period may be stronger if intrasubject variation was minimized by the averaging of multiple plasma measurements.
In Figure 3, we report among individuals without HF or renal failure that those with higher BNP levels had higher incidence of cardiovascular drug use. It is important to note that most drugs used to treat hypertension, including angiotensin-converting enzyme inhibitors,29 aldosterone antagonists,30 and angiotensin II receptor blockers,31 can reduce plasma BNP levels. Currently there is conflicting evidence regarding the effect of β-blockers on plasma BNP levels.32,33 The use of these drugs may have reduced both mortality and BNP over the 7 years of the study thereby potentially obscuring an even tighter association between BNP and mortality during the study period.
Our in-depth clinical and echocardiographic findings offer insight into the mechanism of modestly increased BNP in the adult general population. We report for the first time the phenotypic characteristics of individuals without HF or renal failure who are at increased risk for death during the study period as determined by their plasma BNP levels. In the current study, in the absence of HF and renal failure, there was an incremental and significant increase in the prevalence of traditional risk factors and echocardiographic abnormalities with increasing BNP values. Because our echocardiographic findings documented widespread structural changes in those with increasing BNP levels, it is tempting to speculate that the elevation of plasma BNP represents a plasma biomarker for asymptomatic cardiac abnormalities. Elevated BNP levels may, thus, represent a final common pathway for many cardiovascular pathologic disease states. Clearly, studies of myocardial structure and function in such populations free of HF are warranted together with careful assessment of myocardial secretion of BNP to better understand the underlying mechanism for enhanced plasma concentrations.
The strengths of our study include a large, well-characterized, community-based sample and thorough echocardiographic evaluation of cardiac function and structure. The use of 3 widely used BNP assays is also an important strength. There are several limitations to our study. The Olmsted County population is predominately white, and our results may not be generalized to nonwhites. Although we do confirm and report the predictive value of BNP for death, the mechanism of increased mortality was not defined. In addition, we report all-cause mortality, because of the number of specific-cause deaths was too few for statistical analysis. Finally, we do not assess any potential synergistic role of combining BNP with other biomarkers, such as C-reactive protein, which has been shown to enhance the predictive value of BNP for cardiovascular events in previous reports.26 However, we do assess the relative predictive value of 3 different assays.
The current study has clinical implications that deserve further investigation. Our studies and those of Wang et al4 suggest that a modest elevation of BNP predicts increased mortality during the study period, and the latter report would suggest that it also is predictive of future HF, stroke, and atrial fibrillation. Our echocardiographic and clinical data would suggest that the mechanism of the elevation may be structural abnormalities, such as LVH, diastolic dysfunction, and other myocardial abnormalities. It is important that future studies be directed at the use of BNP to detect people at risk and for populations in which BNP is modestly elevated but below HF and renal failure values. Such use of this cardiac hormone may prompt echocardiographic examination so as to detect structural changes in the heart and to identify clinical risk factors, both of which could be more aggressively treated. In addition, it will be important to then undertake human therapeutic studies to determine whether reduction of BNP in such a population results in improved outcomes and survival.
Perspectives
From a clinical perspective, the current findings may change the way we use NT-proBNP and BNP as biomarkers. It is common clinical practice to use these biomarkers in patients with HF to confirm diagnosis, assess prognosis, and guide therapy. Our findings go beyond HF and especially extend to the setting of general practice. Today, health care providers in the community assess cardiovascular risk factors and attempt to optimally control blood pressure, diabetes, and lipids so as to positively impact survival. Our studies suggest that the use of NT-proBNP or BNP could serve as a blood test to aid in identifying the “high-risk” subject with cardiovascular risk in the general population prompting more aggressive primary prevention. Such use of NT-proBNP or BNP in the general community might well prompt more use of echocardiography to look at underlying structural changes of the heart, which could also have an impact of the cost of care. Most importantly, if future studies clearly demonstrate that a reduction in NT-proBNP or BNP improves mortality in this type of general population, studies that clearly need to be done, then the use of NT-proBNP and BNP would evolve into established clinical practice in the care of the general population without HF.
In summary, we report the first study to compare NT-proBNP and BNP using 3 distinct assays in the same population-based cohort as a biomarker for mortality. Specifically, the NT-proBNP assay is most predictive of mortality even after adjustment for clinical phenotypes and echocardiographic abnormalities. Our findings further underscore that elevated NT-proBNP and BNP may also serve as biomarkers for underlying cardiac remodeling secondary to diverse cardiovascular disease entities. This study, thus, confirms the potential use of these peptides as biomarkers for future events and may enhance efforts at primary prevention.
Acknowledgments
This work was supported by grants from the National Institutes of Health(HL36634), Miami Heart Research Institute, Stanley J. Sarnoff Endowment for Cardiovascular Science, Roche, and Mayo Foundation. P.M.M. acknowledges the generous support of the Stanley J. Sarnoff Endowment for Cardiovascular Research. We acknowledge the outstanding assistance of Linda C. Combs and Jocelyn Jack.
