Summary
The mechanisms that give rise to ocular dominance columns (ODCs) during development are controversial. Early experiments indicated a key role for retinal activity in ODC formation. However, later studies showed that in those early experiments, the retinal activity perturbation was initiated after ODCs had already formed. Moreover, recent studies concluded that early eye removals do not impact ODC segregation. Here we blocked spontaneous retinal activity during the very early stages of ODC development. This permanently disrupted the anatomical organization of ODCs and led to a dramatic increase in receptive field size for binocular cells in primary visual cortex. Our data suggest that early spontaneous retinal activity conveys crucial information about whether thalamocortical axons represent one or the other eye and that this activity mediates binocular competition important for shaping receptive fields in primary visual cortex.
Introduction
How do precise patterns of synaptic connections arise during development? In binocular species, retinal ganglion cell axons terminate in segregated eye-specific domains within the lateral geniculate nucleus (LGN), and LGN axons terminate in ocular dominance columns (ODCs) in layer 4 of primary visual cortex (V1) (Wiesel et al., 1974; also see Adams and Horton, 2003). Eye-specific retinogeniculate projections and ODCs are established model systems for exploring both how precise circuits develop in the CNS and the role of neural activity in axonal refinement (Feller and Scanziani, 2005).
Eye-specific projections to the LGN are known to emerge from an initially overlapping state (Rakic, 1976; Linden et al., 1981). By contrast, the anatomical events that underlie ODC development remain unclear. Early studies that used transneuronal (retino-LGN-V1) transport of radioactive proline to label LGN axons found that, in young animals, the pattern of proline label in V1 was continuous, whereas in more mature animals, an alternating pattern of proline label (which revealed ODCs) was seen (Rakic, 1976; LeVay et al., 1978; Ruthazer et al., 1999). Thus, it was concluded that LGN axons representing the two eyes intermingle extensively before segregating into ODCs. In young animals, however, transneuronal tracers “spill over” into neighboring eyespecific layers in the LGN (LeVay et al., 1978; Stryker and Harris, 1986; Ruthazer et al., 1999), which can lead to spurious conclusions regarding the presence or absence of ODCs in V1. Indeed, optical imaging (Crair et al., 1998, 2001) and focal injections of anterograde tracers into the LGN (Crowley and Katz, 2000) reveal that ODCs are present earlier than transneuronal labeling can detect them (LeVay et al., 1978; Stryker and Harris, 1986; Ruthazer et al., 1999), but these findings still do not resolve whether ODCs form precisely from the outset or through a process of refinement.
Spontaneous retinal activity is present throughout the period of eye-specific retinogeniculate segregation and ODC development (Wong et al., 1993). This activity is required for eye-specific segregation in the LGN (Penn et al., 1998), but its role in ODC development remains unclear. Previous experiments concluded that blocking retinal activity with tetrodotoxin (TTX) disrupts the formation of ODCs (Stryker and Harris, 1986). However, optical imaging (Crair et al., 1998, 2001) later showed that those TTX experiments (and indeed all previous experiments that tested the role of spontaneous activity in ODC formation) were carried out after ODCs had already formed. Crowley and Katz (1999) removed both eyes from early postnatal ferrets, allowed the animals to mature into adulthood, and then labeled thalamocortical axons with injections of anterograde tracers directly into one or the other eye-specific layer of the LGN. Remarkably, in the animals lacking eyes from birth, patches resembling ODCs were observed in V1. Although these eye removal experiments did not completely rule out a role for neural activity in ODC formation, they did directly challenge the traditional model in which retinal activity controls the process.
To clarify the role of spontaneous retinal activity in ODC development, we blocked all spontaneous retinal activity in both eyes of ferrets from P1 to P10 using intravitreal injections of epibatidine (epi) (Penn et al., 1998; Huberman et al., 2002). We then allowed the ferrets to survive into adulthood (P100+), when transneuronal tracing of retino-LGN-V1 projections was used to label ODCs. We labeled ODCs in adulthood in order to avoid the spillover of transneuronal label known to occur in young animals. The results presented here indicate that eliminating early spontaneous retinal activity severely and permanently disrupts patterning of ODCs and leads to a dramatic increase in receptive field size for binocular V1 neurons.
Results
Impact of Early Spontaneous Retinal Activity Blockade on Retinogeniculate Projections
The effects of blocking spontaneous retinal activity from P1 to P10 on eye-specific projections to the LGN have been described previously (Penn et al., 1998; Huberman et al., 2002). Immediately after activity block (on P10), axons from the two eyes are intermingled in the LGN (Penn et al., 1998). During a period of recovery from P11 to P25, however, spontaneous retinal activity quickly returns to normal and axons from the two eyes completely segregate from one another, but into an abnormal “patchy” pattern of eye-specific territories (Huberman et al., 2002) (Figure 1). Previous experiments indicated that LGN neurons in epi-recovery ferrets are driven by one or the other eye, just as in normal ferrets, and that these LGN cells exhibit normal levels of activity, receptive field sizes, and On-center or Off-center responses. Also, the overall topography of visual inputs to the LGN is intact (Huberman et al., 2002).
