Abstract
α-Synuclein is a small presynaptic protein (14,460 D) that is abundantly distributed in the brain. Although, its function is unknown, the aggregated form of α-synuclein is a pathological hallmark of several neurodegenerative diseases, including Parkinson’s disease (PD). Epidemiological studies have shown that smoking can lessen the incidence of Parkinson’s disease, indicating that smoke may contain chemicals that are neuro-protective. The fibrillation of α-synuclein was studied in relation to five different compounds found in cigarette smoke: anabasine, cotinine, hydroquinone, nicotine and nornicotine. Thioflavin T assays, gel electrophoresis, size exclusion chromatography-high performance liquid chromatography (SEC-HPLC) and atomic force microscopy (AFM) were utilized to monitor the rate of α-synuclein fibrillation and the inhibitory effects of the cigarette smoke components. We show that nicotine and hydroquinone inhibit α-synuclein fibril formation in a concentration-dependent manner, with nicotine being more effective. The SEC-HPLC data show that nicotine and hydroquinone stabilize soluble oligomers. The morphology of the oligomers stabilized by nicotine was evaluated by AFM, which showed the presence of three stable oligomers with an average height of 16 nm, 10 nm and 4 nm. Comparable results were obtained for the effect of the cigarette smoke components on the A53T mutant fibrillation. These results show that nicotine and hydroquinone inhibit α-synuclein fibrillation and stabilize soluble oligomeric forms. This information can be used to understand the molecular mechanism of the nicotine and hydroquinone action to develop therapeutic solutions for PD.
Keywords: α-Synuclein, intrinsically disordered protein, smoking, Parkinson’s disease, nicotine, hydroquinone, fibrillation, misfolding
1. Introduction
Parkinson’s disease (PD) is the most common ageing-related movement disorder and second most common neurodegenerative disorder after Alzheimer’s disease. PD is a slowly progressive neurodegenerative disease caused by the loss of nerve cells in a part of the mid-brain known as the substantia nigra. The cells in the substantia nigra are responsible for the dopamine production, a chemical messenger involved in the movement coordination. PD is developed when these cells are damaged or destroyed causing different signs of PD: resting tremors, slowness of movement, stiffness of the limbs and balance problems [1]. The surviving nigral dopaminergic neurons contain specific proteinaceous inclusions, Lewy bodies (LB) and Lewy neurites (LN), which are also found in several neurodegenerative diseases [2, 3]. It is estimated that ~1.5 million Americans are affected by PD. Since only a small percentage of patients are diagnosed before age 50, PD is generally considered as an aging-related disease, and approximately one of every 100 persons over the age of 65 in the US suffers from this disorder [1].
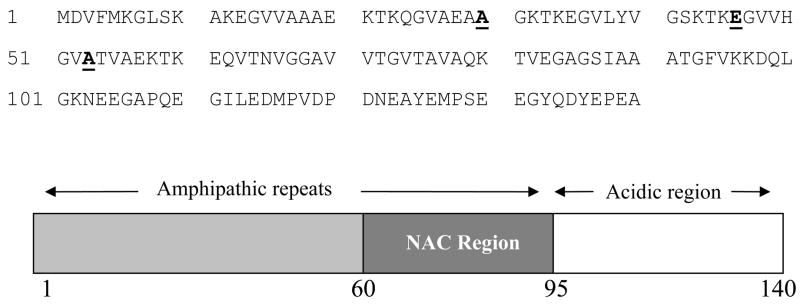
The precise molecular mechanism that leads to the death of the cells in the substantia nigra is still unknown. However, substantial evidence has suggested that the fibrillation of α-synuclein is a critical step in the pathogenesis of PD (reviewed in [4–9]). α-Synuclein is a major fibrillar component of Lewy bodies and Lewy neuritis [10]. It is a typical natively unfolded protein, which possess little ordered structure at physiological conditions [6, 7, 9, 11, 12]. α–Synuclein contains 140 amino acid residues and lacks both cysteine and tryptophan residues [13]. The sequence of α-synuclein is divided into three regions (Scheme 1):
Scheme 1.
α-Synuclein sequence. The three sites of early-onset PD-linked mutations (positions 30, 46 and 53) are highlighted.
The N-terminal region, which represents residues 1–60, contains 11-amino acid imperfect repeats with a consensus motif (KTKEGV).
The central region, which represents residues 61–95, contains a highly amyloidogenic NAC region and two additional repeats.
The C-terminal region, which represents residues 96–140, is rich in acidic residues and prolines, which are suggested to adopt a disordered conformation [14].
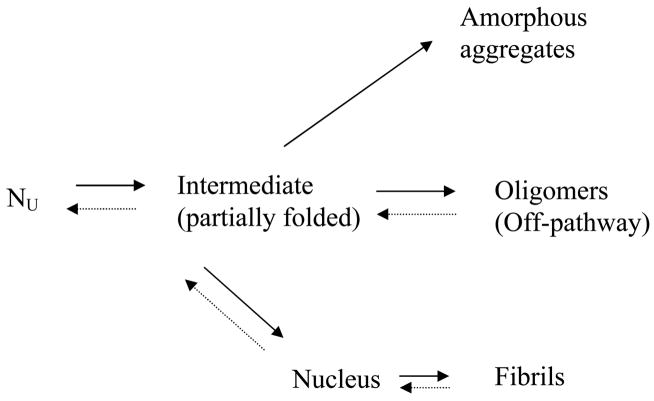
The competing kinetic pathways for the aggregation of α-synuclein begin with a natively unfolded α-synuclein monomer, which partially folds into an aggregation-prone intermediate [12]. Depending on the conditions, this intermediate can form three different products: soluble oligomers, insoluble amorphous aggregates or insoluble fibrils [6, 7, 9] (Scheme 2). The state that α-synuclein adopts depends on changes in the environmental conditions such as decrease in pH, or temperature increase, evolving to the formation of the partially folded intermediate [6, 7, 9, 12].
Scheme 2.
Multiple pathways for α-synuclein aggregation. Nu represents the natively unfolded α-synuclein monomer.
