Abstract
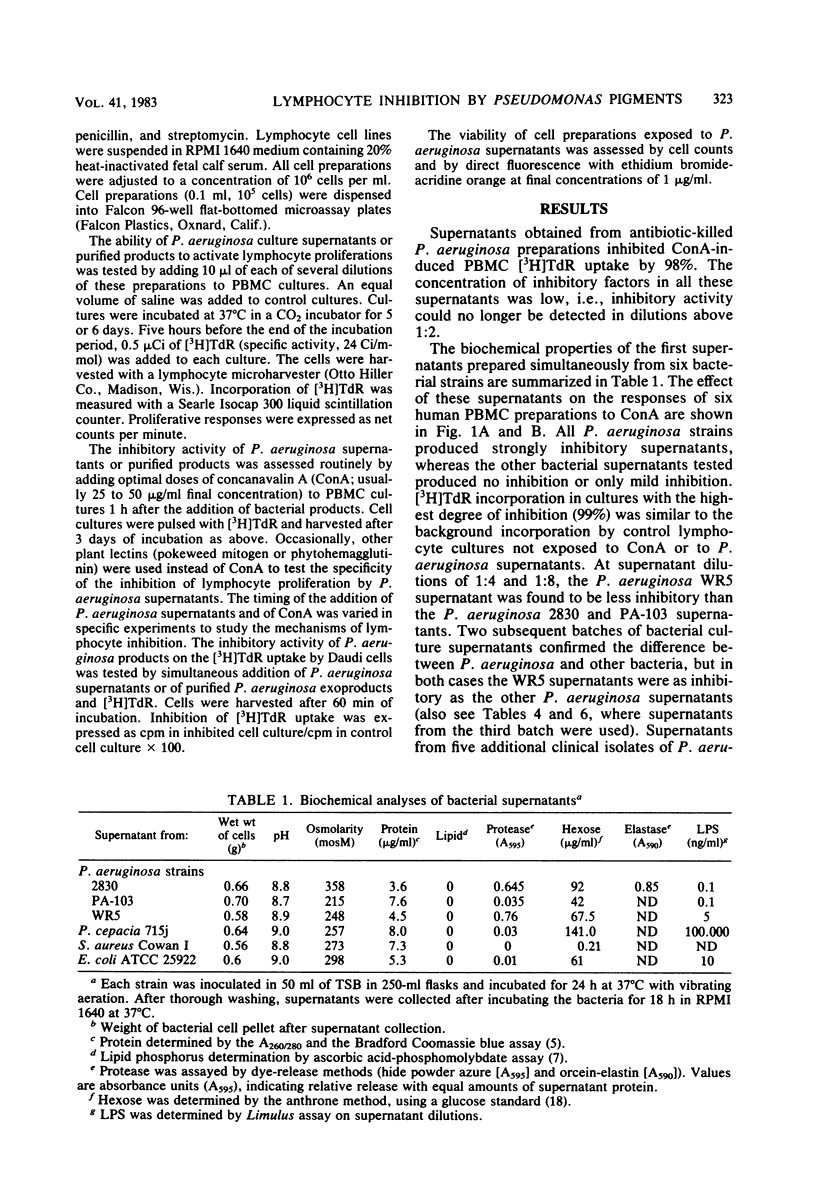
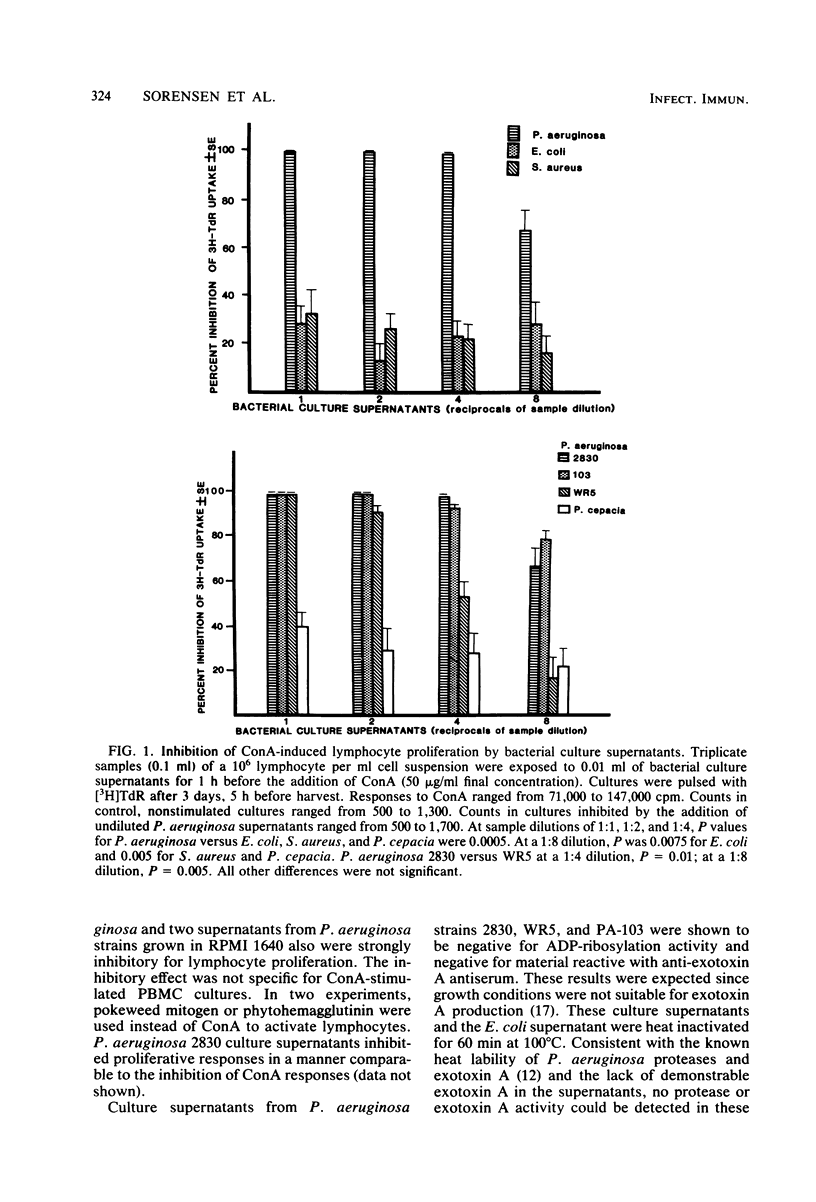
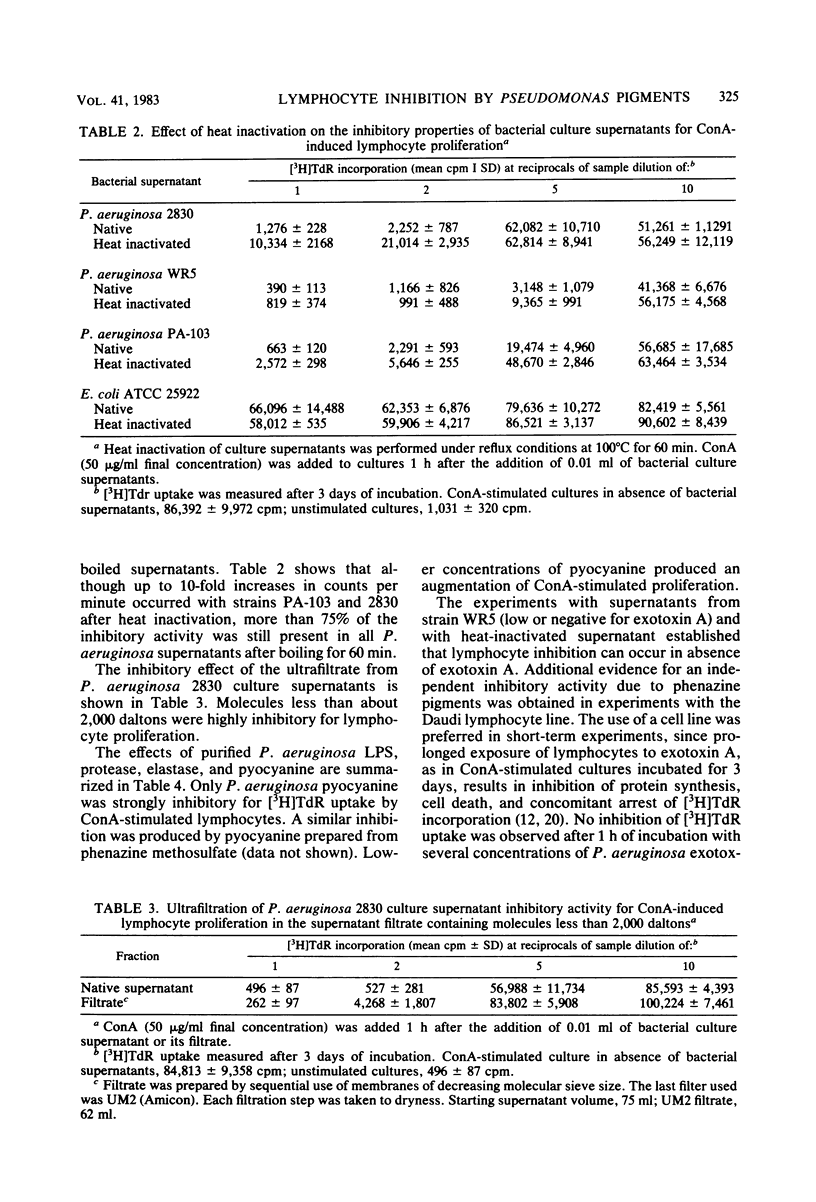
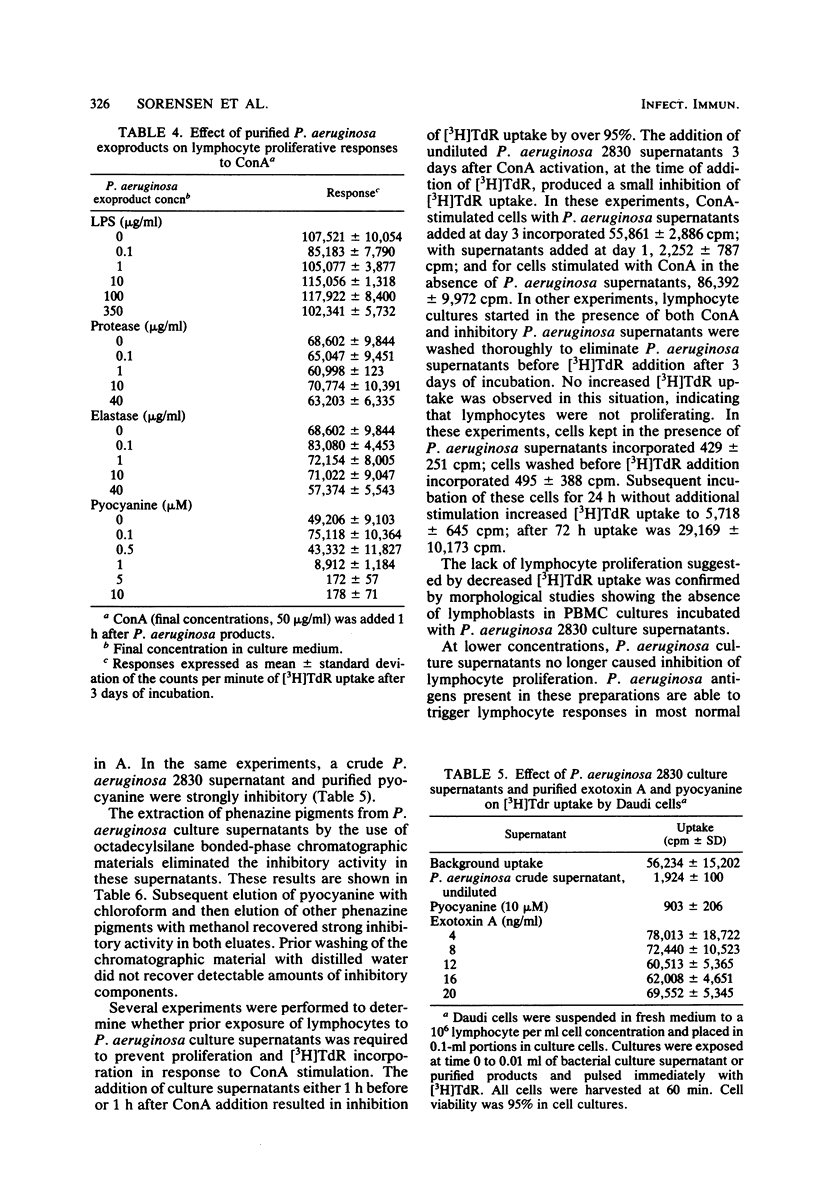
Human lymphocyte proliferation is inhibited in vitro in the presence of killed Pseudomonas aeruginosa or cell-free P. aeruginosa culture supernatants. A comparison of culture supernatants obtained under similar conditions from Staphylococcus aureus, Escherichia coli, P. aeruginosa, and Pseudomonas cepacia strains demonstrated that all P. aeruginosa supernatants were strongly inhibitory, whereas supernatants from other bacteria were mildly inhibitory or not inhibitory at all. These P. aeruginosa inhibitors prevent proliferative responses of resting cells upon mitogen activation and decrease [3H]thymidine uptake when added to human lymphocytes undergoing active proliferation in culture. The inhibitory effect is reversible and not due to cytotoxicity. Most of the inhibitory activity present in crude supernatants was detected in ultrafiltrates of molecular weights below 2,000. Purified P. aeruginosa pyocyanine, a low-molecular-weight phenazine pigment present in culture supernatant, was strongly inhibitory for lymphocyte proliferation. Extraction of pyocyanine and phenazine pigments from inhibitory P. aeruginosa supernatants eliminated their inhibitory activity. Inhibitors were recovered from reverse-phase chromatographic cartridges by both chloroform and methanol elution, indicating that pyocyanine and other phenazine pigments present in