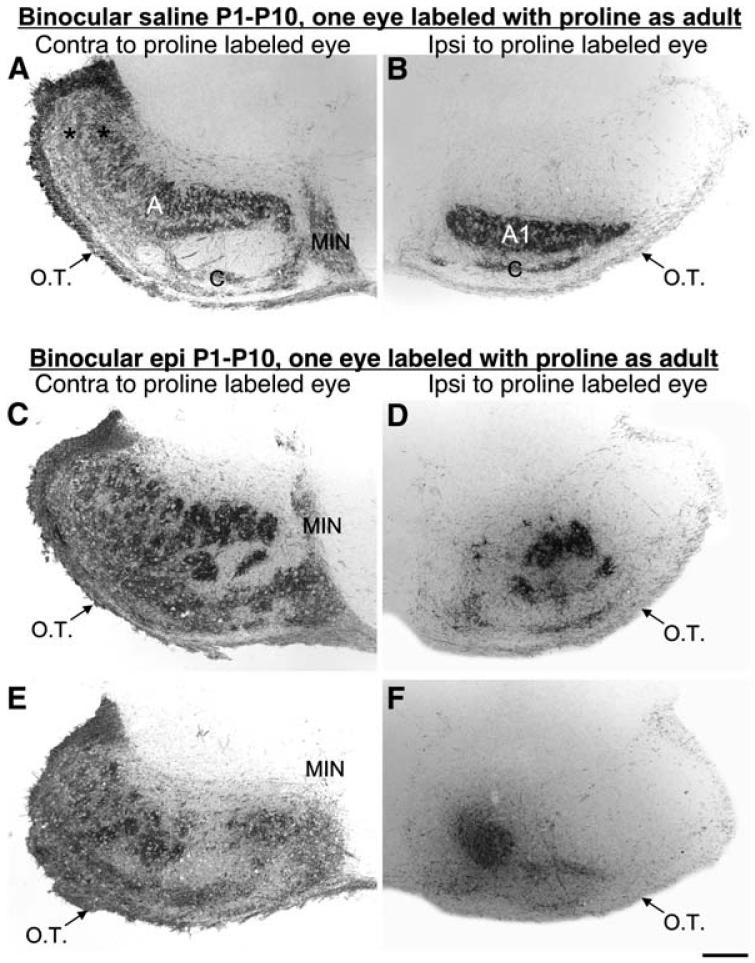
Figure 1. Eye-Specific Retinogeniculate Projections Are Segregated, but Their Patterning Is Altered in Epi-Recovery Ferrets.
(A-F) Photomicrographs of the pattern of proline labeling of retinal axons in horizontal sections through the mature ferret LGN. Scale bar, 1 mm. O.T., Optic Tract. MIN, medial intralaminar nucleus. Rostral is toward the top, and medial is toward the center of each panel. ([A], [C], and [E]) Contralateral and ([B], [D], and [F]) ipsilateral to the proline-injected eye of control ([A] and [B]) and epi-recovery ([C]-[F]) ferrets. (A) In the control contralateral LGN, normal A and C layers (retinal afferent termination zones) are labeled. (B) In the control ipsilateral LGN, layers A1 and C are clearly seen as well. ([C]-[F]) In the LGN of epi-recovery ferrets, patchy, variable patterned retinal inputs are always seen. For further explanation of the anatomy and physiology of the LGN in epi-recovery ferrets, see Huberman et al. (2002).
Impact of Early Spontaneous Retinal Activity Blockade on Ocular Dominance Columns
What is the effect of early retinal activity blockade on ODCs? To address this question, we injected epi into both eyes of ferrets from P1 to P10, and then labeled ODCs when the animals reached adulthood (P100+) using intravitreal injections of tritiated proline (see Experimental Procedures). In control ferrets that were injected with saline in both eyes from P1 to P10, the pattern of ODCs we observed in adulthood matched what has been described in previous reports on normal adult ferrets (Figures 2A-2C) (Ruthazer et al., 1999; White et al., 1999). Relative to macaques or cats, the width and shape of ferret ODCs is rather variable, making it difficult to precisely compare the width of individual ODCs across animals. However, there are some general characteristic features of the ODC map present in all normal ferrets that serve as useful frames of reference for assessing the effects of early activity blockade. For instance, in caudal V1 of normal and saline-injected ferrets, the pattern of radioactive label contralateral to the proline-labeled eye is always uniform (Figure 2A, double asterisks) (Ruthazer et al., 1999). This cortical region receives axonal input from neurons situated in the monocular segment of the LGN (asterisks in Figure 1A) (White et al., 1999). Just anterior to this V1 region is a cortical zone that receives axonal input from LGN neurons situated in the “binocular segment” of the LGN, where central visual space is mapped (White et al., 1999). In this cortical region, ~200-500 μm wide ODCs are readily seen as an alternating, interdigitated pattern of radioactive label (Figure 2A, anterior to dashed line). The bright regions are LGN axons contralateral to the proline-injected eye, and the dark, unlabeled regions are ODCs representing the other (non-proline-injected) eye. Anterior to this ODC-rich region, the “rostral eye bands” can be seen as thick, variably shaped ODCs that span several millimeters (Figure 2A, arrow) (White et al., 1999). Rostral eye bands are a consistent feature of the ODC map in normal ferrets and are therefore useful for assessing the effects of early activity perturbation (see below).
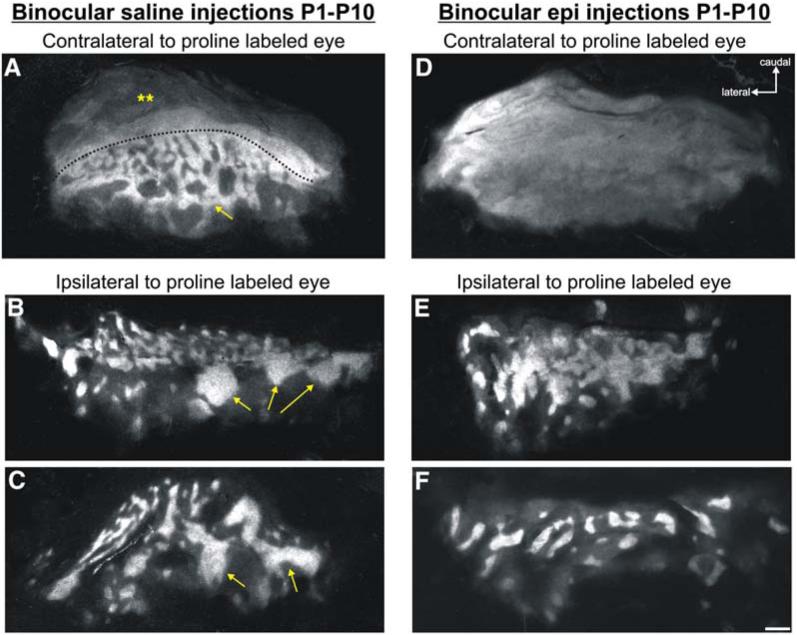
Figure 2. Ocular Dominance Columns Are Perturbed in Adult Ferrets that Lack Spontaneous Retinal Activity from P1 to P10.
