Abstract
Objective
This study examines the tissue engineering potential of passaged (P3) and primary (P0) articular chondrocytes (ACs) and costal chondrocytes (CCs) from skeletally-mature goats for use in the temporomandibular joint (TMJ).
Design
These four cell types were assembled into scaffoldless tissue engineered constructs and cultured for 4 wks. The constructs were then tested for cell, collagen, and glycosaminoglycan (GAG) content with biochemical assays, and collagen types I and II with enzyme-linked immunosorbent assays. Constructs were also tested under tension and compression to determine biomechanical properties.
Results
Both primary and passaged CC constructs had greater GAG/wet weight than AC constructs. Primary AC constructs had significantly less total collagen and contained no collagen type I. AC P3 constructs had the largest collagen I/collagen II ratio, which was also greater in passaged CC constructs relative to primary groups. Primary AC constructs were not mechanically testable, while passaged AC and CC constructs had significantly greater tensile properties than primary CC constructs.
Conclusions
Primary CCs are considerably better than primary ACs and have potential use in tissue engineering when larger quantities of collagen type II are desired. The poor performance of the ACs, in this study, which contradicts the results seen with previous studies using immature bovine ACs, may thus be attributed to the animals’ maturity. However, CC P3 cells appear particularly well-suited for tissue engineering fibrocartilage of the TMJ due to the high quantity of collagen and GAG, and tensile and compressive mechanical properties.
Keywords: Tissue engineering, Articular chondrocyte, Costal chondrocyte, Cartilage, Fibrocartilage, Mechanical properties, Extracellular matrix
Introduction
Tissue engineering presents a potential solution to the complex problem of temporomandibular joint (TMJ) disorders. Current approaches to treating TMJ disorders include pain medication and physical therapy, but surgical approaches are often necessary when the disorder becomes severe or the patient has extensive trauma. These treatment options, described in greater detail elsewhere, are not always universally accepted.1, 2 Reconstruction of the joint requires the use of non-biologic materials, which can restore some function to the joint, but lack of integration of the materials with the soft tissues prevents perfect functional restoration. In some situations, the disc is removed, which may temporarily alleviate pain but will frequently result in degeneration of the joint after time. A possible long-term, non-immunogenic solution for severe TMJ problems is the creation of autogenous, functional tissues. Regeneration of various tissue types within the joint may be necessary; however, this work focuses on the creation of cartilaginous tissues found in the joint: fibrocartilage of the disc and articular cartilage of the mandibular condyle and temporal bone.
Creating these tissues requires a solid understanding of the structural and functional characteristics of the tissues. The properties of the TMJ disc and articular cartilage are distinct from one another and other tissues, as reviewed elsewhere.3, 4 Some key distinctions include the primary type of collagen present in the tissues and the mechanical function of the tissues. While articular cartilage mainly supports compressive loading, the TMJ disc has an important additional tensile role. Articular cartilage contains nearly 100% collagen type II, while the TMJ disc is almost 100% collagen type I. However, other tissues fall between these, containing significant quantities of both collagens type I and II, including the mandibular condylar cartilage5, 6 and the knee meniscus.7, 8 As with collagen content, mechanical properties follow a similar spectrum. The TMJ disc has a relatively low aggregate modulus—around 20 kPa for the porcine disc with indentation testing9—while articular cartilage has a modulus over 1 MPa.10 In tension, the TMJ disc elastic modulus ranges between 1–100 MPa, depending on the species and direction tested, while articular cartilage has a modulus less than 20 MPa.4 Both contain a substantial quantity of glycosaminoglycans (GAGs). These characteristics must be considered when evaluating potential replacements for the tissues.
Previous attempts to tissue engineer the TMJ disc have frequently used TMJ disc cells.11–14 Despite numerous attempts, these cells have yet to approach the quantitative biochemical content or mechanical strength necessary to function in a tissue replacement. Additionally, these cells are difficult to obtain and very limited in quantity, which is not likely remedied through in vitro cell expansion and passaging.15, 16 Recent work with costal chondrocytes (CCs), however, suggests their potential in TMJ disc tissue engineering both in functionality and clinical translatability.17 CCs have been shown to produce substantial, relevant matrix—dramatically more than seen previously with TMJ disc cells. Collagen/wet weight at 3 wks after culture was approximately 1% while GAG/wet weight was 3%.17 These values are below the collagen seen in native tissues [20% collagen/wet weight for cartilage18 and 30% collagen/wet weight for the TMJ disc19] but near to those seen for GAG content in the native tissues [4–7% GAG/wet weight for cartilage18 and 2% GAG/wet weight for the TMJ disc20]. Constructs made from these CCs were mechanically testable and manipulatable with surgical tools, which was not true of previous TMJ disc cell constructs.17, 21, 22 The improvements in translatability include the surgeons’ familiarity harvesting costal cartilage, limited complications of this surgery, and abundance of healthy tissue.23, 24
While tissue engineering of the TMJ disc is still in its infancy, articular cartilage regeneration has been explored considerably longer. The initial paradigm for tissue engineering used cells seeded on a scaffold, but previous work with articular chondrocytes (ACs) has shown the ability of these cells to form neotissue without a scaffold and with properties approaching those of native tissue.25–27 The importance of the cell in this approach is apparent, and while functional tissue has been created with young, calf chondrocytes, the usefulness of this approach with adult ACs remains uncertain. Finding a functional, skeletally-mature cell source would surge forward the clinical translatability of this approach.