References
- 1.Heidenreich PA, Gubens MA, Fonarow GC, Konstam MA, Stevenson LW, Shekelle PG. Cost-effectiveness of screening with B-type natriuretic peptide to identify patients with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2004;43:1019–1026. doi: 10.1016/j.jacc.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 2.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA, Nowak RM, McCord J, Hollander JE, Herrmann HC, Steg PG, Duc P, Westheim A, Omland T, Knudsen CW, Storrow AB, Abraham WT, Lamba S, Wu AH, Perez A, Clopton P, Krishnaswamy P, Kazanegra R, Maisel AS. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106:416–422. doi: 10.1161/01.cir.0000025242.79963.4c. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 5.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, c-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 6.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 7.Mark DB, Felker GM. B-type natriuretic peptide - a biomarker for all seasons? N Engl J Med. 2004;350:718–720. doi: 10.1056/NEJMe038233. [DOI] [PubMed] [Google Scholar]
- 8.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 9.Costello-Boerrigter LC, Boerrigter G, Redfield MM, Rodeheffer RJ, Urban LH, Mahoney DW, Jacobsen SJ, Heublein DM, Burnett JC., Jr Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen SJ, Mahoney DW, Redfield MM, Bailey KR, Burnett JC, Jr, Rodeheffer RJ. Participation bias in a population-based echocardiography study. Ann Epidemiol. 2004;14:579–584. doi: 10.1016/j.annepidem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 13.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109:3176–3181. doi: 10.1161/01.CIR.0000130845.38133.8F. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987;59:956–960. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]
- 17.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–1043. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 18.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 19.Collinson PO, Barnes SC, Gaze DC, Galasko G, Lahiri A, Senior R. Analytical performance of the N terminal pro B type natriuretic peptide (NT-proBNP) assay on the Elecsys 1010 and 2010 analysers. Eur J Heart Fail. 2004;6:365–368. doi: 10.1016/j.ejheart.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro BP, Chen HH, Burnett JC, Jr, Redfield MM. Use of plasma brain natriuretic peptide concentration to aid in the diagnosis of heart failure. Mayo Clin Proc. 2003;78:481–486. doi: 10.4065/78.4.481. [DOI] [PubMed] [Google Scholar]
- 21.Davis M, Espiner E, Richards G, Billings J, Town I, Neill A, Drennan C, Richards M, Turner J, Yandle T. Plasma brain natriuretic peptide in assessment of acute dyspnoea. Lancet. 1994;343:440–444. doi: 10.1016/s0140-6736(94)92690-5. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto K, Burnett JC, Jr, Redfield MM. Effect of endogenous natriuretic peptide system on ventricular and coronary function in failing heart. Am J Physiol. 1997;273:H2406–H2414. doi: 10.1152/ajpheart.1997.273.5.H2406. [DOI] [PubMed] [Google Scholar]
- 23.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B-type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul study. Circulation. 2003;108:2987–2992. doi: 10.1161/01.CIR.0000103681.04726.9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura M, Endo H, Nasu M, Arakawa N, Segawa T, Hiramori K. Value of plasma B type natriuretic peptide measurement for heart disease screening in a Japanese population. Heart. 2002;87:131–135. doi: 10.1136/heart.87.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silver MA, Pisano C. High incidence of elevated B-type natriuretic peptide levels and risk factors for heart failure in an unselected at-risk population (stage A): implications for heart failure screening programs. Congest Heart Fail. 2003;9:127–132. doi: 10.1111/j.1527-5299.2003.02589.x. [DOI] [PubMed] [Google Scholar]
- 26.Campbell DJ, Woodward M, Chalmers JP, Colman SA, Jenkins AJ, Kemp BE, Neal BC, Patel A, Macmahon SW. Prediction of heart failure by amino terminal-pro-B-type natriuretic peptide and C-reactive protein in subjects with cerebrovascular disease. Hypertension. 2005;45:69–74. doi: 10.1161/01.HYP.0000151103.02424.c3. [DOI] [PubMed] [Google Scholar]
- 27.McKie PM, Burnett JC., Jr B-type natriuretic peptide as a biomarker beyond heart failure: speculations and opportunities. Mayo Clin Proc. 2005;80:1029–1036. doi: 10.4065/80.8.1029. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi H, Yoshida J, Yamamoto K, Sakata Y, Mano T, Akehi N, Hori M, Lim YJ, Mishima M, Masuyama T. Elevation of plasma brain natriuretic peptide is a hallmark of diastolic heart failure independent of ventricular hypertrophy. J Am Coll Cardiol. 2004;43:55–60. doi: 10.1016/j.jacc.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 29.Brunner-La Rocca HP, Weilenmann D, Kiowski W, Maly FE, Follath F. Plasma levels of enalaprilat in chronic therapy of heart failure: relationship to adverse events. J Pharmacol Exp Ther. 1999;289:565–571. [PubMed] [Google Scholar]
- 30.Macdonald JE, Kennedy N, Struthers AD. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart. 2004;90:765–770. doi: 10.1136/hrt.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latini R, Masson S, Anand I, Judd D, Maggioni AP, Chiang YT, Bevilacqua M, Salio M, Cardano P, Dunselman PH, Holwerda NJ, Tognoni G, Cohn JN. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2002;106:2454–2458. doi: 10.1161/01.cir.0000036747.68104.ac. [DOI] [PubMed] [Google Scholar]
- 32.Luchner A, Burnett JC, Jr, Jougasaki M, Hense HW, Riegger GA, Schunkert H. Augmentation of the cardiac natriuretic peptides by β-receptor antagonism: evidence from a population-based study. J Am Coll Cardiol. 1998;32:1839–1844. doi: 10.1016/s0735-1097(98)00478-1. [DOI] [PubMed] [Google Scholar]
- 33.Frantz RP, Olson LJ, Grill D, Moualla SK, Nelson SM, Nobrega TP, Hanna RD, Backes RJ, Mookadam F, Heublein D, Bailey KR, Burnett JC. Carvedilol therapy is associated with a sustained decline in brain natriuretic peptide levels in patients with congestive heart failure. Am Heart J. 2005;149:541–547. doi: 10.1016/j.ahj.2004.07.036. [DOI] [PubMed] [Google Scholar]