(A-F) Flat-mount reconstructions of the entire thalamocortical ODC map in layer 4 of V1 from ([A]-[C]) control ferrets and ([D]-[F]) epi-recovery ferrets. Brightly labeled regions are the LGN axons representing the proline-injected eye. (A) In control ferrets, contralateral cortex shows a large monocular region (double asterisks) in caudal V1. The slight modulations in signal intensity (dimmer in the more caudal regions) are the result of varying ganglion cell densities across the central versus peripheral retina, which affects the amount of tracer uptake and transport. Nevertheless, the entire monocular zone is labeled. Anterior to the dashed line are clearly delineated ODCs (alternating pattern of white/black label) that arise from axons in layer A (labeled) and A1 (unlabeled) in the LGN. Further anterior lie the rostral eye bands (arrow) characteristic of ferret ODC maps. (B and C) ODC maps in the ipsilateral cortex from two different control ferrets. Alternating ODCs are readily seen. Anteriorly, the thick rostral eye bands are seen (arrows). (D) Proline label in the contralateral cortical hemisphere of an epi-treated ferret. The proline label is continuous and no ODCs are evident. (E) Ipsilateral hemisphere of an epi-recovery ferret. ODCs are wider and their borders are less distinct than in those of controls. (F) Ipsilateral hemisphere of another epi-recovery ferret. ODCs appear distinct, but are further apart and wider than normal, and there is no evidence of the rostral eye bands characteristic of normal ferrets. Scale bar, 1 mm.
ODCs are also evident in the cortical hemisphere ipsilateral to the proline-injected eye. ODCs from two control ferrets are shown in Figures 2B and 2C. The thin columns arising from the binocular segment of the LGN are readily seen, and the thick rostral eye bands that lie just anterior to them are evident as well (Figures 2B and 2C, arrows).
In epi-recovery ferrets, altered patterns of ODCs were always observed. The most striking alteration in the ODC map was seen in the cortical hemisphere contralateral to the proline-injected eye (Figure 2D). There, no evidence of ODCs was present. Indeed, the boundaries of the monocular versus binocular portions of the map were impossible to discern. Very faint modulation of proline label can be seen in the middle portion of area 17, where the ODCs are normally found. Overall, however, blockade of early spontaneous retinal activity induced an overwhelming lack of eye-specific segregation in the pattern of thalamocortical terminations.
In the cortical hemisphere ipsilateral to the proline-injected eye of the same epi-recovery ferret (Figure 2E), the pattern of proline label was not completely continuous. Nevertheless, the ipsilateral ODCs were much broader than normal. In fact, the ODCs in caudal V1 (which are normally relatively thin) could not be differentiated from the thick rostral eye bands, and overall, the ODCs representing the ipsilateral eye appeared expanded and less distinct when compared with those of controls (compare Figure 2E with Figures 2B and 2C).
In a different epi-recovery ferret, the ipsilateral ODCs were quite distinct; individual ODCs had sharp borders and were well separated (Figure 2F). However, in this animal too the ODCs were much wider than normal, and there was no evidence of rostral eye bands whatsoever (compare Figures 2B and 2C with Figure 2F). A third epi-recovery ferret exhibited both expanded and very poorly defined ODCs similar to those shown in Figure 2E and no rostral eye bands (see Figure S1 in the Supplemental Data).
Electrophysiological Assessment of the Effects of Early Spontaneous Retinal Activity Blockade on Ocular Dominance in V1
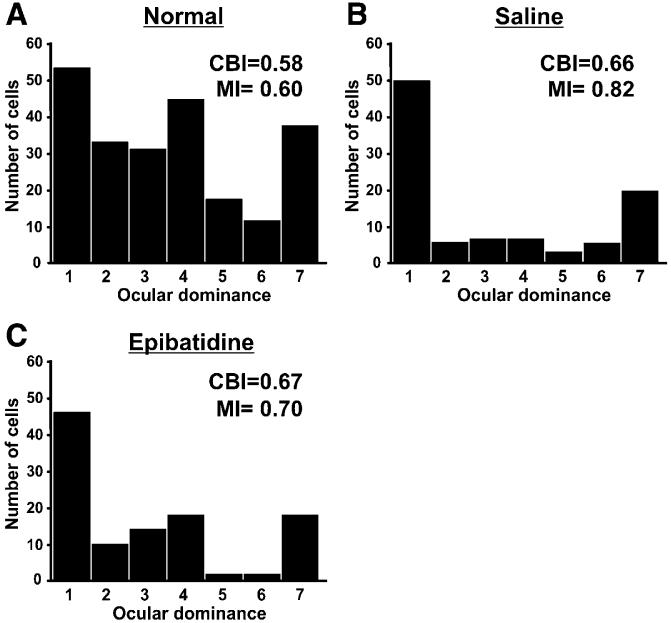
To further explore the effects of early spontaneous retinal activity blockade on the organization of visual cortex, we carried out extracellular microelectrode recordings from cortical neurons in normal, control (saline-injected), and epi-recovery adult ferrets. Using a combination of conventional (Issa et al., 1999) and m-sequence reverse correlation (Reid et al., 1997) mapping, we assessed the ocular dominance, visuotopic location, and size of receptive fields for neurons in area 17 (Law et al., 1988; Issa et al., 1999; White et al., 1999). Pooled ocular dominance histograms for normal, saline-injected, and epi-recovery ferrets are shown in Figure 3. In all three groups, cells responsive to the contralateral eye only (category “1” cells), the ipsilateral eye only (category “7” cells), and to varying degrees, both eyes (category “2-6” cells) were recorded in V1 (Figure 3A) (Hubel and Wiesel, 1962). There is a well-recognized contralateral eye bias of cells in normal ferret V1 (Figure 3A) (Law et al., 1988; Issa et al., 1999). The contralateral bias index (CBI) is a measure of the degree to which cells in V1 tend to be dominated by the contralateral eye (Issa et al., 1999). A CBI of 1 indicates that cells respond only to visual stimulation from the contralateral eye, and a CBI of 0 indicates that cells respond only to visual stimulation from the ipsilateral eye. The CBI values for normal, saline-injected, and epi-recovery ferrets were not significantly different from one another (Figure 3).
