Abstract
Background
The neurobiological relationship between schizophrenia and psychotic mood disorders is not well understood. Neurocognitive deficits have been described in both types of disorders and have been proposed to reflect underlying neurobiological dysfunction. Examining the relationship between neurocognitive function and psychopathology could help illuminate the neurobiological relationship between schizophrenia and psychotic mood disorders.
Methods
Participants included 72 individuals with DSM-IV schizophrenia, 25 individuals with schizoaffective disorder or bipolar disorder with psychotic features, and 72 community controls. Standardized scores and correlations between four domains of neurocognition and psychopathology were examined.
Results
Individuals with schizophrenia and psychotic mood disorders scored similarly on several dimensions of neurocognitive function and psychopathology. The relationships between neurocognitive function and psychopathology were similar in the two groups.
Conclusions
Individuals with schizophrenia and psychotic mood disorders were similar in terms of both the level of impairment in neurocognitive function and psychopathology, as well as in the relationship between the two dimensions of illness. These results suggest that schizophrenia and psychotic mood disorders such as schizoaffective disorder and bipolar disorder with psychotic features are on a neurobiological continuum.
Keywords: schizophrenia, neurocognitive functioning, psychopathology
1. Introduction
A major issue facing the developers of the DSM-V and a topic of considerable debate in the literature is whether individuals with schizophrenia (SCZ) or psychotic mood disorders (PMD) are separate and distinct disorders, or whether they occur on a “psychosis” continuum (e.g., Allardyce et al., 2007). Some experts define this continuum as a spectrum of psychotic disorders with mood disorders, such as bipolar disorder with psychotic features (BDP), on one pole and schizophrenia (SCZ) on the other pole with schizoaffective disorder (SA) in the middle (e.g., Lake & Hurwitz, 2007; Kempf et al., 2005). Support for this view is based on reports of increased genetic risk for the continuum of disorders among first-degree relatives of SCZ, SA, and BDP. For instance, when compared to controls, the risk for bipolar disorder is higher in the relatives of SCZ and SA, the risk of schizophrenia is higher in the relatives of BDP and SA, and the risk of schizoaffective disorder is higher in the relatives of BDP and SCZ (Valles et al., 2000; Kendler et al., 1998; Rice et al., 1987; Tsuang et a., 1980). Linkage studies also suggest that the genes related to the risk of developing SCZ, SA, and BDP may be similar (e.g., Potash, 2006; Hamshere et al., 2005).
The question of whether PMD, such as SA or BDP, are on the same continuum as schizophrenia has also been addressed by examining the severity of neurocognitive deficits and clinical symptoms across the disorders. Prior research has found several similarities between SCZ and PMD. For instance SA and SCZ had similar deficits in neurocognitive performance on individual tasks measuring working memory, executive functioning, and intelligence (e.g., Gooding & Tallent, 2002; Reichenberg et al., 2002). Similarly, SCZ and BDP had similar performance deficits on working memory tasks (Glahn et al., 2006). Also, although Heinrichs and colleagues (2008) found differences between SCZ and SA on specific neurocognitive tests, they concluded that these differences were of insufficient magnitudes to validate two distinguishable disorders. However, few studies compared neurocognitive domain scores between SCZ and PMD (Evans et al., 1999). Additional research is needed to compare the performance of PMD to SCZ with respect to neurocognitive domains, which may be more reliable and robust than scores from individual tests.
Studies comparing the psychopathology of SCZ to PMD indicated that the clinical boundaries between these disorders remain unclear. For instance, some studies found that SCZ, SA, and BDP had similar levels of negative symptoms (Evans et al., 1999; Cuesta & Peralta, 1995), while others did not share this finding (Peralta & Cuesta, 2008; Kendler et al., 1995). The extent of positive and disorganized symptoms among SCZ, SA, and BDP also remains unclear. For instance, Peralta and Cuesta (2008) found that measures of psychosis and disorganization in SA were intermediate between SCZ and BDP, while Evans and colleagues (1999) reported that SCZ scored higher than PMD on measures of positive symptoms. Others found them to have similar ratings on positive (Cuesta & Peralta, 1995; Kendler et al., 1995) and disorganized symptoms (Cuesta & Peralta, 1995). It is possible that these findings were mixed given that few groups used the same methods to assess psychopathology. Thus, our study will use standardized measures to assess psychopathology in SCZ and PMD.