The involvement of α-synuclein in PD was suggested by previous studies of families with autosomal dominant early-onset of familial PD (FPD) in which three separate mutations in the α-synuclein gene A53T, A30P and E46K were linked to the disease [15–17]. All three FPD mutations alter the rate of α-synuclein aggregation in vitro, but only the A53T mutation accelerates fibril formation as compared to the wild type proteins, whereas A30P and E46K increase rates of amorphous aggregation [18–20].
It is believed that many environmental and genetic factors affect α-synuclein fibrillation. Factors that accelerate the α-synuclein fibrillation include certain pesticides, metals, lipids, membranes, polycations, glycosaminoglycans (GAGs), and macromolecular crowding [6, 7, 9]. This acceleration is due to the conditions that increase the concentration of the amyloidogenic intermediate. Oxidative modification was suggested to play an important role in the PD pathogenesis and one of the major factors triggering α-synuclein aggregation was shown to be the formation of free radicals (e.g., see [21]).
On the contrary, inhibition of fibrillation occurs when the monomer or non-fibrillogenic oligomers are stabilized [6, 7, 9]. Some compounds in the cigarette smoke might cause such inhibition, as epidemiological studies revealed that the smoke compounds significantly decrease the risk of PD [22, 23]. In cigarette smoke, out of the more than 3,800 identified compounds, anabasine, cotinine, hydroquinone, nornicotine, and especially nicotine, potentially represent such inhibitory compounds. Nicotine is a good candidate for investigation because of several reasons. First, nicotine stimulates striatal dopamine neurons that are damaged in PD [23]. Second, epidemiological studies have shown that PD is less prevalent in smokers which suggests that nicotine exposure prevents against neuronal insults [23]. Furthermore, nicotine administered orally by gum or transdermally by the patch has been shown to improve symptoms of Parkinson’s disease [24–26]. Intriguingly, it has been reported that nicotine possesses both pro-oxidant and antioxidant properties [27]. It has been pointed out that because of the nicotine’s beneficial role in the treatment of certain neurodegenerative diseases, its involvement in free radical production or its ability to act as an antioxidant requires careful study to further evaluate its overall therapeutic usefulness [27]. In addition to nicotine, numerous agents in tobacco products could modulate biological functions and the development of PD [23].
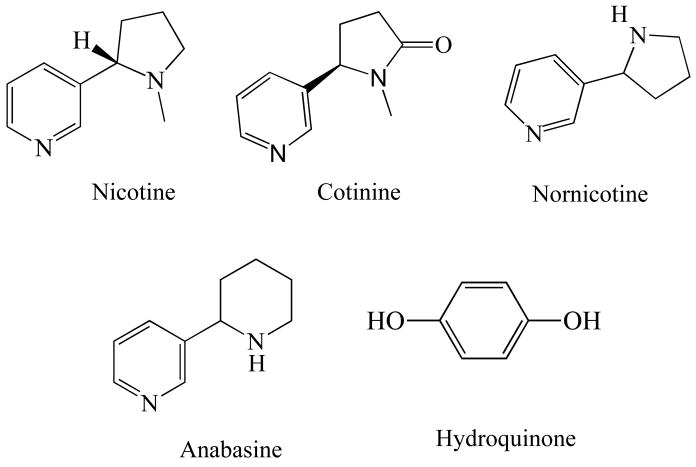
In this study, the effect of nicotine, and three structurally similar compounds, anabasine, cotinine and nornicotine (Scheme 3), on α-synuclein fibrillation were investigated. The goal was to understand how these compounds affect the rate of fibrillation. The results were compared to those obtained for hydroquinone, a known inhibitor of α-synuclein fibrillation, which is structurally different from the four smoke compounds (Scheme 3). Thioflavin T (ThT) assays, gel electrophoresis, SEC-HPLC and atomic force microscope (AFM) were used to investigate the inhibitory effects. Our results revealed that nicotine and hydroquinone show strong inhibitory effects on α-synuclein fibrillation by stabilizing oligomers. These findings may shed light on novel therapeutic solutions for PD based on the structure of the compounds.
Scheme 3.
Chemical structures of nicotine, cotinine, anabasine, nornicotine, and hydroquinone.
2. Materials and Methods
2.1. Materials
The chemical compounds such as nicotine and cotinine were obtained from Sigma, hydroquinone was purchased from Acros Organics, whereas nornicotine and ThT were from Fluka.
2.2. Expression and purification of human recombinant α-synuclein
Human wild type α-synuclein was expressed using E. coli BL21 (DE3) cell line transfected with pRK172/α-synuclein plasmid (generously donated by M. Goedert, MRC Cambridge). Expression and purification of human recombinant α-synuclein and its mutant from E. coli were performed as previously described [28]. Culture flasks containing 1 L of LB media were inoculated with 6 ml of a night pre-culture each and subsequently incubated at 37°C at 250 rpm until the media reached A500= 0.9–1. The culture was then induced with isopropyl-b-D-thiogalactopyranoside (IPTG) and incubated for another 5 hrs. The cells were collected by centrifugation at 4000 rpm for 15 minutes at 4°C. The cell pellets were frozen and kept overnight at −20°C. At the next day, pellets were thawed at room temperature and redissolved in 40 ml of lysis buffer (50 mM NaCl, 20 mM Tris-HCl, 0.10% Triton- X 100, 0.20 mM phenylmethyl sulfonyfluoride (PMSF) pH 7.5).
Sonication at 60% power for 3.5 minutes total with seven 30 second sonication bursts and 40 seconds of rest in between each burst was used to lyse the cells. Subsequently, ammonium sulfate was slowly added to achieve 30% saturation with vigorous stirring on ice. Ten minutes of gentle stirring completed the precipitation. In order to precipitate the cell debris, the solution was centrifuged at 13,000 rpm for 15 minutes at 4°C. The supernatant was separated from the pellet and the pellet was discarded. The supernatant was brought to 50 % saturation with ammonium sulfate. The solution was centrifuged at 13,000 rpm for 15 minutes at 4°C. The supernatant was separated from the pellet and the discarded. The pellet was saved, dissolved in 50 mL of 10 mM Tris-HCl (pH 7.5) and dialyzed against 4L of 50 mM NaCl, 20 mM Tris-HCl (pH 7.5) for 2 hours. The dialysis buffer was changed after two hours and then changed three more times overnight. In order to purify the protein, the sample from the dialysis was loaded onto a DEAE-Sepharose Fast Flow column previously equilibrated with ~5X-bed volume of 50 mM NaCl, 20 mM Tris-HCl, pH 7.2. About 80 fractions were collected, and the presence of α-synuclein was verified by using SDS-PAGE. In order to remove the salt from the protein, the fractions that contained α-synuclein were dialyzed against deionized water for at least 36 hours at 4°C. After the dialysis, the precipitate was removed by centrifugation. Protein purity evaluated by mass-spectrometry, SEC-HPLC and SDS-PAGE was close to 98%. The protein concentration was then determined by A275. The protein was frozen in liquid nitrogen and then lyophilized.