Figure 3. Ocular Dominance Histograms of Control and Activity-Blocked Ferrets.
(A-C) Histograms of the number of cells per OD category recorded in (A) normal, (B) P1-P10 saline-injected, and (C) P1-P10 epi-injected adult ferrets. The 1-7 category OD metric was applied to determine whether a given cell in V1 was responsive to the contralateral eye only (category 1 cells), the ipsilateral eye only (category 7 cells) or to varying degrees, to both eyes (category 2-6 cells). Category 4 cells respond equally to both eyes (Hubel and Wiesel, 1962; Issa et al., 1999). The pooled contralateral bias index (CBI) and monocularity index (MI) is shown for each group. The CBI and MI of these groups are not significantly different from normal ferrets or each other. (CBI: normal versus saline, p > 0.55; normal versus epi, p > 0.55; epi versus saline, p > 0.55. MI: normal versus saline, p > 0.40; normal versus epi, p > 0.55; saline versus epi, p > 0.85.) (Normal, n = 5 hemispheres; saline, n = 4 hemispheres; epi, n = 3 hemispheres; Mann-Whitney U test).
The monocularity index (MI) is a measure of the degree to which cells respond to only one eye (Stryker and Harris, 1986). A MI of 0 indicates that all cells respond equally to both eyes [ocular dominance category “4” in the Hubel and Wiesel (1962) seven-point scale]. An MI of 1 indicates that all cells respond only to visual input through the contralateral or ipsilateral eye (“1s” or “7s”). Minor damage to the extra ocular muscles caused by early eye injections rendered the saline- and epi-injected ferrets slightly strabismic (less binocular convergence) relative to normal ferrets. This is reflected by the slightly lower fraction of binocular (category 2-6) cells encountered in V1 of saline-injected and epi-recovery ferrets compared with normal, uninjected ferrets (Figure 3). Nevertheless, a full range of OD values were encountered in saline-injected and epi-recovery ferrets, and the MI values of these groups were not significantly different from normal ferrets (Figure 3).
Based on the lack of OD segregation seen in epi-recovery animals by anatomical tracing (Figure 2), one might expect an increased number of binocular cells in V1 of these animals. However, anatomical segregation of ODCs in layer 4 does not appear necessary to generate physiological OD, even in layer 4. Adams and Horton (2006) discovered that, in squirrel monkeys that lack ODCs, cells in layer 4c of V1 can still be monocular. Thus, even though epi-recovery ferrets exhibit degraded anatomical segregation of ODCs, it is not surprising that the physiological OD of individual cells and the overall OD profile of cells in V1 of these animals appears normal.
Electrophysiological Assessment of the Effects of Early Spontaneous Retinal Activity Blockade on Receptive Field Size and Structure of Neurons in V1
Next we examined the location and size of receptive fields in V1 of control and epi-recovery ferrets. In both saline-injected and epi-recovery ferrets, cells in this region responded preferentially to visual stimuli presented in central visual space. In saline-injected ferrets, monocular cells (category 1 or category 7) and binocular cells (category 2-6) in V1 had receptive field areas comparable to those described for normal ferrets in previous reports (Figure 4)(Law et al., 1988).
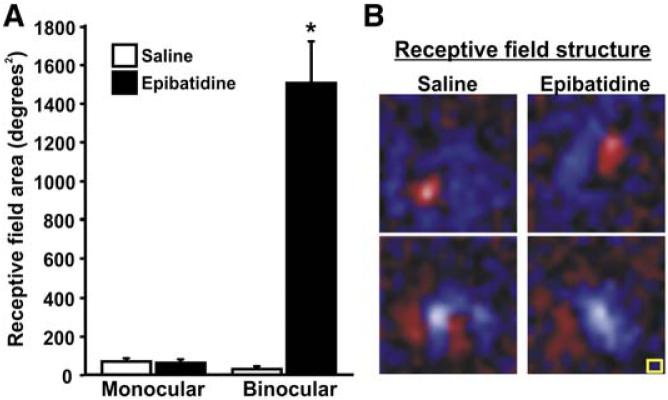
Figure 4. Spontaneous Retinal Activity Blockade Increases Receptive Field Size for Binocular V1 Neurons.
(A) Mean receptive field size for monocular V1 cells is not significantly different for epi-recovery ferrets compared with saline-injected controls (mean saline-injected monocular receptive field area = 79.77 degrees2 ± 9.34, n = 70 cells; mean epi-recovery monocular receptive field area = 73.27 degrees2 ± 10.78, n = 64 cells; p = 0.64 for saline versus epi, Student's t test), and is within normal range of previously reported values (Law et al., 1988). The size of receptive fields for binocular cells in V1 of epi-recovery ferrets, however, is dramatically increased (mean epi-recovery binocular receptive field area = 1514.69 degrees2 ± 206.64, n = 42 cells; mean saline-injected binocular receptive field area = 41.00 degrees2 ± 6.95, n = 28 cells; p < 0.00001 for saline versus epi, Student's t test). (B) Maps of receptive field structure obtained with m-sequence reverse correlation mapping for monocular V1 cells in control and epi-recovery ferrets. Normal response profiles, orientation bias, and spatial organization of receptive fields are seen in monocular V1 cells from both groups. Areas of the receptive fields of the cells excited by light stimuli (On subregions) are shown in red, and areas excited by dark stimuli (Off subregions) are shown in blue. Brightness corresponds to the strength of the responses of the cells. Yellow square, 2 × 2 degrees of visual angle. Error bars = SEM.