Skeletally-mature CCs and young ACs have shown previous functional potential and do not require cells from the TMJ, suggesting their clinical usefulness to patients in need of TMJ surgery, whose TMJ cells may be phenotypically or functionally abnormal. However, the quantity of chondrocytes needed is quite substantial, and so there is considerable interest in expanding and passaging these cells. Previous work on both these cell types showed the dedifferentiation of the cells from a chondrocyte-like to a more fibroblast-like phenotype.28, 29 Articular chondrocytes showed about 10 times more collagen I gene expression at passage 3 than at passage 1 and 7 times less collagen II expression.30 While this response is often considered an undesirable result of the cell passaging process, in the case of fibrocartilage tissue engineering, it may be beneficial. The shift from collagen type II to collagen type I and decrease in GAGs yields a fibrochondrocyte-like cell type. These chondrocytes, phenotypically altered by passaging, may function most effectively in a tissue engineered construct for the purposes of fibrocartilage replacement.
This study examines ACs and CCs at both passage 0 (P0) and passage 3 (P3) in a scaffoldless tissue engineering approach. The hypotheses of this study are twofold: 1) CCs will produce constructs that are equal to or better than AC constructs in biological and biomechanical properties. 2) P3 constructs will have characteristics that are more amenable to fibrocartilage tissue engineering, while P0 constructs will be better suited for cartilage tissue engineering; specifically, P3 constructs will have greater collagen I than collagen II and vice versa for P0 constructs. P3 constructs will have greater tensile properties, while P0 constructs will have greater compressive properties.
Materials and Methods
Cell isolation
Tissue was obtained from three skeletally-mature (approximately 1 year), Spanish, female goats from a local abattoir within 4hrs postmortem. Costal cartilage was scraped from the non-floating ribs, and articular cartilage was taken from the distal femur. Both cartilages were minced and digested overnight in 0.2% type II collagenase (Worthington, Lakewood, NJ) in Dulbecco’s modified Eagle medium (DMEM) (Gibco, Carlsbad, California) supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-Products, Woodland, CA), 1% Penicillin-Streptomycin-Amphotericin B (PSF), 1% non-essential amino acids (NEAA) (Life Technologies, Carlsbad, CA), and 25 μg/mL L-ascorbic acid (Sigma, St. Louis, MO). Some cells were expanded on tissue culture treated plastic, while the remaining primary cells were frozen in liquid nitrogen in DMEM with DMSO and an additional 10% FBS. Freezing of the primary chondrocytes allowed for simultaneous seeding of the groups for three-dimensional culture. Cells were passaged at 70–90% confluence with trypsin-EDTA (Gibco) until passage 3 with approximately 5 times expansion occurring at each passage.
Construct culture
Constructs were formed from passage 3 and primary ACs and CCs by a modified method, described previously.25, 31 Two million cells were seeded into 5 mm diameter agarose molds. Passage 3 chondrocytes were judiciously chosen based on their behavior in previous work, which showed considerable dedifferentiation without complete loss of the original phenotype.29, 30 After 2 wks, constructs were removed and transferred into agarose-coated tissue culture treated plates. Chondrogenic medium consisting of DMEM supplemented with 1% PSF, 1% NEAA, 1% insulin-transferrin-selenium+ premix (BD Biosciences, San Jose, CA), 0.1 μM dexamethasone, 40 μg/mL L-proline (EMD Chemicals, Gibbstown, NJ), 50 μg/mL ascorbate 2-phosphate (Sigma), and 100 μg/mL sodium pyruvate (Fisher) was changed everyday. Constructs were examined at 4 wks using the various assays described below.
Histology
Samples were frozen in HistoPrep™ Frozen Tissue Embedding Media (Fisher) and cut to 14 μm sections in a cryotome. Sections were fixed in formalin and stained with picrosirius red for collagen, safranin-O/fast green for GAGs and von Kossa stain for calcification using standard histological protocols. Immunohistochemistry (IHC) was also used to examine the localization of collagen types I and II, using methods described previously.32
Biochemistry
Six samples per group were used to determine the biochemical content. Samples were weighed and dried to determine wet and dry weights. Dry samples were then digested at 4°C with constant mechanical agitation in 125 μg/mL papain (Sigma) for 7 days followed by 1mg/mL elastase (Sigma) digestion for 2 days. Cell number was determined by quantifying DNA with a PicoGreen® dsDNA reagent (Molecular probes) and converting with a factor of 7.7 pg DNA/cell, as determined previously.33 Collagen was quantified with a modified colorimetric hydroxyproline assay, described previously.34 In brief, samples were hydrolyzed with NaOH. The reaction was then neutralized with HCl. Chloramine T and Ehrlich’s reagents were added and incubated at 60°C. Sulfated GAGs were measured with a dimethylmethlylene blue (DMMB) Blyscan kit, according to the manufacturer’s protocol (Biocolor, Newtownabbey, Ireland). Enzyme-linked immunosorbent assays (ELISA) were also performed to quantify the amounts of collagen type I and type II. Type I collagen was quantified using an indirect ELISA, described previously. 28 Briefly, digested samples and standards were incubated overnight on high-affinity plates at 4°C. A primary mouse antibody (Accurate Chemical) was added for 2hrs, followed by a HRP-conjugated secondary antibody (Chemicon). Tetramethyl bizidine (Chemicon) was used for visualization. A Chondrex (Redmond, WA) collagen detection kit was used for collagen type II quantification, according to the manufacturer’s protocol.