Although existing research has examined the relationships between neurocognitive deficits and psychopathology within SCZ, research has yet to examine how these relationships might vary across different psychotic disorders. Prior research suggests that the domains of psychopathology in SCZ show various relationships to neurocognitive function (e.g., Basso et al., 1998). For example, research on SCZ suggests that positive symptoms show little relationship to neurocognitive function (e.g., Nieuwenstein et al., 2001), while disorganized symptoms have been consistently related to impairments in working memory, executive function and episodic memory (e.g., Bozikas et al., 2004; Cameron et al., 2002). Findings for negative symptoms are mixed, but a number of studies suggest a relationship between negative symptoms and deficits in working memory, executive function and episodic memory (e.g., Nieuwenstein et al., 2001; Palmer & Heaton, 2000). Understanding whether psychopathology and neurocognitive deficits are similarly related among SCZ and PMD will inform theories of diagnosis, psychopharmacologic treatment, and illness pathophysiology. Thus, it is necessary to examine whether the pattern of relationships between neurocognitive deficits and psychopathology is similar between SCZ and PMD.
In the present study, we examined whether individuals with schizophrenia differ from individuals with psychotic mood disorders with respect to [1] the type and severity of neurocognitive deficits, [2] level of psychopathology, and [3] the relationship between neurocognitive function and psychopathology.
2. Methods
2.1. Participants
Participants included 72 individuals with schizophrenia (SCZ), 72 community controls (CON), and 25 individuals with psychotic mood disorders (PMD), including schizoaffective disorder (n=18: bipolar subtype (n=10), depressive subtype (n=8)) and bipolar disorder with psychotic features (n=7). We recomputed all study analyses using the participants with schizoaffective disorder (n=18) for the comparison group (data not shown). All of the same findings were significant and in the same direction as the total PMD sample (n=25). Thus, we elected to use the larger sample for this study to maximize statistical power.
All groups were similar on age, gender, race, and parental socioeconomic status. SCZ and PMD were psychiatrically stable and recruited from a St. Louis metropolitan psychiatric center and its outpatient clinics. CON were recruited through local advertisements. All subjects gave written informed consent for participation after the study’s risks and benefits were explained to them. Participants were excluded to avoid biasing results if they had: an unstable medical condition, a neurologic disorder, a head injury with loss of consciousness (at any point in lifetime), or substance abuse or dependence in the three months preceding the study. CON were also excluded if they had a first-degree relative with a psychotic disorder.
2.2. Measures
All participants were assessed by Master’s or Doctoral clinicians, blind to the diagnosis of the participant, who regularly participated in training and reliability sessions. DSM-IV Axis I diagnoses of each participant were determined by the consensus of a research psychiatrist and trained research clinicians who used the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 2002). The participants’ age of illness onset (operationalized as first appearance of psychotic symptoms) was assessed using self-report.
Participants completed a battery of neuropsychological tests. Based on prior research (Nuechterlein et al., 2004), we converted raw scores from the neuropsychological tests into standardized scores (based on current sample) for four domains: crystallized IQ, working memory, episodic memory, and executive functioning. Crystallized IQ was based on a single scaled variable measuring vocabulary from the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) (Wechsler, 1997a). Working memory was a sum of scaled scores on letter-numbering sequencing, spatial span, and digit span, subtests from the Wechsler Memory Scales-Third edition (WMS-III) (Wechsler, 1997b), and the four-item d’ score from the continuous performance task (Barch et al., 2004). Episodic memory was a sum of scaled scores from the WMS-III subtests on immediate recall of family pictures and logical memory (Wechsler, 1997b). Executive function included the time to completion on Trails B (Reitan & Wolfson, 1985), the number of novel words generated on the category and verbal fluency tasks (Benton et al., 1976), a scaled score on the matrix reasoning subtest from the WAIS-III (Wechsler, 1997a), and the score for perseverative errors (reversed in sign) from the Wisconsin Card Sort Test (Heaton, 2003).
Psychopathology (i.e., positive, negative, disorganized symptoms) was assessed using global ratings from the Scale for the Assessment of Positive Symptoms (SAPS: Andreasen, 1983a) and the Scale for the Assessment of Negative Symptoms (SANS: Andreasen, 1983b). All ratings were z-scored using the mean and standard deviation of the current sample and averaged within symptom clusters. The positive symptom cluster included the SAPS global ratings of hallucinations and delusions. The negative symptom cluster included the SANS global ratings of affective flattening, alogia, anhedonia, and avolition. The disorganized symptom cluster included the SAPS global ratings of positive formal thought disorder and bizarre behavior, and the SANS global rating of attention (Andreasen et al., 1995).