2.3. Fibrillation of WT α-synuclein and the ThT Assay
The lyophilized α-synuclein was dissolved in 1 ml of 0.02 M NaPO4 (Pi) buffer, 0.1M NaCl and 0.01% NaN3, pH 7.5. In order to remove any insoluble material, the sample was centrifuged in the cold room for 30 minutes at 13.2 RPM. The supernatant was then analyzed by UV spectrophotometry to estimate protein concentration and by mass-spectrometry, SEC-HPLC and SDS-PAGE to evaluate the presence of aggregated material. At the beginning of the fibrillation studies, protein samples were predominantly monomeric and did not contain noticeable amounts of any oligomeric forms. Assay solutions contained α-synucelin at a concentration of 1.0 mg/ml, 20 μM ThT with various concentrations of smoke compounds as indicated. A volume of 150 μl of the mixture was pipetted into a well of a 96-well plate (white plastic, clear bottom), and a 1/8th in. diameter. Teflon sphere (McMaster-Carr, Los Angeles) was added. Each sample was run in triplicate or quadruplicate. The plates were sealed with Mylar plate sealers (Dynex). The plate was loaded into a fluorescence plate reader (Fluoroskan Ascent) and incubated at 37°C with shaking at 600 rpm with a shaking diameter of 2 mm. The fluorescence was measured at 30 min intervals with excitation at 450 nm and emission at 485 nm, with a sampling time of 100 ms.
The data were fit to a sigmoidal curve described by the empirical equation [29] using SigmaPlot software:
| (1) |
where F is the fluorescence intensity and t50 is the time to 50% of maximal fluorescence. The initial baseline during the lag time is described by Fi + mit. The final baseline after the growth phase has ended is described by Ff + mft. The apparent rate constant, kapp, for the growth of fibrils is given by 1/τ, the lag time is calculated as t50 − 2τ and the amplitude, amp, is given by Ff − Fi. Although eq.1 gave very good fits for the ThT kinetic profiles, the expression is strictly a simple empirical means of providing kinetic parameters for comparing rates of fibrillation from different samples and does not directly reflect the underlying complex kinetic scheme.
2.4. SDS-PAGE
Aliquots of the supernatant were airfuged for 30 minutes at 20 Psi (75,000 rpm) at the end of the fibrillation. 3 μL of the supernatant and 1 μL of staining gel were added into an Eppendorf tube, boiled for five minutes and spun down at 14,000 rpm for 1 minute. The amount of soluble protein present in the supernatant was monitored using the Coomassie-blue staining of the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels.
2.5. SEC-HPLC
Oligomers and monomers were separated using SEC-HPLC. After incubation, an aliquot of sample was removed. The samples were loaded onto a BioSep-SEC 2000 column in 50mM phosphate/100 mM NaCl (pH 7.0) buffer. The samples were eluted at a flow rate of 0.7 ml/min using a Waters 2695 separations module and the presence of protein in the sample was evaluated by UV absorption.
2.6. AFM Measurements
Aliquots of 5 μl of sample and 5 μl of 1 M NaCl were placed on a mica plate. The mica plate was left to incubate for 24 hours and then was rinsed with water to remove salt and any proteins not bound to the mica. The mica plate was then dried with N2. The Atomic Force Microscopic (AFM) images were obtained using a PicoScan Plus microscopy, which was equipped with the MAC mode. For the MAC mode imaging, probes with a 2.8 newton/m spring constant and a 75-kHz resonance frequency were used. A scan rate of 0.5–1 line/s with 512 data points per line, at a driver current of 10 ± 5 Å were used. The height range of 1.0 to 100 was estimated by section analysis. At least four regions of the mica surface were examined to verify that similar structures existed through out the sample.
3. Results
3.1. Nicotine and hydroquinone inhibit the fibrillation of α-synuclein
The kinetics of α-synuclein fibril formation was monitored by the characteristic increase in the ThT fluorescence intensity. ThT is a fluorescent dye that interacts preferentially with the cross-β-enriched amyloid-like fibrils and is commonly used to detect the amyloid fibrils and to follow the fibrillation process. ThT binds to β-sheet and non-β-sheet cavities with the diameters of 8–9 Å [30]. ThT fluorescence is an efficient method to monitor fibrillation kinetics because the fibrils of α-synuclein contain cross-β filaments that specifically interact with ThT. As more fibrils are formed, there is an increase in ThT fluorescence intensity. The kinetics of α-synuclein fibrillation is represented by a sigmoidal curve, which begins with a lag phase, followed by a growth phase, and ends with an equilibrium phase.