Mechanical testing
Six samples per group were tested for tensile and compressive properties. Tensile testing was performed on an Instron 5565 (Norwood, MA) with samples glued to a paper frame. This allowed for a defined gauge length and proper securing of the samples to the Instron. After cutting the paper, samples were loaded at 1% strain/sec until failure, as described previously.35, 36 Ultimate tensile strength (UTS) and elastic modulus (E) were determined from the stress/strain curves. Compressive properties were determined with creep indentation testing.37 Samples were cut through the diameter to make a level testing surface and put in saline solution. A tare load of 0.00196 N was applied until equilibrium was reached (deformation less than 10−6 mm/s) or 10 min elapsed. Afterwards, an instantaneous step load of 0.00686 N was added until equilibrium was again reached or 1 hr passed. The creep load was then removed, and the sample equilibrated once again. These data were used to determine aggregate modulus, Poisson’s ratio, and permeability.
Statistics
Data were analyzed with a one-way analysis of variance. When an F-test indicated significance (p < 0.05) a Tukey’s post hoc test was performed to determine differences among the groups.
Results
Morphology and histology
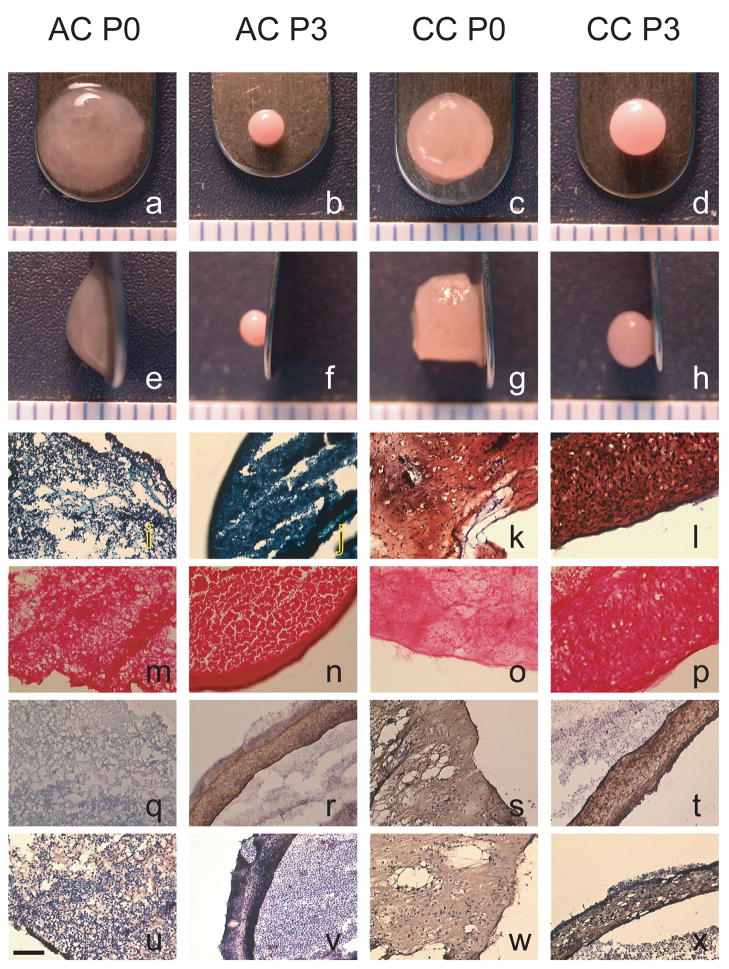
Weight and diameter data for the constructs are shown in Table 1, and gross morphology is illustrated in Fig. 1. Wet weight was significantly greatest for CC P0 constructs (p < 0.0001). AC P0 constructs had greater wet weights than AC P3 and CC P3 constructs. Both P0 groups had the greatest percent water, followed by CC P3 and then AC P3, which were statistically different (p < 0.0001). AC P0 constructs had the greatest diameter, around 5.5 mm (p < 0.0001). The CC P0 constructs had diameters around 4.5 mm, which were significantly greater than either of the P3 groups. CC P3 constructs had significantly greater diameters than the AC P3 constructs, measuring about 3 mm and 2 mm, respectively. CC P0 constructs remained cylindrical in shape, while CC P3 and AC P3 formed more spherical shapes. AC P0 constructs were of a hydrated, gelatinous consistency, which did not hold a solid shape, but rather relaxed to the position seen in Fig. 1a,e. Histological micrographs are also shown in Fig. 1. Safranin O staining (Fig. 1i–l) was positive for both CC groups but only lightly stained in the AC P0 group and not stained in the AC P3 group. The picrosirius red stain (Fig. 1m–p) was positive for all groups, but particularly intense on the outer few micrometers of the constructs. Collagen I (Fig. 1q–t) was positive for all groups except AC P0. Collagen II (Fig. 1u–x) was positive for all groups, although AC P3 stained only faintly.
Table 1.