2.3. Data Analyses
An ANOVA was used to estimate the main effect of group status on the domains of neurocognition and psychopathology, and generate effect sizes. Levene’s test of homogeneity of variance was conducted given that bias in sample size increases the probability of violating normality and homogeneity of variances. A Welch variance weighted ANOVA was used when unequal variances were present.
Pairwise comparisons between groups were conducted with a Bonferroni (with equal variances) or Tamhane (with unequal variances) correction for multiple comparisons when a significant main effect was present. Pearson correlations were computed to examine the relationships between the domains of neurocognition and psychopathology. Statistical differences in r between SCZ and PMD were estimated using Fisher’s r-to-Z transformation (Cohen & Cohen, 1983). Fisher’s r-to-Z transformation converts the difference between Pearson’s correlations to a standardized Z score from the normal distribution.
3. Results
SCZ and PMD had similar ages of illness onset, while drug and alcohol use disorders were more prevalent in PMD. Specifically, they had a significantly higher lifetime prevalence of an alcohol use disorder. PMD also showed a trend for higher rates of a lifetime prevalence of cocaine or stimulant use disorders (Table 1).
Table 1.
Demographic and Clinical Characteristics of Study Sample
| SCZ (n=72) | PMD (n=25) | CON (n=72) | χ2/F Statistic | |
|---|---|---|---|---|
| Age (mean±SD) | 39.1±12.1 | 41.4±9.7 | 39.9±13.3 | 0.35 |
| Age of Onset-Psychosis (mean±SD) | 23.8±9.6 | 24.7±7.4 | na | 0.19 |
| Gender (% male) | 40.3% | 52.0% | 45.8% | 1.51 |
| SES (mean±SD) | 3.5±1.00 | 3.2±1.0 | 3.2±1.0 | 2.37 |
| Race (% African-American) | 56.0% | 56.9% | 41.7% | 3.74 |
| Substance Use Disorder (Abuse or Dependence) | ||||
| Alcohol | 18.1% | 44.0% | na | 6.71** |
| Cannabis | 19.4% | 36.0% | na | 2.81 |
| Cocaine | 9.7% | 24.0% | na | 3.26+ |
| Stimulants | 2.8% | 12.0% | na | 3.23+ |
| Hallucinogen | 4.2% | 8.0% | na | 0.56 |
| Sedatives | 2.8% | 4.0% | na | 0.09 |
| Opioids | 1.4% | 0.0% | na | 0.35 |
p<.07,
p<.01,
p<.001.
We found unequal variances in the main effect of group status on positive (F=15.6, p<.001) and negative symptoms (F=6.6, p=.002) and executive functioning (F=3.5, p=.034). Thus, the Welch ANOVA was used to test the effect of group status on these dimensions. Overall, we found that the main effects of group status on the domains of psychopathology and neurocognitive functioning were statistically significant (Table 2). Upon examining the pairwise comparisons, SCZ (MD=1.08, p<.001) and PMD (MD=.85, p=.001) had higher positive symptoms than CON. SCZ (MD=.76, p=.001) and PMD (MD=.77, p=.004) also had higher disorganized symptoms than CON. PMD were found to have levels of negative symptoms that were intermediate between SCZ (MD=−.41, p=.012) and CON (MD=.70, p<.001). SCZ also had higher levels of negative symptoms than CON (MD=1.11, p<.001).
Table 2.
Standardized Scores for Psychopathology and Neurocognition
| SCZ (n=72) | PMD (n=25) | CON (n=72) | ANOVA F Statistic(df1, df2)a,b | Homogeneity F Statistic | Effect Size (ηp2)c | |
|---|---|---|---|---|---|---|
| Psychopathology | ||||||
| Positive Symptoms | .318 | .082 | −.769 | 81.1 (2,48)***d | 15.6*** | .17/.02 |
| Negative Symptoms | .331 | −.081 | −.777 | 55.5(2,47)*** e | 6.6** | .26/.08 |
| Disorganized Symptoms | .226 | .230 | −.531 | 7.3(2,107)***d | 2.6 | .12/.00 |
| Neurocognition | ||||||
| Crystallized IQ | −.467 | .094 | .483 | 23.4(2,166)***f | 0.7 | .22/.08 |
| Working Memory | −.510 | −.290 | .399 | 31.0(2,166)***g | 0.8 | .27/.02 |
| Episodic Memory | −.625 | −.498 | .756 | 78.8(2,166)***g | 0.6 | .49/.01 |
| Executive Functioning | −.484 | −.342 | .401 | 32.3(2, 66)***g | 3.5* | .27/.01 |
p<.05,
p<.01,
p<.001
Note. CON: n=13 for psychopathology and n=72 for neurocognition
Pairwise Comparisons (Bonferroni): p<.05
Welch ANOVA with Tamhane Pairwise Correction for Unequal Variances used when homogeneity non-significant.