In epi-recovery ferrets, the size of receptive fields for monocular V1 cells was normal (Figure 4). The size of receptive fields for binocular cells in epi-recovery ferrets, however, was dramatically increased (Figure 4A). Abnormally large receptive fields were observed for these binocular cells even when boundaries of the receptive field were mapped by visual stimulation of just one eye. Overall, early retinal activity blockade caused a greater than 30-fold expansion in receptive field area of binocular cells. We are confident that we did not inadvertently record from outside area 17 (receptive fields are known to be larger in area 18) because we always recorded along the caudal-most aspect of occipital cortex, where ODCs of area 17 are found. Also, within single-electrode penetrations, we often encountered monocular cells with normally sized receptive fields and binocular cells with receptive fields that were extremely large, interspersed with one another along the depth of the electrode penetration. We thus conclude that early retinal activity block dramatically increases the size of receptive fields for binocular, but not monocular, cells in ferret V1.
The massive enlargement of receptive field size in binocular cells of epi-recovery animals made it impossible to obtain maps of receptive field structure for binocular cells using m-sequence reverse correlation mapping or to carefully map the orientation preference of each binocular cell. However, we successfully obtained m-sequence reverse correlation maps for monocular V1 cells in epi-recovery ferrets, and these were comparable to receptive fields observed in saline-injected control (Figure 4B) and normal adult ferrets (Usrey et al., 2003). In both groups, monocular cells exhibited preferred orientation bias and well-defined subfields, further supporting the finding that monocular cells are relatively unaltered by early retinal activity blockade.
Discussion
The data presented here indicate that spontaneous retinal activity that occurs before the onset of vision is required for anatomical segregation of ODCs and for the establishment of normal receptive field sizes of binocular V1 neurons. As a result of early retinal activity blockade, LGN axons representing the contralateral eye showed continuous labeling in V1 with no ODC segregation, and the ODCs representing the ipsilateral eye were broader than normal. Also, in some cases the rostral eye bands characteristic of normal ferret ODC maps failed to form. The greater effects of activity blockade on thalamocortical projections representing the contralateral eye are likely due to the fact that early in development, the LGN axons representing the contralateral eye are more widespread and effective in driving V1 cells than the LGN axons representing the ipsilateral eye (Crair et al., 1998, 2001).
The pattern of eye-specific retinogeniculate projections is altered in epi-recovery ferrets (Huberman et al., 2002). Thus it is conceivable that ODCs were normal in epi-recovery ferrets until eye-opening (~P30) and then degraded in response to altered activity in the visual system during the critical period. However, we have shown previously that after retinal activity is blocked with epi from P1 to P10, (1) normal patterns and levels of spontaneous retinal activity return by P12, (2) segregation of eye-specific projections to the LGN occurs by P25 (before eye opening), and (3) the receptive fields and topography of visual inputs to the LGN are normal (Huberman et al., 2002). Thus, it is likely that spontaneous and visually driven retino-LGN transmission was intact from P12 into adulthood, at which point ODCs were labeled. One caveat to this assertion is that damage to the lateral eye muscles caused by early eye injections appears to have rendered the epi-recovery ferrets slightly strabismic (they have less binocular overlap in the visual field viewed by both eyes) (Figure 3). If anything, however, strabismus of this sort should render ODCs more segregated than normal (Lowel, 1994). We never observed this; epi-recovery ferrets exhibited abnormally broad and less distinct ODCs in adulthood.
Crowley and Katz (1999, 2000) removed one or both eyes from ferrets at ages ranging from P1 to P14, and then traced ODCs early in development (~P17) or in adulthood using focal injections of anterograde tracers into the contralateral (“A”) or ipsilateral (“A1”) eye-specific layer of the LGN. They reported no impact of early eye removal on ODC segregation. Why does early retinal activity blockade perturb ODC development whereas early eye removal does not? The answer is not clear. It is conceivable that focal injections of anterograde tracers into the LGN reveal a patchy organization of thalamocortical connectivity unrelated to ODCs, such as On- or Off-center domains (Zahs and Stryker, 1988). However, the periodicity of label reported by Crowley and Katz (1999, 2000) closely matches that of ODCs labeled by transneuronal retino-LGN-V1 tracing (Crowley and Katz, 1999, 2000; Ruthazer et al., 1999), so this seems unlikely. One possibility is that eye-removal is different than epi-induced spontaneous retinal activity blockade in terms of its impact on the levels and patterns of activity in the remaining thalamocortical pathway. Eye removals at older ages (P24-P27) degrade (but not eliminate) correlated firing of neurons within the same eye-specific layers of the LGN (Weliky and Katz, 1999). The effects of early eye removal or epi-induced retinal activity blockade on spontaneous activity in the LGN and V1 are not known for ages relevant to ODC formation (P1-P17) because such activity can only be assessed in awake animals (anesthetics shut down early spontaneous activity), and successful in vivo recordings from the LGN and/or V1 of ferrets younger than P22 have not proved possible (Weliky and Katz, 1999; Chiu and Weliky, 2001). However, a recent study showed that early spontaneous retinal activity drives spindle burst activity in V1 in rodents, indicating that early retinal activity is propagated to V1 and suggesting that spindle bursts may be important for thalamocortical development (Hanganu et al., 2006). Experiments are now needed to compare how activity blockades and eye removals affect activity in the LGN and cortex of animals with ODCs. The results we present here nevertheless provide direct evidence that spontaneous retinal activity that occurs before ODCs form is necessary for normal patterning of ODCs.