Quantitative size data for all groups
| Wet weight (mg) | % water | Diameter | |
|---|---|---|---|
| AC P0 | 20.4 ± 5.7B | 94.8 ± 0.58A | 5.41 ± 0.62A |
| AC P3 | 3.91 ± 0.083C | 83.0 ± 0.038C | 2.07 ± 0.040D |
| CC P0 | 59.8 ± 10.4A | 93.3 ± 0.86A | 4.52 ± 0.32B |
| CC P3 | 9.66 ± 2.4C | 84.9 ± 0.19B | 3.12 ± 0.31C |
Data are shown as mean ± standard deviation (n = 6). Groups separated by different letters are considered significantly different (p < 0.05). CC P0 constructs were significantly heavier in wet weight than any other group. AC P0 constructs had greater wet weights than both types of P3 constructs. Primary groups had the greatest percent water, and the CC P3 constructs also had more water than AC P3 constructs. All diameters were statistically different from one another with AC P0 being the greatest, followed by CC P0, CC P3, and AC P3.
Figure 1.
Morphological and histological images of representative constructs from each group at 4 wks. Safranin-O/fast green staining (i–l), picrosirius red staining (m–p), collagen type I IHC (q–t), and collagen type II IHC (u–x) illustrate the localization of the ECM. AC P0 had limited safranin-O staining throughout the construct, and AC P3 showed none. CC P0 and CC P3 had dense staining throughout the construct, although CC P3 did not stain the very outer edge of the construct. All constructs stained positively and uniformly for picrosirius red. AC P0 did not stain for collagen type I, while collagen type II staining was seen throughout the construct. AC P3 stained positively for collagen I, though only a little for collagen type II. CC constructs from both passages stained positive for collagen types I and II throughout the constructs. Scale bar = 0.1 mm
Biochemistry
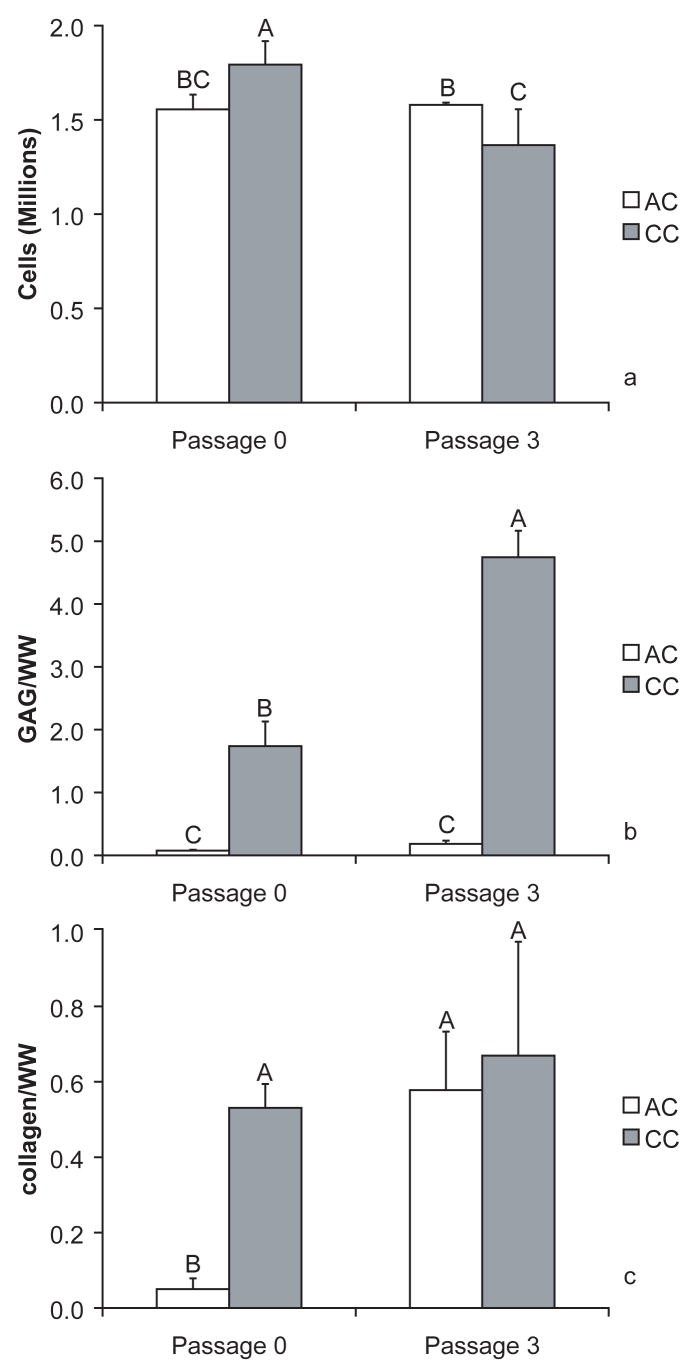
Cell numbers at 4 wks were all around 1.5 million (Fig. 2a). The number of CC P0 was significantly greater than the other groups, and the AC P3 number was significantly greater than the CC P3 number (p < 0.0001). The CC P3 group, however, had the greatest GAG per wet weight (Fig. 2b) (p < 0.0001). CC P0 constructs also had significantly greater GAG per wet weight than either passage of AC constructs. Collagen per wet weight (Fig. 3c) was significantly greater in CC P3, AC P3, and CC P0 than AC P3 constructs (p < 0.0001). The collagen type I ELISA did not detect any collagen I in the AC P0 samples. In contrast, AC P3 constructs had 86 ± 5 times the collagen I relative to the collagen II content measured with the ELISAs. The type I to type II ratios for CC P3 and CC P0 were 0.87 ± 0.3 and 0.05 ± 0.009, respectively, suggesting the prevalence of collagen type II in the primary constructs.