First effect size reports difference between all three groups/second effect size reports contrast for SCZ vs. PMD.
SCZ, PMD>CON
SCZ>PMD>CON
SCZ<PMD, CON
SCZ, PMD<CON
We also found that there were no differences between SCZ and PMD with respect to working memory (MD=−.22, p=.545), episodic memory (MD=−.13, p=1.0), and executive functioning (MD=−.14, p=1.0). SCZ and PMD were lower than CON on working memory (MD=−.91, p<.001; MD=−.69, p<.001), episodic memory (MD=−1.4, p<.001; MD=−1.3, p<.001), and executive functioning (MD=−.88, p<.001; MD=−.74, p<.001). Crystallized IQ was found to be higher in PMD (MD=.56, p=.013) and CON (MD=.95, p=<.001) when compared to SCZ (Table 2). A moderate effect size of group status (all three groups) on episodic memory was present (ηp2=.49), while small effect sizes were present for the remaining domains (ranging from ηp2=.12 to ηp2=.27). Furthermore, small effect sizes were also found when comparing SCZ to PMD (ranging from ηp2=.00 to ηp2=.08) (Table 2).
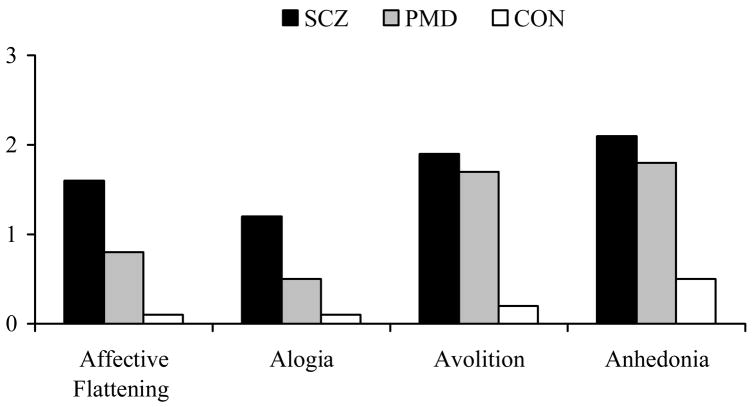
Given that negative symptoms differed significantly between SCZ and PMD, we conducted a post hoc analysis of the ratings of negative symptoms between groups to better understand why PMD was intermediate between SCZ and CON on this domain (Figure 1). We found a significant group effect on affective flattening (F2,110=14.95, p<.001), alogia (F2,110=9.43, p<.001), avolition (F2,110=10.00, p<.001), and anhedonia (F2,110=7.74, p=.001). Among the Bonferroni-corrected pairwise comparisons, we found that PMD was lower than SCZ (MD= −.81 SE=.24, p=.003) and similar to CON (MD=0.68 SE=.35, p=.158) on the rating of affective flattening. Also, PMD was lower than SCZ (MD=−.71 SE=.24, p=.009) and similar to CON with respect to alogia (MD=0.40 SE=.35, p=.26). SCZ and PMD displayed similar levels of avolition (MD=−.22 SE=.30, p=1.0) and anhedonia (MD=−.38 SE=.31, p=.730), which were significantly higher than CON (MD=1.7 SE=0.4, p<.000; MD=1.5 SE=0.4, p=.002; respectively) (Figure 1).
Figure 1. Post Hoc Analysis of Negative Symptom Global Ratings.
aF2,110=14.95, p<.001.
bF2,110=9.43, p<.001.
cF2,110=10.00, p<.001.
dF2,110=7.74, p=.001.