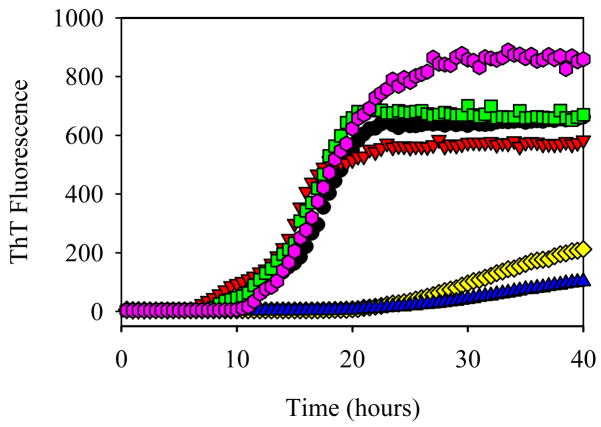
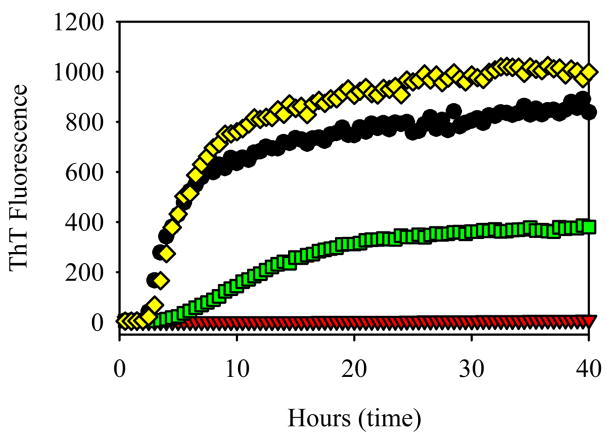
Figure 1 demonstrates the time-dependent changes in the ThT fluorescence intensity during the α-synuclein fibrillation in the absence or presence of five smoke compounds at the same concentration of 200 μM. This graph shows that anabasine, nornicotine and cotinine do not affect the kinetics of fibril formation as compared to the control. It also illustrates hydroquinone and nicotine has a noticeable inhibitory effect on the fibrillation. Nicotine shows a much stronger inhibition, which is reflected in longer lag-time and in the smaller ThT signal.
Figure 1.
Effect of different cigarette smoke compounds on the fibrillation kinetics of α-synuclein: α-Synuclein (1 mg/ml) alone (black circles); α-synuclein in the presence of 200 μM cotinine (red triangles); 200 μM anabasine (green squares); 200 μM hydroquinone (yellow diamonds); 200 μM nicotine (blue triangles); and 200 μM nornicotine (pink pentagons).
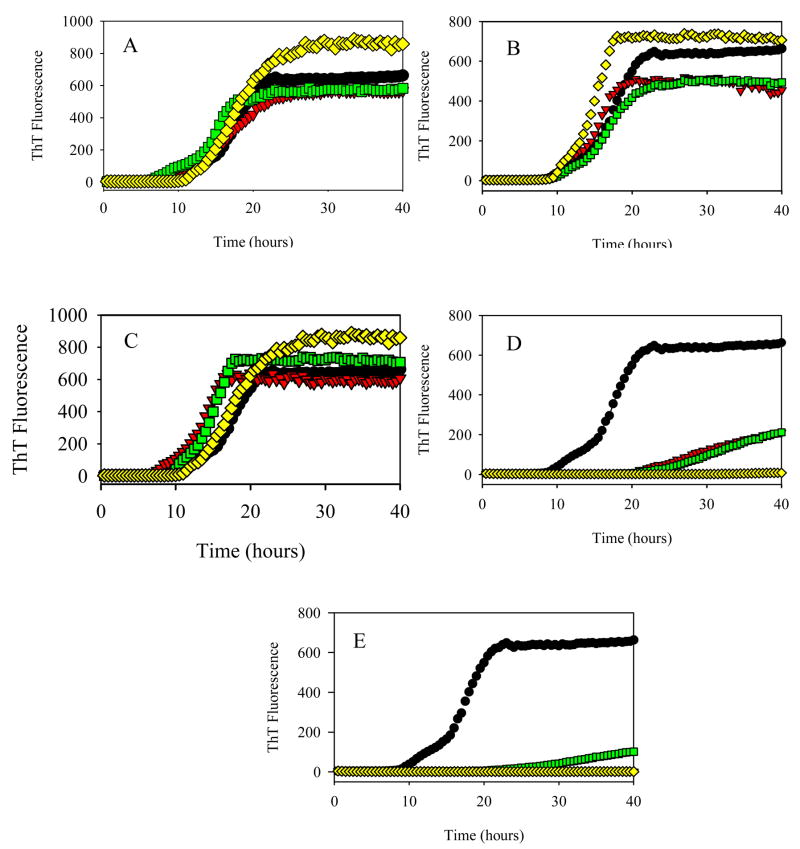
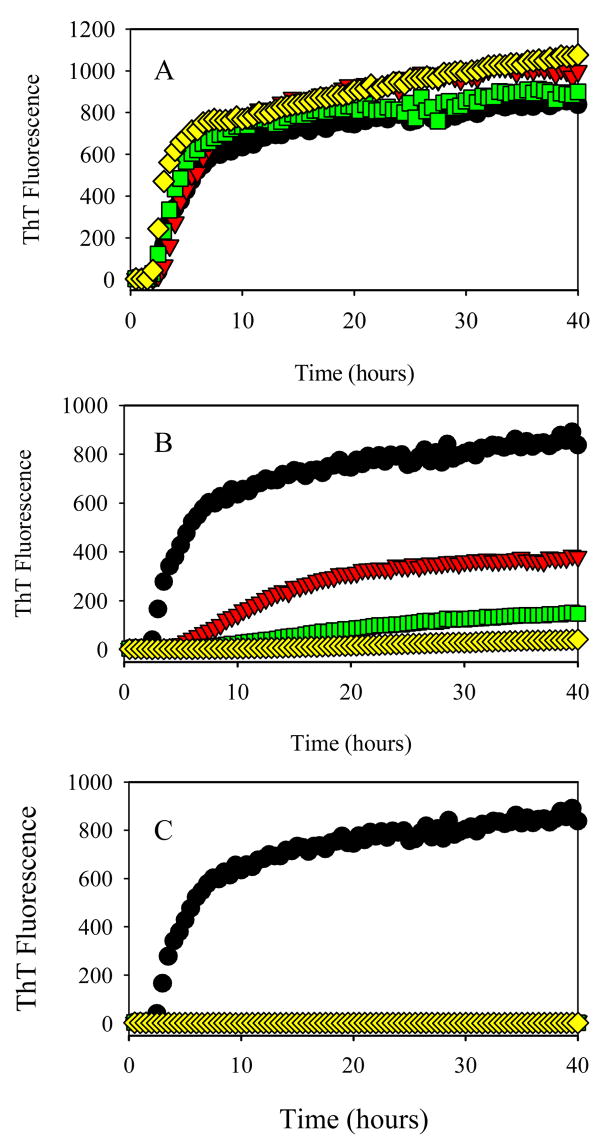
Figure 2 shows the time-dependent changes in the ThT fluorescence intensity during the α-synuclein fibril formation as a function of different concentrations (100 μM, 200 μM and 400 μM) of cotinine (Figure 2A), anabasine (Figure 2B), nornicotine (Figure 2C), hydroquinone (Figure 2D) and nicotine (Figure 2E) as compared to the control. Figures 2A, 2B and 2C show that even at concentration of 400 μM cotinine, anabasine and nornicotine did not affect the α-synuclein fibrillation. On the other hand, Figures 2D and 2E demonstrate that as the hydroquinone and nicotine concentration increases, the ThT fluorescence signal decreases, suggesting the effective inhibition of the α-synuclein fibrillation. Compared to hydroquinone, nicotine is a slightly better inhibitor as reflected in lower ThT signal and slower fibrillation kinetics.