Figure 2.
Quantitative biochemical data for all groups (n = 6). Data are shown as mean ± standard deviation. Groups separated by different letters are considered significantly different (p < 0.05). While some statistical differences are observed between the groups for the number of cells (Fig. 2a), the groups aggregate around 1.5 million cells. Dramatic differences were seen in the GAG per wet weight (WW) (Fig. 2b) with CC P3 and CC P0 being significantly more concentrated than either of the AC groups. CC P3 also had significantly more GAG per wet weight than CC P0. AC P0 was significantly lower than the other groups for collagen per wet weight (Fig. 2c).
Figure 3.
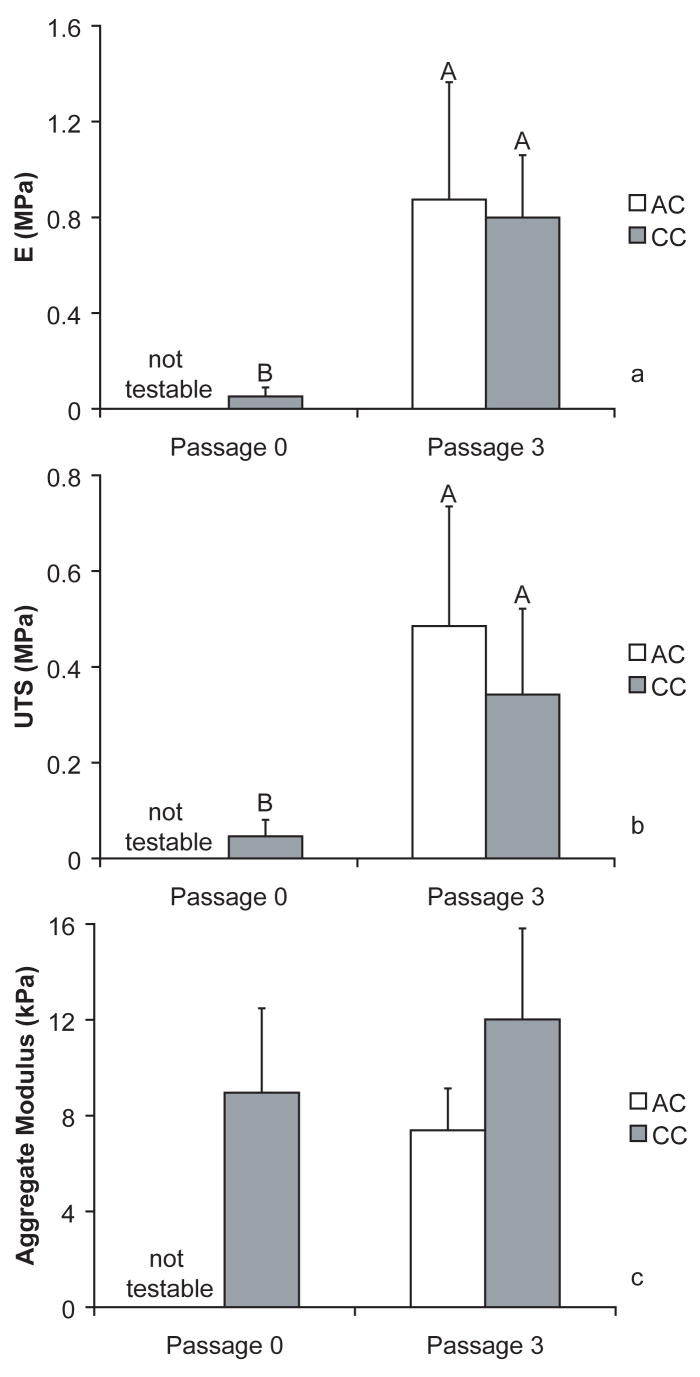
Mechanical data for all groups (n = 6). Data are shown as mean ± standard deviation. Groups separated by different letters are considered significantly different (p < 0.05). AC P0 groups were not testable in tension or compression. For the tensile properties, UTS and E, CC groups were significantly better than AC P3. No statistical differences were seen between the groups for aggregate modulus (Fig. 3c).
Mechanical properties
As was explained in the morphology description of the AC P0 constructs, this group was not solid and unable to withstand mechanical loading; therefore, these constructs were not testable in either tension or compression. Tensile elastic modulus (Fig. 3a) and UTS (Fig. 3b) were significantly greater for passage 3 groups than primary. Aggregate modulus was not significantly different for any of the testable groups (p = 0.0632). Poisson’s ratio was not significant with means of 0.0044, 0.042, 0.11, for CC P0, CC P3, and AC P3, respectively. CC P3 constructs had a significantly greater permeability than AC P3 or CC P0 groups with results of 5.46·10−13 ± 4.32·10−13, 1.52·10−13 ± 1.02·10−13, and 5.20·10−14 ± 1.71·10−14, respectively (p = 0.0171).