SCZ and PMD demonstrated relationships between negative symptoms and several neurocognitive domains (working memory, episodic memory, and executive function) that were similar in magnitude (in some cases somewhat larger), though the significance was less in PMD in some cases, due in part to their smaller sample size (table 3). Further, Fisher’s r-to-Z transformations did not indicate any significant differences between SCZ and PMD in the magnitude of these correlations between negative symptoms and neurocognitive function. SCZ also demonstrated significant inverse correlations between disorganization symptoms and working memory, episodic memory, executive function and crystallized IQ. Although these correlations were in the same direction in PMD, none of them were significant and they were consistently smaller in magnitude. Fisher’s r-to-Z transformation analyses did not reveal significant group differences in the magnitude of these correlations.
Table 3.
Correlations between Neurocognition and Psychopathology Among SCZ and PMDa
| SCZ (n=72) | PMD (n=25) | Fisher’s z | |
|---|---|---|---|
| Working Memory & Negative Symptoms | −0.262* | −0.383+ | 0.55 |
| Working Memory & Disorganized Symptoms | −0.335** | −0.179 | −0.68 |
| Episodic Memory & Negative Symptoms | −0.275* | −0.489* | 1.03 |
| Episodic Memory & Disorganized Symptoms | −0.370** | −0.062 | −1.33 |
| Executive Function & Negative Symptoms | −0.305** | −0.238 | −0.30 |
| Executive Function & Disorganized Symptoms | −0.284* | −0.177 | −0.46 |
| IQ & Disorganized Symptoms | −0.231* | −0.091 | −0.59 |
p<.01,
p<.05,
p<.06 denote significance within group; Fisher’s z signifies statistical difference between groups.
4. Discussion
Our findings suggest that SCZ and PMD share several similarities, including age of illness onset, severity of neurocognitive deficits, and severity of psychopathology. We also found that PMD had higher levels of a prior alcohol or a cocaine use disorder when compared to SCZ. The differences in rates of substance use disorder among PMD could be explained by the greater risk for a comorbid substance use disorder in individuals with a mood disorder (Compton et al., 2007).
SCZ and PMD scored similarly (with small effect sizes) in working memory, episodic memory, and executive functioning, with both groups showing significant impairments when compared to CON. Thus, our findings are consistent with previous work indicating that SCZ and PMD had similar deficits in neurocognitive performance (e.g., Glahn et al., 2006; Gooding & Tallent, 2002; Reichenberg et al., 2002). Additionally, given that our domains of working memory, episodic memory and executive functioning reflect multiple component processes; our findings may be more reliable than those of prior research. Furthermore, our results support Heinrichs and colleagues’ (2008) conclusion that schizophrenia and schizoaffective disorder may not be neuropsychologically distinguishable, and the work of Schretlen and colleagues (2007) which suggested that schizophrenia and bipolar disorder (75% of their sample had a history of psychotic symptoms) may share qualitatively similar neurocognitive deficits, although these deficits are more severe in schizophrenia. The one domain of neurocognition in which we found differences between SCZ and PMD was in crystallized IQ, with PMD scoring significantly higher than SCZ. This result cannot be explained by differences in age of onset or parental SES, as these were similar across groups. It is possible that this difference in crystallized IQ reflects variation in the degree of premorbid neurocognitive impairment, with a greater level in SCZ. Further work in prodromal or at-risk populations will be needed to examine this issue.
SCZ and PMD also had similar levels of positive and disorganized symptomatology (with small effect sizes). These results are consistent with prior research suggesting that individuals with schizophrenia and schizoaffective disorder had similar ratings of hallucinations and delusions (Cuesta & Peralta, 1995; Kendler et al., 1995), and positive formal thought disorder (Cuesta & Peralta, 1995). We also found that PMD had levels of negative symptomatology that were lower than SCZ and higher than CON. This is consistent with prior research indicating that individuals with schizoaffective disorder had lower levels of negative symptoms than SCZ (Kendler et al., 1995). Upon further analysis we found that PMD was similar to SCZ, but different from CON, on ratings of anhedonia and avolition. However, PMD was similar to CON with respect to alogia, a negative symptom reflecting poverty of speech, and affective flattening. This finding is consistent with the work of Fennig and colleagues (1996) who indicated that higher levels of alogia were specific to schizophrenia when compared to other psychotic disorders. This finding is also consistent with the diagnoses of bipolar or schizoaffective disorder, which are typically associated with elevated or depressed moods rather than affective flattening. Our findings were similar to other studies using the SANS or SAPS (Cuesta & Peralta, 1995; Kendler et al., 1995), thus, the use of alternate measures of psychopathology may explain the quantitative differences that have previously been found between groups (Peralta & Cuesta, 2008; Evans et al., 1999).