Figure 2.
Effect of different concentrations of various smoke compounds on the fibrillation kinetics of 1 mg/ml α-synuclein. (A) cotinine, (B) anabasine, (C) nornicotine, (D) hydroquinone, (E) nicotine. Black circles, 0 μM; red triangles, 100 μM; green squares, 200 μM; yellow diamonds, 400 μM.
3.2. Hydroquinone- and nicotine-induced stabilization of the oligomeric form of α-synuclein: SDS-PAGE analysis
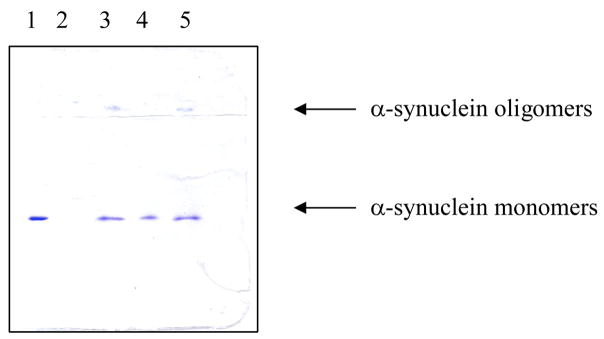
To confirm the inhibitory effect of hydroquinone and nicotine on the α-synuclein fibrillation, the samples at the end of the incubation period were checked by SDS-PAGE. Incubated samples were subjected to the centrifugation to pellet the mature fibrils and the resulting supernatants were used for the SDS-PAGE analysis (Figure 3). This analysis was carried out for all the hydroquinone concentrations. Figure 3 clearly shows that soluble protein is present in the supernatant of α-synuclein incubated in the presence of all the hydroquinone concentrations. In these cases, in addition to the band corresponding to the monomeric α-synuclein, an oligomeric form of the protein was also detected (see lines 3–5 in Figure 3). This indicates that non-fibrillar α-synuclein is present in the supernatant, suggesting that the fibrillation of α-synuclein is effectively inhibited by hydroquinone. Similar results were also obtained for nicotine (data not shown).
Figure 3.
Determination of the concentration of α-synuclein in the supernatant using SDS-PAGE. (1) α-synuclein, 1mg/ml; (2) control experiments where α-synuclein was fibrillated alone, (3) α-synuclein after fibrillation in the presence of 100 μM hydroquinone; (4) α-synuclein after fibrillation in the presence of 200 μM hydroquinone; (5) α-synuclein after fibrillation in the presence of 400 μM hydroquinone.
3.3. Hydroquinone- and nicotine-induced stabilization of the oligomeric form of α-synuclein: SEC-HPLC analysis
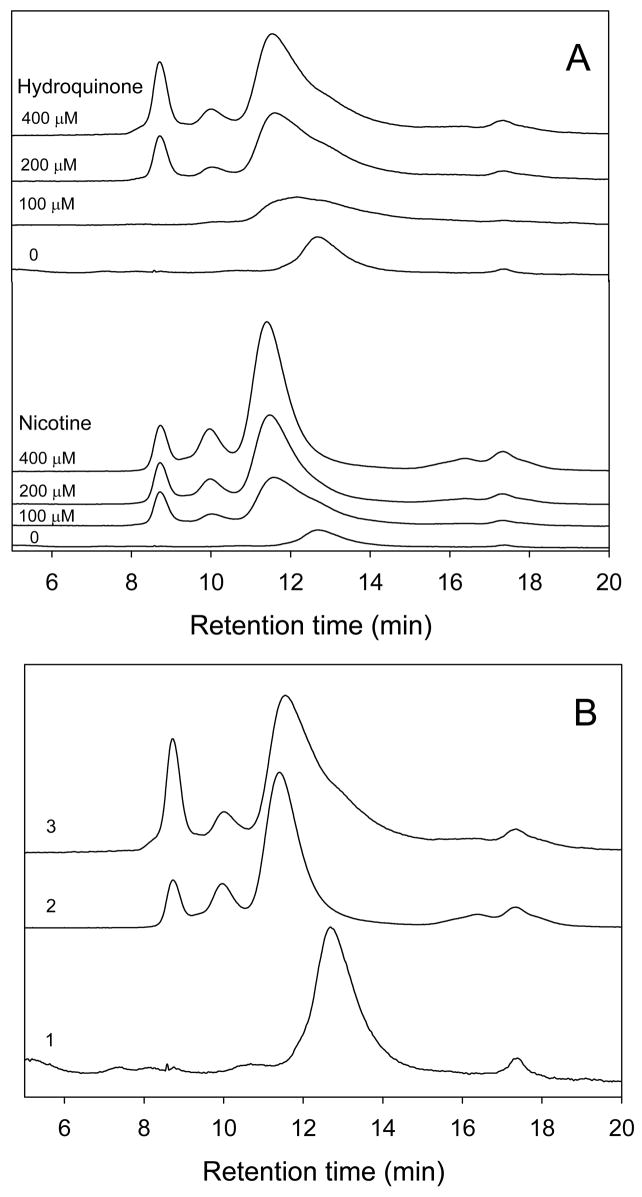
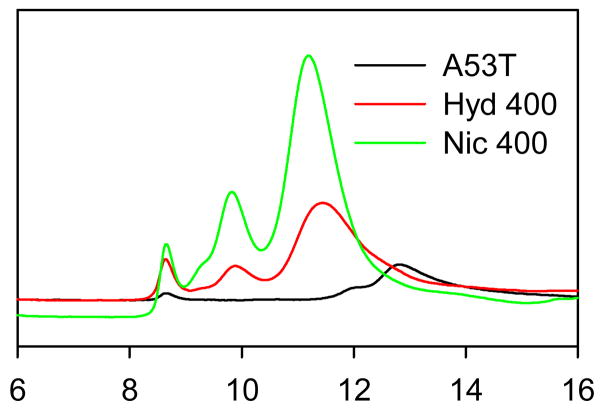
In order to gain further information on the oligomeric state of α-synuclein incubated in the presence of hydroquinone and nicotine, SEC-HPLC analysis was performed for samples incubated with different concentrations of these compounds. Figure 4A shows that compared to the control, most of the α-synuclein in the presence of nicotine or hydroquinone is in a form of higher molecular weight oligomers. Figure 4B compares the elution profiles of the monomeric α-synuclein with the protein samples incubated in the presence of 400 μM nicotine or hydroquinone. Figure 4B clearly shows that several different oligomer types were formed in the presence of nicotine and hydroquinone. Interestingly, although these smoke compounds seem to stabilize similar oligomers, the relative populations of these oligomers were different for samples containing nicotine and hydroquinone. In addition to oligomers, hydroquinone samples contained noticeable amount of the monomeric α-synuclein as evidenced by the presence of the measurable shoulder in the corresponding elution profile.