How might P1-P10 spontaneous retinal activity contribute to ODC development? At the age when we eliminated spontaneous retinal activity (P1-P10), LGN axons are in the process of innervating layer 4, and they extend collateral synaptic inputs to the subplate (Herrmann et al., 1994). Thus, early spontaneous retinal activity appears crucial for normal interactions between LGN axons and layer 4 and/or subplate neurons. Others have shown that an intact subplate is crucial for ODC formation (Ghosh and Shatz, 1992; Kanold et al., 2003). Eye-specific information relayed from the retinas to the LGN may be instructive for sorting thalamocortical axons into ODCs in the subplate or the cortex proper. From P1 to P10, eye-specific layers have not yet formed in the LGN (Linden et al., 1981). Thus, at these early ages, activity-based information regarding whether a given thalamocortical axon will eventually represent the left eye or the right eye can only arise through readout of differences in activity arriving from the retinas. Early retinal activity blockade from P1 to P10 would eliminate the only source of eye-specific information provided to LGN at this stage and thereby perturb sorting of LGN axons in into ODCs (Miller et al., 1989).
The other major effect of early retinal activity blockade we observed here was a dramatic enlargement of receptive fields for binocular neurons in V1. Receptive fields of monocular neurons were normal in epi-recovery ferrets, and enlargement of binocular receptive fields was never observed in saline-injected ferrets. Thus, the enlarged binocular receptive fields observed in epi-recovery ferrets result from the early retinal activity blockade rather than any nonspecific effects of eye injections or global disruptions in visual transmission caused by epi treatment. How might spontaneous retinal activity selectively mediate binocular receptive field refinement? During the early stages of thalamocortical development, spontaneous activity arising from the eye helps coordinate firing of cells located in the same eye-specific layer of the LGN (Weliky and Katz, 1999). In the absence of this retinal influence, the thalamocortical connections that underlie binocular receptive fields may fail to undergo refinement. We have shown previously that receptive field sizes of LGN cells in epi-recovery ferrets are normal (Huberman et al., 2002), and here we found that receptive field sizes for monocular V1 cells are normal in epi-recovery ferrets as well (Figure 4). Thus, it is likely that the connections underlying binocular receptive field structure in V1 remain (or become) exceptionally diffuse following early activity block compared with those serving monocular cells. New techniques that allow one to simultaneously monitor the visually evoked activity of many individual cells in V1 (Ohki et al., 2005) would be informative for determining if this is indeed the case.
Cang et al. (2005a) recently reported that in β2 nicotinic acetylcholine receptor knockout (β2 nAChR-/-) mice, where spontaneous retinal activity is defective from P1 to P8 but then recovers (McLaughlin et al., 2003), retinotopic refinement in V1 is perturbed. Others have also shown a decrease in visual acuity in the binocular region of V1 in β2 nAChR-/- mice (Rossi et al., 2001). β2 nAChR-/- mice exhibit an LGN phenotype very similar to ferrets injected with epi from P1 to P10 in that eye-specific segregation is absent immediately following the retinal activity blockade (ferret: Penn et al., 1998; mouse: Rossi et al., 2001), but then, normal retinal activity quickly resumes and eye-specific segregation occurs in the LGN, albeit in an abnormal patchy pattern (ferret: Huberman et al., 2002; mouse: Muir-Robinson et al., 2002; Grubb et al., 2003). Mice do not have ODCs, so these previous studies were unable to assess ODCs or systematically compare receptive field sizes for monocular versus binocular cells. However, our finding that binocular receptive fields are enlarged in epi-recovery ferrets is consistent with the results of Cang et al. (2005a) and Rossi et al. (2001).
Previous experiments have shown that early binocular enucleations in ferrets do not disrupt the overall organization of retinotopy in the ferret geniculocortical pathway (Guillery et al., 1985), and our electrophysiological experiments generally confirmed this: central visual space was mapped to the appropriate portion of V1, and cells recorded in a given electrode penetration mapped to roughly the same location in visual space. However, based on both the expanded receptive field size of binocular cells we observed (Figure 4) and the results reported by Cang et al. (2005a), we expect that the fine precision of the retinotopic map is degraded in V1 of epi-recovery ferrets.
In summary, here we observed a severe and persistent disruption of ODCs and receptive field properties of binocular cells in V1 after early spontaneous retinal activity blockade. We favor a model of ODC development similar to that emerging for retinotopic mapping in V1 (Cang et al., 2005a, 2005b) and eye-specific segregation in the LGN (Penn et al., 1998; Huberman et al., 2005; Pfeiffenberger et al., 2005), in which retinal activity and axon guidance cues, such as ephrin-As, induce segregation of axons representing the two eyes and organize them into an overall feature map. In future experiments it will be interesting to compare the expression of ephrin-As in V1 of animals which do not have ODCs, such as mice and some squirrel monkeys (Adams and Horton, 2003), with the expression of ephrin-As in V1 of animals that have robust ODCs, such as ferrets, other squirrel monkeys, macaques, and humans.
Experimental Procedures
Animals
Fitch-coat ferrets were born from timed-pregnant dams (Marshall Farms) in our colony at UC Davis. All procedures were in accordance with approved institutional protocols. P0 represents the day of birth. All the tissue shown in this study is from age-matched, identically housed animals that were 180-190 days old at time of sacrifice. Two saline-injected ferrets and three epibatidine-treated ferrets were used for the anatomical experiments. Physiological recordings were carried out on three normal (uninjected), two saline-injected, and three epi-recovery ferrets.