Given the clinical importance of the relationship between neurocognitive deficits and functional outcomes associated with schizophrenia (Green et al., 2000) and the debate as to whether neurocognitive impairment should be included as a part of a dimensional definition of schizophrenia (Keefe & Fenton, 2007), we felt it critical to examine whether the relationship between psychopathology and neurocognitive function varied among individuals with disorders along the schizophrenia spectrum. Consistent with prior research (e.g., Nieuwenstein et al., 2001), SCZ demonstrated inverse relationships between negative symptoms and performance in working memory, episodic memory and executive functioning. PMD demonstrated these same relationships between the severity of negative symptoms and neurocognitive function. Our results were also consistent with research reporting that individuals with schizophrenia demonstrated inverse relationships between disorganization symptoms and performance in all neurocognitive domains (e.g., Bozikas et al., 2004; Cameron et al., 2002). Although the direction of these correlations were similar in PMD, the magnitude was somewhat smaller (though not significantly different) than in SCZ.
Thus, these findings support the hypothesis that psychotic mood disorders are on a neurobiological continuum with schizophrenia, given that the structure of the relationships between neurocognition and psychopathology were similar across these diagnostic categories. Furthermore, Robins and Guze (1970) suggested that “when consistent with a defined clinical picture” psychological testing can help validate a psychiatric classification. Given that neurocognitive deficits appear to be a common pathophysiology among SCZ and PMD, neurocognitive deficits and their relationship to psychopathology can assist in clarifying the psychosis continuum.
Our findings also have several implications that inform diagnosis, psychopharmacologic treatment, and illness pathophysiology. In accordance with Kendell & Jablensky (2003), our finding that a similar relationship exists between psychopathology and neurocognition among individuals with schizophrenia and psychotic mood disorders suggests there may be continuous variation between disorders of psychosis, thus contrasting theory suggesting boundaries exist between related disorders. Hence, a dimensional approach to diagnosing schizophrenia and psychotic mood disorders warrants attention. The overlap in relationship between psychopathology and neurocognitive functioning may also contribute to misdiagnosis and inappropriate psychopharmalogic treatment (Lake, 2008). For example, individuals with psychotic mood disorders (who are misdiagnosed with schizophrenia) would be at risk for receiving inadequate mood-stabilizing medication and developing neurotoxicity from higher doses of antipsychotic medication (Lake & Hurwitz, 2006). The findings of this study also have implications for the pathophysiology of schizophrenia. Given that neurocognitive deficits in schizophrenia are related to structural and functional neuroabnormalities (Barch, 2005) and functional outcome (Green et al., 2004), future research is needed to examine whether similar impairments exist in the pathophysiology of individuals with psychotic mood disorders.
There were several limitations to this study. First, the sample was highly selective as all participants agreed to participate in a study that required completion of extensive clinical and neurocognitive testing. Thus, it is possible that our results would not generalize to an unselected sample of SCZ and PMD. Second, all participants were medicated--the most obvious implication being that the psychopathology assessed was residual, and may have reflected the capacity of the participants to respond to treatment as well as the psychopathology associated with their disorders. However, similar findings may emerge in an unmedicated sample since prior research has found similar levels of neurocognitive impairment in medication naïve individuals with SCZ and PMD (Barch et al., 2003). Third, the PMD sample is relatively small compared to our sample of SCZ. Finally, our sample did not include individuals with all psychotic disorder, in particular, major depressive disorder with psychotic features, and delusional disorder. Thus, our findings may be specific to the continuum between schizophrenia and psychotic mood disorders including schizoaffective disorder and bipolar disorder with psychotic features.
In conclusion, our findings provide evidence that individuals with schizophrenia and PMD share several clinical similarities such as age of onset, neurocognitive functioning, and severity of psychopathology. Furthermore, these results suggest that individuals with schizophrenia and psychotic mood disorders share a similar pattern in the relationship between neurocognitive impairment and psychopathology. Future research examining these relationships in a larger sample of individuals with psychotic mood disorders may shed further light on a dimensional definition of psychosis.