Figure 4.
SEC-HPLC demonstration that nicotine and hydroquinone stabilize α-synuclein oligomers. (A) α-Synuclein (1 mg/ml) alone or incubated in the presence of 100 μM, 200 μM, 400 μM nicotine, and 100 μM, 200 μM, and 400 μM hydroquinone. (B) α-Synuclein (1 mg/ml, 1) alone or incubated in the presence of 400 μM nicotine (2) or 400 μM hydroquinone (3).
3.4. Nicotine-induced stabilization of the oligomeric form of α-synuclein: AFM analysis
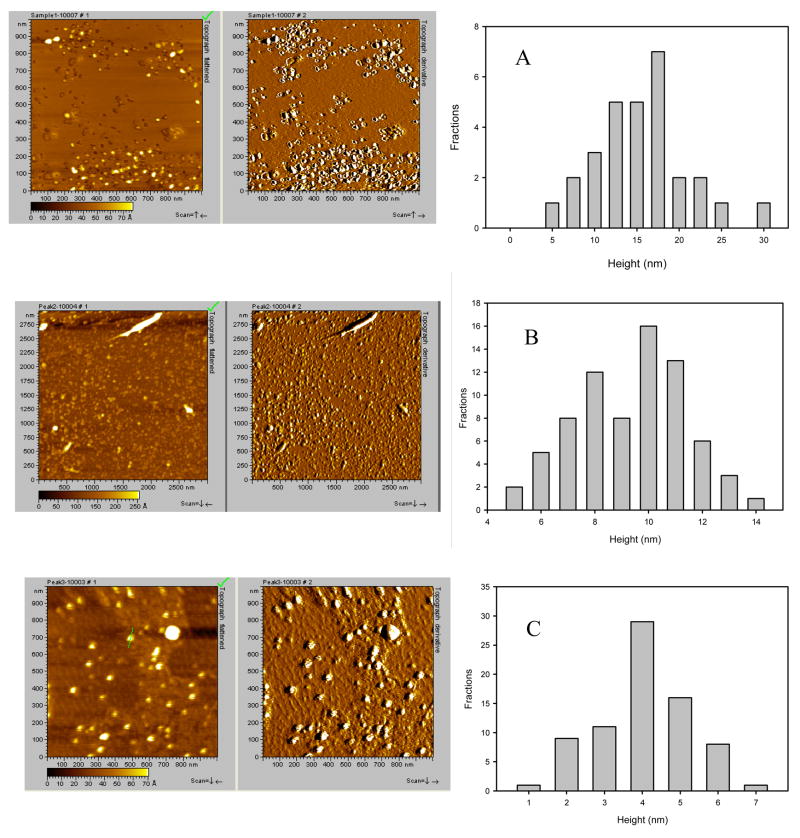
Since the nicotine sample contained a higher amount of oligomers than the hydroquinone sample (this conclusion follows from the fact that SEC-HPLC profile for 400 μM hydroquinone preserves small shoulder at the monomer peak position (see Figure 4B), indicating that some monomers are still present), each of the three peaks of the α-synuclein sample containing the 400 μM nicotine (Figure 4B) were subjected to the AFM imaging in order to evaluate the size and the shape of these oligomers. Figure 5 represents the results of this analysis both as AFM images and the corresponding height histogram. Figures 5A, 5B, and 5C correspond to the samples from the first, second and third SEC-HPLC peaks respectively. As expected, the average height of the oligomers in the first peak (~16 nm) was greater than the height of the oligomers in the second peak (~ 10 nm), and higher than the height of the oligomers in the third peak (~ 4 nm).
Figure 5.
AFM images and height distributions of an α-synuclein sample containing 400 μM nicotine. Samples were collected from the first (A), second (B) or third peak (C) eluting from the SEC-HPLC column (Figure 4B).
3.5. Nicotine and hydroquinone inhibit the α-synuclein A53T mutant fibrillation
The early onset of familial PD is associated with the different missense mutations in the α-synuclein gene, corresponding to A53T, A30P and E46K mutations in the α-synuclein protein. Therefore, at the next stage, we evaluated the effect of hydroquinone, nicotine and nornicotine on fibrillation of the A53T mutant. Figure 6 represents the time-dependence changes in the ThT fluorescence intensity during the A53T fibril formation in the presence of different compounds at a concentration of 100 μM. In agreement with earlier data, Figure 6 shows that the A53T mutant fibrillates faster than the wild type protein. A53T fibrillation was no affected by nornicotine, whereas hydroquinone and nicotine possessed an inhibitory effect. Similar to the wild type protein, nicotine was a better inhibitor of the A53T fibrillation.
Figure 6.
Effect of different smoke compounds on the fibrillation kinetics of the A53T mutant. Mutant α-synuclein incubated at 1mg/ml alone (black circles) or in the presence of 100 μM nicotine (red triangles); 100 μM hydroquinone (green squares); or 100 μM nornicotine (yellow diamonds).