Discussion
This work examined the biochemical and mechanical properties of scaffoldless, tissue engineered constructs created from passaged and primary costal chondrocytes and articular chondrocytes. The constructs made from primary cells were generally larger, had less mechanical integrity, and contained less ECM per wet weight. The AC P0 constructs, in particular, had significantly less collagen, and were not mechanically testable in either tension or compression. While smaller and rounded in shape, the P3 groups had significantly greater tensile strength, and the CC P3 constructs had the most GAG/wet weight. ELISA and IHC analyses demonstrated the lack of collagen type I in AC P0 constructs and limited quantity of collagen type II in AC P3 constructs.
These results supported the hypotheses of this study. The first, that CCs would produce constructs equal to or better than AC constructs, was particularly apparent in the primary constructs. CC P0 constructs were better than AC P0 constructs for cell quantity, overall size, mechanical properties, collagen/wet weight, and GAG/wet weight. CC P3 constructs also had significantly greater size and GAG/wet weight than AC P3 constructs, but only trended higher in aggregate modulus and collagen/wet weight. In regard to the second hypothesis, which suggested that P3 constructs would be more like fibrocartilage and the P0 constructs more like articular cartilage, there are both significant and trending results in support of this. AC P3 constructs had a significantly higher collagen type I/collagen type II ratio than AC P0 constructs. This ratio was also greater for CC P3 constructs compared to CC P0 constructs but not significantly different. Additionally, the P3 constructs had significantly greater tensile properties than the P0 constructs, but compressive properties were not significantly different.
The results seen for the primary costal chondrocytes were similar to those seen previously.17 While the previous study used similar methods, samples were tested biochemically at 3 and 6 wks. The quantitative results of this 4 wk study fell between the previous values for both hydroxyproline and DMMB assays. Tensile results were 3–5 times lower in this study than those seen previously at 6 wks, but the previous study used smaller agarose molds with the same number of cells to form the constructs, resulting in smaller constructs. This change in construct size may alter the organization of the ECM and therefore the mechanical properties.38, 39 In addition, tensile properties of CC constructs have been seen to increase over time (data not shown).
In contrast, the primary articular chondrocyte constructs produced in this study are quite different from those created previously. Previous work showed substantially higher GAG, total collagen, and collagen type II content for the constructs.25, 27, 40 While the constructs produced here were gelatinous, difficult to handle, and not mechanically testable, previous constructs have produced aggregate moduli around 200 kPa, elastic modulus approaching 1.4 MPa, and UTS near 350 kPa.27 While these mechanical properties were seen on constructs created with 5.5 million cells, even when previous work used 2 million cells (as was used here), an aggregate modulus of 74 kPa was obtained.40 The primary change in the present study from previous ones, which may account for these differences, is the source of the chondrocytes. Both studies obtained the ACs with similar methods from the distal femur, but previous work used ACs from 1 wk old, male calves, while this study used cells from skeletally mature, female goats. While the sex or species may account for the differences, the more likely explanation is the age difference. There is considerable work that demonstrates age-related changes in ECM structure, which may relate to the changes in mechanical properties seen with these different-aged cells.41–45 Previous work also showed decreases in collagen type II, aggrecan core protein, and Sox 9 gene expression of cultured, mature chondrocytes compared to immature chondrocytes.46 This suggests that the immature cells have a greater productive capacity for the relevant ECM than mature chondrocytes.
Despite the somewhat disappointing results for primary chondrocytes relative to previous results, the passaged ACs and CCs showed considerable improvements in their constructs’ biochemical and mechanical properties. The decrease in size of the passaged constructs, in addition to the decrease in wet weight, may indicate that the passaged constructs are simply a contracted form of the primary constructs; however, the changes in mechanical properties suggest this is not the case. The increases in tensile strength and stiffness, which occurred for both types of chondrocytes, suggest that the passaged constructs contain more organized ECM than the primary constructs at 4 wks. While both cell types have been shown to dedifferentiate with passage, this effect appears to have benefits, particularly for this current use of the cells.29, 47 Passaged AC constructs, unlike their primary counterparts, were mechanically testable in both tension and compression and contained 11 times more total collagen/wet weight. AC P3 constructs also had almost 2.5 times more GAG/wet weight than AC P0 constructs, although the differences were not significant. Passaged CC constructs had almost 3 times more GAG/wet weight, 16 times greater elastic modulus, and 7 times greater UTS than primary constructs. The aggregate modulus was not significantly different between the CC P3 and CC P0 constructs.
While these passaged constructs were dramatically improved from P0 constructs, the characteristics of the tissues of interest for tissue engineering must be considered. As reviewed previously, articular cartilage contains primarily collagen type II, while the TMJ disc contains primarily collagen type I.3 Additionally, articular cartilage is most important under compressive loading, while fibrocartilage is also important under tensile loading. In this study, only the AC P0 constructs did not contain any collagen type I, while the AC P3 cells produced the lowest amount of collagen II, particularly relative to the large quantity of collagen type I produced in this group. CC constructs, however, had measurable quantities of both types of collagen. CC P0 constructs had around 20 times more collagen II than collagen I, while CC P3 had less than 1.5 times the collagen II/collagen I ratio. These data, combined with the CC constructs’ mechanical properties, suggest the potential usefulness of primary CCs for cartilage tissue engineering and passaged CCs for fibrocartilage tissue engineering.