Acknowledgments
The authors would like to acknowledge the assistance of the staff of the Conte Center for the Neuroscience of Mental Disorders for clinical and neurocognitive assessments, and for database management. We would like to thank Michael P. Harms, Ph.D., from the Conte Center for his assistance with statistical analyses. The authors would also like to thank Linda B. Cottler, Ph.D. and Catherine Striley, Ph.D. from the Epidemiology and Prevention Research Group in the Department of Psychiatry at Washington University School of Medicine, and Thomas Oltmanns, Ph.D. from the Department of Psychology at Washington University for providing feedback on the manuscript.
Support for the preparation of this paper was provided by grants from the National Institute of Mental Health (R01 MH056584 and P50 MH071616, Principle Investigator: Dr. Csernansky) through the Conte Center for the Neuroscience of Mental Disorders, at the Department of Psychiatry, Washington University in St. Louis. Support was also provided by an NIMH training grant (T32 MH17104, Principle Investigator: Dr. Linda B. Cottler, Ph.D.). NIH had no additional role in study design, collection, analysis, and interpretation of the data; and in dissemination of the findings.
Footnotes
Conflict of Interest
The authors report no competing interests. Dr. Csernansky has received research grants from the NIMH and NIA, royalties from Medtronic for a patent held jointly with Washington University School of Medicine, has been a paid consultant for Eli Lilly and Sanofi-Aventis, and has received speaker’s honoraria from Janssen Pharmaceutica, Eli Lilly and Bristol-Myers Squibb.
Contributors
All authors have made significant scientific contributions to this manuscript. Matthew J. Smith contributed to the conceptualization of the study, conducted the statistical analyses, and wrote the first draft of the manuscript. Deanna M. Barch contributed to the conceptualization and implementation of the study, securing funding and contributed to the editing of the manuscript. John G. Csernansky contributed to the conceptualization and implementation of the study, securing funding, and contributed to the editing of the manuscript. All authors approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allardyce J, Gaebel W, Zielaske J, van Os J. Deconstructing psychosis conference February 2006: the validity of schizophrenia and alternative approaches to the classification of psychosis. Schizophr Bull. 2007;33:863–67. doi: 10.1093/schbul/sbm051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1983a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983b. [Google Scholar]
- Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M. Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch Gen Psychiatry. 1995;52:341–51. doi: 10.1001/archpsyc.1995.03950170015003. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, MacDonald AW, III, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112:132–43. [PubMed] [Google Scholar]
- Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. J Abnorm Psychol. 2004;113:556–68. doi: 10.1037/0021-843X.113.4.556. [DOI] [PubMed] [Google Scholar]
- Basso MR, Nasrallah HA, Olson SC, Bornstein RA. Neuropsychological correlates of negative, disorganized and psychotic symptoms in schizophrenia. Schizophr Res. 1998;31:99–111. doi: 10.1016/s0920-9964(98)00023-1. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. Iowa City: AJA Associates; 1976. [Google Scholar]
- Bozikas VP, Kosmidis MH, Kioperlidou K, Karavatos A. Relationship between psychopathology and cognitive functioning in schizophrenia. Compr Psychiatry. 2004;45:392–400. doi: 10.1016/j.comppsych.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Oram J, Geffen GM, Kavanagh DJ, McGrath JJ, Geffen LB. Working memory correlates of three symptom clusters in schizophrenia. Psychiatry Res. 2002;110:49–61. doi: 10.1016/s0165-1781(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavior sciences. Lawrence Erlbaum Associates; 1983. [Google Scholar]
- Compton WM, Thomas YF, Stinson F, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:566–76. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V. Are positive and negative symptoms relevant to cross-sectional diagnosis of schizophrenic and schizoaffective patients? Compr Psychiatry. 1995;36:353–61. doi: 10.1016/s0010-440x(95)90116-7. [DOI] [PubMed] [Google Scholar]
- Evans JD, Heaton RK, Paulsen JS, McAdams LA, Heaton SC, Jeste DV. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60:874–82. [PubMed] [Google Scholar]