Retinal Activity Blockade
Intravitreal epibatidine (Sigma) injections were carried out according to previously described protocols at concentrations that block all ganglion cell spiking and calcium waves (1 mM dissolved in saline; volumes as follows: 1.0 μl/eye on P1, 1.25 μl/eye on P3, 1.50 μl/eye on P5, 1.75 μl/eye on P7, 2.0 μl/eye on P9) (Penn et al., 1998; Huberman et al., 2002). Equivalent volumes of saline were injected in controls. Note that we opted to not block retinal activity from P12 to P25 because this leads to complete desegregation of retino-LGN axons in the LGN (Chapman, 2000), and thus, anatomically labeling ODCs representing only one eye becomes impossible in these animals using transneuronal or other labeling techniques.
Labeling of Retino-LGN-V1 Projections
In adulthood, ferrets received injections of tritiated proline into one eye for labeling of ODCs (Ruthazer et al., 1999; Adams and Horton, 2003). We let two weeks elapse to permit transport of the tracer. Both retinas of each animal were inspected for damage with an ophthalmoscope (by J.C. Horton). Retinas were also inspected for damage after perfusion by flat-mount inspection of the retinal ganglion cell layer. No retinal damage was observed for any of the animals used in this study.
Cortical Flat-Mounting/Visualization of ODC and LGN Label
Procedures were essentially as described previously (LeVay et al., 1985; Adams and Horton, 2003, 2006). After perfusion with 1% paraformaldehyde, the brain was removed, the white matter removed, each cortical hemisphere physically flattened between slides, and sectioned at 40 μm, parallel to the cortical surface. Sections were mounted, dipped in photographic emulsion (Kodak-2), stored in the dark for 8 weeks, then developed, coverslipped, and imaged under darkfield optics using a digital CCD camera (Spot). The complete map of ODCs was digitally reconstructed from serial images of the pattern of radioactive proline label in layer 4. The thalamus was sectioned horizontally at 40 μm, mounted onto slides, dipped in photographic emulsion, and kept in the dark for 2 weeks, after which time the sections were developed, coverslipped, and imaged. Images were obtained with a CCD camera (Ziess Axiovision).
Cortical Electrophysiology
Animals were prepared for electrophysiological recordings as described previously (Huberman et al., 2002). Recordings were made from neurons in area 17 with penetrations directed into caudal aspect of the lateral gyrus (Law et al., 1988; Issa et al., 1999) using lacquer-coated tungsten electrodes (MicroProbe Inc., MD; impedance = 5 MΩ). The electrode was directed mediolaterally so as to penetrate across ODCs. Penetrations were separated by at least 500 μm, and cells were sampled every 100 μm along the depth of each penetration. Single units were isolated using waveform analysis in Spike 2 (Cambridge Electronic Design, Cambridge, UK) (further details below). Receptive field position, receptive field boundaries (area), and ocular dominance of each V1 cell were mapped using previously described techniques (Issa et al., 1999). Ocular dominance was determined using the seven-point scale of Hubel and Wiesel (1962), in which a unit with an ocular dominance rating of 1 is entirely dominated by the contralateral eye, 4 indicates driven equally by both eyes, and 7 indicates entire domination by the ipsilateral eye. Two or three independent observers were used to establish the OD profile of each cell.
The CBI is a measure of the degree to which the entire population of units recorded is dominated by the contralateral eye and is expressed as the ratio
in which NT is the total number of visually responsive units and Nx is the number of units with ocular dominance rating x. A CBI of 0 indicates that the ipsilateral eye dominates the population of measured units, whereas a CBI of 1 indicates that the contralateral eye dominates the population.
The MI reflects the degree to which responses are dominated by one eye or the other, but not by both (Stryker and Harris, 1986). The MI is defined as:
An MI of 0 suggests that all individual cells are driven equally by both eyes, whereas an MI of 1 suggests that all cells are driven exclusively by one eye or the other.
The Mann-Whitney U test was used to test for statistical significance of CBI and MI values obtained for each hemisphere across experimental groups.
m-Sequence Reverse Correlation Mapping of Receptive Field Structure
Neuronal responses were amplified, filtered, and recorded to a PC computer equipped with a Power 1401 data acquisition interface and the Spike 2 software package (Cambridge Electronic Design, Cambridge, UK). Spike isolation was based on waveform analysis (on-line and off-line) and presence of a refractory period, as indicated by the autocorrelogram. Visual stimuli were created with a VSG2/5 visual stimulus generator (Cambridge Research Systems, Rochester, UK). Receptive fields of cortical neurons were mapped quantitatively by reverse correlation using pseudorandom spatio-temporal white-noise stimuli (m-sequences) (Reid et al., 1997). The white-noise stimulus (100% contrast) consisted of a 16 × 16 grid of squares (pixels) that were white or black one half of the time during an m-sequence of length 215-1. The size of individual pixels was 1.4° squared. The speed of presentation was varied at 1, 3, 5, or 7 frames per term. At 1 frame per term, the stimulus was updated every 15.625 ms; at 3 frames per term, every 46.875 ms; at 5 frames per term, every 78.125 ms; and at 7 frames per term, every 109.375 ms.
Supplementary Material
Acknowledgments
We are grateful to Sheri Harris for technical assistance and Dr. Michael Stryker for allowing us the use of his laboratory space for the proline experiments. We are especially grateful to Dr. Jonathan Horton for conducting eye exams on the ferrets, for expert advice and assistance with the cortical flat-mounting procedure, and for use of his darkfield imaging equipment. We also thank Drs. Dan Adams and Lawrence Sincich for expert advice on imaging and reconstructing cortical OD maps. Daniel Rathbun and Drs. Henry Alitto and Faran Briggs provided advice on m-sequence reverse correlation mapping, and Chirag Shah assisted with the electrophysiology. Dan Sperka provided help with the programs for generating m-sequences. We also thank Dr. Linda Wilbrecht for assistance with the statistical analysis and comments on this manuscript and Dr. Jianhua Cang for helpful comments on an earlier version of this manuscript. This work was supported by NIH grant EY11369 (B.C.).