Comparing the data to native tissue, the GAG content was similar to both articular cartilage and the TMJ disc, as was seen previously.17 The GAG/wet weight was approximately 2% for CC P0 constructs and 5% for CC P3 constructs. This suggests the potential of either of these cell types to function for a variety of soft tissues in the TMJ. The collagen/wet weight was, again, lower than native tissue with the CC P0 group having 0.5% collagen/wet weight and the CC P3 constructs having 0.7% collagen/wet weight. Mechanical properties were also still many times lower than native. The most dramatic difference between these two groups, which relates their potential to function in either cartilage or fibrocartilage is the collagen II/collagen I ratios, as discussed previously. While improvements in collagen content and mechanical properties must be made before such a construct could be implanted, these cells exhibit considerable potential for functionality.
While the passaged ACs were, for most metrics, statistically equivalent to passaged CC constructs, CC constructs had 8 times more GAG/wet weight. The CC constructs were also significantly larger in size. This makes the translation from bench top to bedside more feasible. Considering the accessibility of this cell source, limited surgical complications, and abundance of costal cartilage tissue, this cell source appears feasible for future use. While considerable aspects of this work should be explored in greater detail (i.e., passage number, expansion conditions, temporal changes in culture), these data are promising. The affirmation of the hypotheses, combined with the significant results seen here, suggest that passaged CCs should be considered as a possible cell source for engineering TMJ fibrocartilage tissues.
Acknowledgments
We gratefully acknowledge funding from NIH grants: NIDCR #R01DE015038-01A2 and NIAMS R01 AR053286. We would also like to thank Kerem Kalpakci and Roman Natoli for their help in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dimitroulis G. The role of surgery in the management of disorders of the temporomandibular joint: a critical review of the literature. Part 2. International journal of oral and maxillofacial surgery. 2005;34(3):231–237. doi: 10.1016/j.ijom.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Wong ME, Allen KD, Athanasiou KA. Tissue engineering of the temporomandibular joint. In: Bronzino JD, editor. Biomedical Engineering Handbook Third Edition: Tissue Engineering and Artificial Organs. CRC Press; 2006. pp. 51-51–52-22. [Google Scholar]
- 3.Johns DE, Athanasiou KA. Design characteristics for TMJ disc tissue engineering: Learning from tendon and articular cartilage. Proceedings of the Institution of Mechanical Engineers. 2007;221(5):509–526. doi: 10.1243/09544119JEIM158. [DOI] [PubMed] [Google Scholar]
- 4.Almarza AJ, Athanasiou KA. Design characteristics for the tissue engineering of cartilaginous tissues. Ann Biomed Eng. 2004;32(1):2–17. doi: 10.1023/b:abme.0000007786.37957.65. [DOI] [PubMed] [Google Scholar]
- 5.Delatte M, Von den Hoff JW, van Rheden RE, Kuijpers-Jagtman AM. Primary and secondary cartilages of the neonatal rat: the femoral head and the mandibular condyle. European journal of oral sciences. 2004;112(2):156–162. doi: 10.1111/j.0909-8836.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 6.Teramoto M, Kaneko S, Shibata S, Yanagishita M, Soma K. Effect of compressive forces on extracellular matrix in rat mandibular condylar cartilage. Journal of bone and mineral metabolism. 2003;21(5):276–286. doi: 10.1007/s00774-003-0421-y. [DOI] [PubMed] [Google Scholar]
- 7.Cheung HS. Distribution of type I, II, III and V in the pepsin solubilized collagens in bovine menisci. Connective tissue research. 1987;16(4):343–356. doi: 10.3109/03008208709005619. [DOI] [PubMed] [Google Scholar]
- 8.Eyre DR, Wu JJ. Collagen of fibrocartilage: a distinctive molecular phenotype in bovine meniscus. FEBS letters. 1983;158(2):265–270. doi: 10.1016/0014-5793(83)80592-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim KW, Wong ME, Helfrick JF, Thomas JB, Athanasiou KA. Biomechanical tissue characterization of the superior joint space of the porcine temporomandibular joint. Ann Biomed Eng. 2003;31(8):924–930. doi: 10.1114/1.1591190. [DOI] [PubMed] [Google Scholar]
- 10.Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9(3):330–340. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 11.Allen KD, Athanasiou KA. Growth factor effects on passaged TMJ disk cells in monolayer and pellet cultures. Orthod Craniofac Res. 2006;9(3):143–152. doi: 10.1111/j.1601-6343.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 12.Almarza AJ, Athanasiou KA. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann Biomec Eng. 2005;33(7):943–950. doi: 10.1007/s10439-005-3311-8. [DOI] [PubMed] [Google Scholar]
- 13.Detamore MS, Athanasiou KA. Use of a rotating bioreactor toward tissue engineering the temporomandibular joint disc. Tissue engineering. 2005;11(7–8):1188–1197. doi: 10.1089/ten.2005.11.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johns DE, Athanasiou KA. Improving culture conditions for temporomandibular joint disc tissue engineering. Cells, tissues, organs. 2007;185(4):246–257. doi: 10.1159/000102173. [DOI] [PubMed] [Google Scholar]
- 15.Allen KD, Athanasiou KA. Effect of passage and topography on gene expression of temporomandibular joint disc cells. Tissue engineering. 2007;13(1):101–110. doi: 10.1089/ten.2006.0094. [DOI] [PubMed] [Google Scholar]