P. aeruginosa supernatants are responsible for the inhibition of lymphocyte proliferation. In addition to the identification of phenazine pigments as lymphocyte proliferation inhibitors, several criteria ruled out major contributions of P. aeruginosa polysaccharide, exotoxin A, and proteases to this phenomenon. P. aeruginosa strains selected for very low protease production or for very low exotoxin A production produced supernatants as inhibitory for lymphocyte proliferation as supernatants obtained from clinical P. aeruginosa isolates. Purified P. aeruginosa lipopolysaccharide and protease preparations failed to induce reversible lymphocyte proliferation inhibition. Finally, heat inactivation of P. aeruginosa supernatants at 100 degrees C for 60 min inactivates exotoxin A and proteases but produced only a moderate decrease of the inhibitory activity for lymphocyte proliferation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong A. V., Stewart-Tull D. E., Roberts J. S. Characterisation of the Pseudomonas aeruginosa factor that inhibits mouse-liver mitochondrial respiration. J Med Microbiol. 1971 May;4(2):249–262. doi: 10.1099/00222615-4-2-249. [DOI] [PubMed] [Google Scholar]
- Armstrong A. V., Stewart-Tull D. E. The site of the activity of extracellular products of Pseudomonas aeruginosa in the electron-transport chain in mammalian cell respiration. J Med Microbiol. 1971 May;4(2):263–270. doi: 10.1099/00222615-4-2-263. [DOI] [PubMed] [Google Scholar]