Footnotes
Supplemental Data The Supplemental Data for this article can be found online at http://www.neuron.org/cgi/content/full/52/2/247/DC1/.
References
- Adams DL, Horton JC. Capricious expression of cortical columns in the primate brain. Nat. Neurosci. 2003;6:113–114. doi: 10.1038/nn1004. [DOI] [PubMed] [Google Scholar]
- Adams DL, Horton JC. Monocular cells without ocular dominance columns. J. Neurophysiol. 2006 doi: 10.1152/jn.00131.2006. in press. Published online July 19, 2006. 10.1152/jn.00131.2006. [DOI] [PubMed] [Google Scholar]
- Cang J, Renteria RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005a;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Kaneko M, Yamada J, Woods G, Stryker MP, Feldheim DA. Ephrin-as guide the formations of functional maps in the visual cortex. Neuron. 2005b;48:577–589. doi: 10.1016/j.neuron.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B. Necessity for afferent activity to maintain eyespecific segregation in ferret lateral geniculate nucleus. Science. 2000;287:2479–2482. doi: 10.1126/science.287.5462.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Weliky M. Spontaneous activity in developing ferret visual cortex in vivo. J. Neurosci. 2001;21:8906–8914. doi: 10.1523/JNEUROSCI.21-22-08906.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Horton JC, Antonini A, Stryker MP. Emergence of ocular dominance columns in cat visual cortex by 2 weeks of age. J. Comp. Neurol. 2001;430:235–249. doi: 10.1002/1096-9861(20010205)430:2<235::aid-cne1028>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Development of ocular dominance columns in the absence of retinal input. Nat. Neurosci. 1999;2:1125–1130. doi: 10.1038/16051. [DOI] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290:1321–1324. doi: 10.1126/science.290.5495.1321. [DOI] [PubMed] [Google Scholar]
- Feller MB, Scanziani M. A precritical period for plasticity in visual cortex. Curr. Opin. Neurobiol. 2005;15:94–100. doi: 10.1016/j.conb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 1992;255:1441–1443. doi: 10.1126/science.1542795. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changuex JP, Thompson ID. Abnormal functional organization in the dorsal latera; geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;30:1151–1172. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Ombrellaro M, LaMantia AL. The organization of the lateral geniculate nucleus and of the geniculocortical pathways that develops without retinal afferents. Brain Res. 1985;352:221–233. doi: 10.1016/0165-3806(85)90109-9. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J. Neurosci. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K, Antonini A, Shatz CJ. Ultrastructural evidence for synaptic interactions between thalamocortical axons and subplate neurons. Eur. J. Neurosci. 1994;6:1729–1742. doi: 10.1111/j.1460-9568.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Stellwagen D, Chapman B. Decoupling eye-specific segregation from lamination in the lateral geniculate nucleus. J. Neurosci. 2002;22:9419–9429. doi: 10.1523/JNEUROSCI.22-21-09419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Murray KD, Warland DK, Feldheim DA, Chapman B. Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nat. Neurosci. 2005;8:1013–1021. doi: 10.1038/nn1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in Ferret's visual cortex. J. Neurosci. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–525. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- Law MI, Zahs KR, Stryker MP. Organization of primary visual cortex (area 17) in the ferret. J. Comp. Neurol. 1988;278:157–180. doi: 10.1002/cne.902780202. [DOI] [PubMed] [Google Scholar]
- LeVay S, Stryker MP, Shatz CJ. Ocular dominance columns and their development in layer IV of the cat's visual cortex: a quantitative study. J. Comp. Neurol. 1978;179:223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- LeVay S, Connolly M, Houde J, Van Essen DC. The complete pattern of ocular dominance stripes in the striate cortex and visual field of the macaque monkey. J. Neurosci. 1985;5:486–501. doi: 10.1523/JNEUROSCI.05-02-00486.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DC, Guillery RW, Cucchiaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J. Comp. Neurol. 1981;203:189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- Lowel S. Ocular dominance column development: strabismus changes the spacing of adjacent columns in cat visual cortex. J. Neurosci. 1994;14:7451–7468. doi: 10.1523/JNEUROSCI.14-12-07451.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Miller KD, Keller JB, Stryker MP. Ocular dominance column development: analysis and simulation. Science. 1989;245:605–615. doi: 10.1126/science.2762813. [DOI] [PubMed] [Google Scholar]
- Muir-Robinson G, Hwang BJ, Feller MB. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J. Neurosci. 2002;22:5259–5264. doi: 10.1523/JNEUROSCI.22-13-05259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise microarchitecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Renteria RC, Copenhagen DR, Flanagan JG, Feldheim DA. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat. Neurosci. 2005;8:1022–1027. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Reid RC, Victor JD, Shapley RM. The use of m-sequences in the analysis of visual neurons: linear receptive field properties. Vis. Neurosci. 1997;14:1015–1027. doi: 10.1017/s0952523800011743. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changuex JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc. Natl. Acad. Sci. USA. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Baker GE, Stryker MP. Development and organization of ocular dominance bands in the primary visual cortex of the sable ferret. J. Comp. Neurol. 1999;407:151–165. [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J. Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Sceniak MP, Chapman B. Receptive fields and response properties of neurons in layer 4 of ferret visual cortex. J. Neurophysiol. 2003;89:1003–1015. doi: 10.1152/jn.00749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE, Bosking WH, Williams SM, Fitzpatrick D. Maps of central visual space in ferret V1 and V2 lack matching inputs from the two eyes. J. Neurosci. 1999;19:7089–7099. doi: 10.1523/JNEUROSCI.19-16-07089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH, Lam DM. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974;79:273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Zahs K, Stryker MP. Segregation of ON and OFF afferents to ferret visual cortex. J. Neurophysiol. 1988;59:1410–1429. doi: 10.1152/jn.1988.59.5.1410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.