Figure 7A compares the effects of various nornicotine concentrations on the A53T fibrillation and shows that this compound did not effect the fibrillation even at 400 μM concentration. On the other hand, Figures 7B and 7C show that hydroquinone inhibited the A53T fibrillation on a concentration-depended manner, whereas the fibrillation process was completely inhibited in the presence of the smallest nicotine concentration (100 μM).
Figure 7.
Effect of different concentrations of various cigarette smoke compounds on the fibrillation kinetics of the 1 mg/mL A53T mutant. (A) nornicotine, (B) hydroquinone, (C) nicotine. Black circles, 0 μM; red triangles, 100 μM; green squares, 200 μM; yellow diamonds, 400 μM.
3.6. Stabilization of the oligomeric form of the A53T α-synuclein with hydroquinone and nicotine
SDS-PAGE carried out for A53T samples incubated in the presence of the different concentrations of hydroquinone revealed that the A53T mutant protein was in the supernatant (Figure 8). Addition of nicotine produced similar inhibitory effect (data not shown). The distribution of the soluble oligomers in A53T samples incubated in the presence of 400 μM of hydroquinone or nicotine was analyzed by the SEC-HPLC (Figure 9). As with the wild type proteins, in addition to the monomer, different size oligomers were detected. Taken together these data suggest that similar to the wild type protein, the fibrillation of the A53T α-synuclein is effectively inhibited by the hydroquinone and nicotine in a concentration dependent manner. In all cases, this inhibition occurs due to the preferential stabilization of the soluble oligomers.
Figure 8.
Determination of the concentration of the soluble A53T mutant in the supernatant after fibrillation using SDS-PAGE. (1) mutant, 1 mg/ml, (2) mutant after incubation alone, (3) A53T after incubation in the presence of 100 μM hydroquinone, (4) A53T after incubation in the presence of 200 μM hydroquinone, and (5) A53T after incubation in the presence of 400 μM hydroquinone.
Figure 9.
SEC-HPLC demonstration that nicotine and hydroquinone, being coincubated with the A53T mutant, stabilize oligomers. Mutant A53T; A53T incubated with 400 μM nicotine, or with 400 μM hydroquinone.
4. Discussion
PD is a neurodegenerative disease occurring in about 1% of the population over the age of 65. It is characterized by the damage of the dopaminergic nigrostriatal neurons that leads to the motor impairment. The most commonly used treatment for PD is dopamine replacement therapy. Although, somewhat effective, the L-dopa only provides symptomatic relief with an unavoidable disease progression. It also loses efficacy with time and can cause drug-induced side effects [31]. The currently available therapies aim at the improvement of the functional capacity of the patient as long as possible but do not affect the progression of the neurodegenerative processes [32]. All this clearly emphasizes the need for newer and more effective therapeutic solutions. This problem is receiving more attention and is being subject to extensive research.
Many epidemiological studies have found that smoking is associated with a lower incidence of PD [22, 23]. The risk of PD in nonsmokers is about twice that of smokers. This means that cigarette smokers are about 50% less likely to have PD and that the patients with PD are about 50% less likely to have smoked cigarettes during their lifetime. These findings are important because, being understood at the molecular level they may provide some clues about novel therapeutic strategies for protection against PD.
The successful inhibition of α-synuclein fibrillation is believed to be important for the prevention or control of PD. In this study, we showed that two compounds of the cigarette smoke, nicotine and hydroquinone, led to the efficient inhibition of the α-synuclein fibril formation in a concentration-dependent manner (Figure 2). The SEC-HPLC analysis revealed that instead of the insoluble amyloid-like fibrils, three stable oligomeric forms were formed (Figure 4). The AFM imaging of these oligomers showed that they are mostly spherical species with heights of ~16 nm, ~10 nm, and ~4 nm (Figure 5). We also established that nicotine and hydroquinone were successful inhibitors of the A53T fibrillation (Figures 6–9). Finally, our data showed that nicotine was a more effective inhibitor than hydroquinone.
Our findings are in a perfect agreement with the results of earlier studies on the nicotine effect on the α-synuclein fibrillation. In fact, using thioflavin S (ThS) it has been shown that the fibrillation of both wild type and A53T α-synucleins (both at the concentration of 140 μM) was effectively inhibited by nicotine in a concentration dependent manner.16 AFM and EM analyses confirmed that nicotine inhibited α-synuclein fibril formation, as samples of α-synuclein (140 μM) incubated with 100 μM nicotine contained smaller amount of fibrils and these fibrils were shorter and thinner in comparison with those formed in the absence of nicotine [33]. We have established that nicotine is able to inhibit α-synuclein fibrillation almost completely. Our experimental settings were quite different from those in the work of Ono et al. [33], as we used smaller α-synuclein concentrations (70 μM) and higher nicotine concentrations (up to 400 μM).
Thus, α-synuclein fibrillation can be effectively inhibited by the cigarette smoke compounds such as nicotine and hydroquinone in a concentration-dependent manner. This inhibition involves the stabilization of soluble oligomeric forms. How can the stabilization of such soluble oligomers be related to the pathology of neurodegenerative diseases? In this respect it is important to remember that for a long time it was believed that the amyloid fibrils are harmful. However, a novel emerging paradigm favors the idea that the deposited proteinaceous inclusions (such as senile plaques in Alzheimer’s disease or Lewy bodies or Lewy neurites in Parkinson’s disease, etc.) are not cytotoxic. Instead, the formation of some small oligomers, known as various protofibrils, is responsible for the neurotoxicity [34–37]. This hypothesis is supported by the fact that the amount of fibrillar deposits found at autopsy does not typically correlate with the clinical severity of Alzheimer’s or Parkinson’s disease [37]. Furthermore, the dopamine-dependent neurotoxicity of α-synuclein in PD was shown to be mediated by 54-83-kD soluble protein complexes that contain α-synuclein and 14-3-3 protein, which are elevated selectively in the Substantia nigra in PD [21]. Furthermore, in animal models of PD and AD, the disease-like phenotypes were shown to develop before the appearance of the fibrillar deposits [38, 39]. Non-fibrillar oligomers, known as protofibrils, of various amyloidogenic proteins (both disease-realted and non-disease-associated) are toxic in cell cultures and are able to disrupt membrane inegrity in vitro [36, 37, 40–43]. Special attention has been paid to the so-called amyloid pores; i.e., morphologically similar annular protofibrils, that resemble a class of pore-forming bacterial toxin, as these oligomers might cause the inappropriate membrane permealization leading to cell disfunction and cell death [36, 37, 43–45].