- Baron S. S., Rowe J. J. Antibiotic action of pyocyanin. Antimicrob Agents Chemother. 1981 Dec;20(6):814–820. doi: 10.1128/aac.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang P. C., Blackwood A. C. Simultaneous production of three phenazine pigments by Pseudomonas aeruginosa Mac 436. Can J Microbiol. 1969 May;15(5):439–444. doi: 10.1139/m69-077. [DOI] [PubMed] [Google Scholar]
- Eagon R. G., Hodge T. W., 3rd, Rake J. B., Yarbrough J. M. The effect of phenazine methosulfate-ascorbate on bacterial active transport and adenosine triphosphate formation: inhibition of Pseudomonas aeruginosa and stimulation of Escherichia coli. Can J Microbiol. 1979 Jul;25(7):798–802. doi: 10.1139/m79-117. [DOI] [PubMed] [Google Scholar]
- Garzelli C., Colizzi V., Campa M., Bozzi L., Falcone G. Depression of contact sensitivity by Pseudomonas aeruginosa-induced suppressor cells which affect the induction phase of immune response. Infect Immun. 1979 Oct;26(1):4–11. doi: 10.1128/iai.26.1.4-11.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. J., Jr, Brodsky R. E., Schultz M. G. Changing patterns of malaria in the United States. J Infect Dis. 1974 Nov;130(5):553–555. doi: 10.1093/infdis/130.5.553. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Parker C. M., Parker C. W. Opposing effects of mitogenic and nonmitogenic lectins on lymphocyte activation. Evidence that wheat germ agglutinin produces a negative signal. J Biol Chem. 1976 Jul 10;251(13):4017–4025. [PubMed] [Google Scholar]