- 16.Allen KD, Erickson K, Athanasiou KA. The effects of protein-coated surfaces on passaged porcine TMJ disc cells. Archives of oral biology. 2008;53(1):53–59. doi: 10.1016/j.archoralbio.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns DE, Athanasiou KA. Engineering the TMJ disc with clinically-relevant cell sources. J Dent Res. doi: 10.1177/154405910808700609. Accepted 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 19.Gage JP, Shaw RM, Moloney FB. Collagen type in dysfunctional temporomandibular joint disks. J Prosthet Dent. 1995;74(5):517–520. doi: 10.1016/s0022-3913(05)80355-5. [DOI] [PubMed] [Google Scholar]
- 20.Sindelar BJ, Evanko SP, Alonzo T, Herring SW, Wight T. Effects of intraoral splint wear on proteoglycans in the temporomandibular joint disc. Arch Biochem Biophys. 2000;379(1):64–70. doi: 10.1006/abbi.2000.1855. [DOI] [PubMed] [Google Scholar]
- 21.Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue engineering. 2004;10(11–12):1787–1795. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- 22.Almarza AJ, Athanasiou KA. Evaluation of three growth factors in combinations of two for temporomandibular joint disc tissue engineering. Archives of oral biology. 2006;51(3):215–221. doi: 10.1016/j.archoralbio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist C, Jokinen J, Paukku P, Tasanen A. Adaptation of autogenous costochondral grafts used for temporomandibular joint reconstruction: a long-term clinical and radiologic follow-up. J Oral Maxillofac Surg. 1988;46(6):465–470. doi: 10.1016/0278-2391(88)90413-2. [DOI] [PubMed] [Google Scholar]
- 24.Yotsuyanagi T, Mikami M, Yamauchi M, Higuma Y, Urushidate S, Ezoe K. A new technique for harvesting costal cartilage with minimum sacrifice at the donor site. J Plast Reconstr Aesthet Surg. 2006;59(4):352–359. doi: 10.1016/j.bjps.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 25.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue engineering. 2006;12(4):969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 26.Revell CM, Athanasiou KA. Defined medium for the self-assembling process: Enhancing functionality of tissue engineered articular cartilage. J Orthop Res. Submitted 2007. [Google Scholar]
- 27.Elder BD, Athanasiou KA. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res. 2008;26(2):238–246. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darling EM, Athanasiou KA. Growth factor impact on articular cartilage subpopulations. Cell and tissue research. 2005;322(3):463–473. doi: 10.1007/s00441-005-0020-4. [DOI] [PubMed] [Google Scholar]
- 29.Tay AG, Farhadi J, Suetterlin R, Pierer G, Heberer M, Martin I. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue engineering. 2004;10(5–6):762–770. doi: 10.1089/1076327041348572. [DOI] [PubMed] [Google Scholar]
- 30.Darling EM, Hu JC, Athanasiou KA. Zonal and topographical differences in articular cartilage gene expression. J Orthop Res. 2004;22(6):1182–1187. doi: 10.1016/j.orthres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Hu JC, Athanasiou KA. Chondrocytes from different zones exhibit characteristic differences in high density culture. Connective tissue research. 2006;47(3):133–140. doi: 10.1080/03008200600685392. [DOI] [PubMed] [Google Scholar]
- 32.Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 2005;24(1):45–57. doi: 10.1016/j.matbio.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Analytical biochemistry. 1988;174(1):168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 34.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 35.Koay EJ, Hoben GM, Athanasiou KA. Tissue engineering with chondrogenically differentiated human embryonic stem cells. Stem cells (Dayton, Ohio) 2007;25(9):2183–2190. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- 36.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS ONE. 2008;3(7):e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12(3):340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 38.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue engineering. 2007;13(9):2195–2205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 39.Calve S, Dennis RG, Kosnik PE, 2nd, Baar K, Grosh K, Arruda EM. Engineering of functional tendon. Tissue engineering. 2004;10(5–6):755–761. doi: 10.1089/1076327041348464. [DOI] [PubMed] [Google Scholar]
- 40.Revell CM, Reynolds CE, Athanasiou KA. Seeding density optimization in self assembly of articular cartilage. Ann Biomed Eng. doi: 10.1007/s10439-008-9524-x. Submitted 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roughley PJ. Age-associated changes in cartilage matrix: implications for tissue repair. Clinical orthopaedics and related research. 2001;391(Suppl):S153–160. doi: 10.1097/00003086-200110001-00015. [DOI] [PubMed] [Google Scholar]
- 42.Adams CS, Horton WE., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. The Anatomical record. 1998;250(4):418–425. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 43.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. The Biochemical journal. 1998;330 ( Pt 1):345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolton MC, Dudhia J, Bayliss MT. Age-related changes in the synthesis of link protein and aggrecan in human articular cartilage: implications for aggregate stability. The Biochemical journal. 1999;337 ( Pt 1):77–82. [PMC free article] [PubMed] [Google Scholar]
- 45.Buckwalter JA, Roughley PJ, Rosenberg LC. Age-related changes in cartilage proteoglycans: quantitative electron microscopic studies. Microscopy research and technique. 1994;28(5):398–408. doi: 10.1002/jemt.1070280506. [DOI] [PubMed] [Google Scholar]
- 46.Hidaka C, Cheng C, Alexandre D, Bhargava M, Torzilli PA. Maturational differences in superficial and deep zone articular chondrocytes. Cell and tissue research. 2006;323(1):127–135. doi: 10.1007/s00441-005-0050-y. [DOI] [PubMed] [Google Scholar]
- 47.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23(2):425–432. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]