- Fennig S, Bromet EJ, Galambos N, Putnam K. Diagnosis and six-month stability of negative symptoms in psychotic disorders. Eur Arch Psychiatry Clin Neurosci. 1996;246:63–70. doi: 10.1007/BF02274895. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap Monkul E, Maples N, Velligan DI, Soares JC. Differential working memory impairment in bipolar disorder and schizophrenia: effects of a lifetime history of psychosis. Bipolar Disord. 2006;8:117–23. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA. Spatial working memory performance in patients with schizoaffective psychosis versus schizophrenia: a tale of two disorders? Schizophr Res. 2002;53:209–18. doi: 10.1016/s0920-9964(01)00258-4. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Bennett P, Willmans N, Segurado R, Cardono A, Norton N, Lambert D, Willmans H, Kirov G, Corvin A, Holmans P, Jones L, Jones I, Gill M, O’Donovan MC, Owen MJ, Craddock N. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p3. Arch Gen Psychiatry. 2005;62:1081–88. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Computerized Wisconsin Card Sort Task Version 4 Research Edition. Odessa, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- Heinrichs RW, Narmeen A, McDermid Vaz S, Miles AA. Are schizophrenia and schizoaffective disorder neuropsychologically distinguishable? Schizophr Res. 2008;99:149–54. doi: 10.1016/j.schres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33:912–20. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf L, Hussain N, Potash JB. Mood disorder with psychotic features, schizoaffective disorder, and schizophrenia with mood features: trouble at the borders. Int Rev Psychiatry. 2005;17:9–19. doi: 10.1080/09540260500064959. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Walsh D. The structure of psychosis: latent class analysis of probands from the Roscommon Family Study. Arch Gen Psychiatry. 1998;55:492–99. doi: 10.1001/archpsyc.55.6.492. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, Walsh D. Examining the validity of DSM-III-R schizoaffective disorder and its putative subtypes in the Roscommon Family Study. Am J Psychiatry. 1995;152:755–64. doi: 10.1176/ajp.152.5.755. [DOI] [PubMed] [Google Scholar]
- Kendell R, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2002;160:4–12. doi: 10.1176/appi.ajp.160.1.4. [DOI] [PubMed] [Google Scholar]
- Lake CR. Disorders of thought are severe mood disorders: the selective attention defect in mania challenges the Kraepelinian dichotomy--a review. Schizophr Bull. 2008;34:109–117. doi: 10.1093/schbul/sbm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake CR, Hurwitz N. Schizoaffective disorders are psychotic mood disorders; there are no schizoaffective disorders. Psychiatry Res. 2006;143:255–287. doi: 10.1016/j.psychres.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Lake CR, Hurwitz N. Schizoaffective disorder merges schizophrenia and bipolar disorder as one disease – there is no schizoaffective disorder. Curr Opin Psychiatry. 2007;20:365–79. doi: 10.1097/YCO.0b013e3281a305ab. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Aleman A, de Haan EHF. Relationship between symptom dimensions and neurocognitive functioning in schizophrenia: a meta-analysis of WCST and CPT studies. J Psychiatr Res. 2001;35:119–125. doi: 10.1016/s0022-3956(01)00014-0. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Heaton RK. Executive dysfunction in schizophrenia. In: Sharma T, Harvey P, editors. Cognition in Schizophrenia: Impairments, Importance and Treatment Strategies. New York, NY: Oxford University Press; 2000. pp. 51–72. [Google Scholar]
- Peralta V, Cuesta MJ. Exploring the borders of the schizoaffective spectrum: a categorical and dimensional approach. J Affect Disord. 2008;108:71–86. doi: 10.1016/j.jad.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14:47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, Kaplan Z, Davidson M. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. Am J Psychiatry. 2002;159:2027–35. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: theory and clinical interpretation. Tuscon, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Rice J, Reich T, Andreasen NC, Endicott J, Van Eedewegh M, Fishman R, Hirschfeld RM, Klerman GL. The familial transimission of bipolar illness. Arch Gen Psychiatry. 1987;44:441–47. doi: 10.1001/archpsyc.1987.01800170063009. [DOI] [PubMed] [Google Scholar]
- Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. 1970;126:983–87. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, Testa SM, Munro CA, Pulver AE, Rivkin P, Rao VA, Diaz-Asper CM, Dickerson FB, Yolken RH, Pearlson GD. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry. 2007;62:179–86. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Winokur G, Crowe RR. Morbidity risks of schizophrenia and affective disorders among first degree relatives of patients with schizophrenia, mania, depression and surgical conditions. Br J Psychiatry. 1980;137:497–504. doi: 10.1192/bjp.137.6.497. [DOI] [PubMed] [Google Scholar]
- Valles V, Van Os J, Guillamat R, Gutierrez B, Campillo M, Gento P, Fananas L. Increased morbid risk for schizophrenia in families of in-patients with bipolar illness. Schizophr Res. 2000;42:83–90. doi: 10.1016/s0920-9964(99)00117-6. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third Edition. San Antonio, TX: Psychological Corporation; 1997b. [Google Scholar]