- Huber C., Sundström C., Nilsson K., Wigzell H. Surface receptors on human haematopoietic cell lines. Clin Exp Immunol. 1976 Sep;25(3):367–376. [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J. C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J., Bjorn M. J., Maxwell E. S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Knight M., Hartman P. E., Hartman Z., Young V. M. A new method of preparation of pyocyanin and demonstration of an unusual bacterial sensitivity. Anal Biochem. 1979 May;95(1):19–23. doi: 10.1016/0003-2697(79)90179-9. [DOI] [PubMed] [Google Scholar]
- Leisinger T., Margraff R. Secondary metabolites of the fluorescent pseudomonads. Microbiol Rev. 1979 Sep;43(3):422–442. doi: 10.1128/mr.43.3.422-442.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Mathé G., Florentin I., Bruley-Rosset M., Hayat M., Bourut C. Heat-killed Pseudomonas aeruginosa as a systemic adjuvant in cancer immunotherapy. Biomedicine. 1977 Dec;27(9-10):368–373. [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol. 1977 Feb;23(2):183–189. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- Petit J. C., Richard G., Albert B., Daguet G. L. Depression by Pseudomonas aeruginosa of two T-cell-mediated responses, anti-Listeria immunity and delayed-type hypersensitivity to sheep erythrocytes. Infect Immun. 1982 Mar;35(3):900–908. doi: 10.1128/iai.35.3.900-908.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Markham R. B. Induction in mice of cell-mediated immunity to Pseudomonas aeruginosa by high molecular weight polysaccharide and vinblastine. J Immunol. 1982 May;128(5):2121–2125. [PubMed] [Google Scholar]
- Rechler M. M. The purification and characterization of L-histidine ammonia-lyse (Pseudomonas). J Biol Chem. 1969 Feb 25;244(4):551–559. [PubMed] [Google Scholar]
- Rubin H. R., Sorensen R. U., Chase P. A., Klinger J. D. Suppression of in vitro lymphocyte DNA synthesis by killed Pseudomonas aeruginosa. Infect Immun. 1983 Feb;39(2):630–637. doi: 10.1128/iai.39.2.630-637.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen R. U., Stern R. C., Chase P., Polmar S. H. Defective cellular immunity to gram-negative bacteria in cystic fibrosis patients. Infect Immun. 1979 Feb;23(2):398–402. doi: 10.1128/iai.23.2.398-402.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Tull D. E., Armstrong A. V. The effect of 1-hydroxyphenazine and pyocyanin from Pseudomonas aeruginosa on mammalian cell respiration. J Med Microbiol. 1972 Feb;5(1):67–73. doi: 10.1099/00222615-5-1-67. [DOI] [PubMed] [Google Scholar]
- Taylor N. S., Pollack M. Purification of Pseudomonas aeruginosa exotoxin by affinity chromatography. Infect Immun. 1978 Jan;19(1):66–70. doi: 10.1128/iai.19.1.66-70.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Cellular transport mechanisms. Annu Rev Biochem. 1978;47:933–965. doi: 10.1146/annurev.bi.47.070178.004441. [DOI] [PubMed] [Google Scholar]
- ZAUGG W. S. SPECTROSCOPIC CHARACTERISTICS AND SOME CHEMICAL PROPERTIES OF N-METHYLPHENAZINIUM METHYL SULFATE (PHENAZINE METHOSULFATE) AND PYOCYANINE AT THE SEMIQUIDNOID OXIDATION LEVEL. J Biol Chem. 1964 Nov;239:3964–3970. [PubMed] [Google Scholar]