Although the hypothesis that the small oligomers are cytotoxic and that the larger insoluble aggregates found in Lewy bodies and other proteinaceous may be protective is very important, it is necessary to remember that protein aggregates (including oligomers) are highly heterogenious. This heterogeneity might be caused either by the heterogeneous starting materials or by multiple pathways of assembly, or by both these factors. Therefore, it is difficult to believe that all the soluble oligomers, with their astonishing morphological variability, will be cytotoxic to the same degree. In fact, we have recently shown that the flavonoid baicalein was able to inhibit the α-synuclein fibrillation via the stabilization of soluble oligomers that possessed very specific structural features [46], being spherical in shape (according to the AFM and EM data), having relatively globular structure with packing density intermediate between that of pre-molten globules and typical globular proteins (according to the Kratky plot analysis of the SAXS data), and having relatively well-developed secondary structure (according to the FTIR and far-UV CD analysis). Furthermore, these oligomers were characterized by high thermodynamic stability and were able to inhibit fibrillation of baicalein-untreated α-synuclein. The most important finding was the fact that these highly stable and fibrillation-inhibiting oligomers did not disrupt the integrity of the biological membrane. Based on these observations it has been concluded that the soluble oligomer formation does not always create harm and can be beneficial [46]. We believe that similar oligomers might be stabilized by other chemical compounds (e.g., by the compounds of the cigarette smoke analyzed on this study), which might act similarly to baicalein; i.e., via the specific stabilization of the thermodynamically stable, non-fibrillating soluble oligomers that can inhibit α-synuclein fibrillation and do not cause the inappropriate membrane permealization.
Obviously, further studies are needed for the deeper understanding of the molecular mechanisms of the inhibition of α-synuclein fibrillation by nicotine and other cigarette smoke compounds. These studies might also be important for the development of novel anti-PD therapeutic strategies. An important question is whether the inhibition observed by us in vitro takes also place inside the living cells. If this inhibition does occur in the cell, then does it follow a receptor-related mechanism or a non-receptor-mediated mechanism? Another question is whether other chemicals in smoke can possess the neuroprotective effect. These and other issues need to be resolved in order to understand the molecular basis for the neuroprotection caused by smoking.
5. Conclusions
We show that compounds of the cigarette smoke such as nicotine and hydroquinone are able to inhibit the formation of α-synuclein fibrils in a concentration-dependent manner, with nicotine being more effective inhibitor. SEC-HPLC, SDS-PAGE, and AFM analyses revealed that nicotine and hydroquinone stabilized soluble oligomers. Similar data were obtained for the A53T mutant. These findings are first steps in better understanding the molecular mechanism of the nicotine- and hydroquinone-mediated inhibition of α-synuclein fibrillation, which potentially might help developing novel therapeutic solutions for PD.
Acknowledgments
This research was supported in part by grants R01 NS39985 (to D.-P.H and A.L.F.), R01 LM007688-01A1 (V.N.U.) and GM071714-01A2 (V.N.U.) from the National Institutes of Health and by a grant from the Program «Molecular and Cellular Biology» of the Russian Academy of Sciences (to V.N.U.). We gratefully acknowledge the support of the IUPUI Signature Centers Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Lewy FH. In: Handbuch der Neurologie. Lewandowski M, editor. Springer; Berlin: 1912. pp. 920–933. [Google Scholar]
- 3.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Trojanowski JQ, Goedert M, Iwatsubo T, Lee VM. Fatal attractions: abnormal protein aggregation and neuron death in Parkinson’s disease and Lewy body dementia. Cell Death Differ. 1998;5:832–837. doi: 10.1038/sj.cdd.4400432. [DOI] [PubMed] [Google Scholar]
- 5.Trojanowski JQ, Lee VM. Parkinson’s disease and related alpha-synucleinopathies are brain amyloidoses. Ann N Y Acad Sci. 2003;991:107–110. doi: 10.1111/j.1749-6632.2003.tb07468.x. [DOI] [PubMed] [Google Scholar]
- 6.Uversky VN. A protein-chameleon: conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. J Biomol Struct Dyn. 2003;21:211–234. doi: 10.1080/07391102.2003.10506918. [DOI] [PubMed] [Google Scholar]
- 7.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 8.Dev KK, Hofele K, Barbieri S, Buchman VL, van der Putten H. Part II: alpha-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45:14–44. doi: 10.1016/s0028-3908(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 9.Fink AL. The aggregation and fibrillation of alpha-synuclein. Acc Chem Res. 2006;39:628–34. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- 10.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 12.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 13.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uversky VN, Fink AL. Amino acid determinants of alpha-synuclein aggregation: putting together pieces of the puzzle. FEBS Lett. 2002;522:9–13. doi: 10.1016/s0014-5793(02)02883-1. [DOI] [PubMed] [Google Scholar]
- 15.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 16.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 17.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Uversky VN, Fink AL. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40:11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 19.Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, Lansbury PT., Jr Accelerated oligomerization by Parkinson’s disease linked alpha-synuclein mutants. Ann N Y Acad Sci. 2000;920:42–45. doi: 10.1111/j.1749-6632.2000.tb06903.x. [DOI] [PubMed] [Google Scholar]
- 20.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 22.Fratiglioni L, Wang HX. Smoking and Parkinson’s and Alzheimer’s disease: review of the epidemiological studies. Behav Brain Res. 2000;113:117–20. doi: 10.1016/s0166-4328(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 23.Quik M. Smoking, nicotine and Parkinson’s disease. Trends Neurosci. 2004;27:561–8. doi: 10.1016/j.tins.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Newhouse PA, Potter A, Levin ED. Nicotinic system involvement in Alzheimer’s and Parkinson’s diseases. Implications for therapeutics. Drugs Aging. 1997;11:206–28. doi: 10.2165/00002512-199711030-00005. [DOI] [PubMed] [Google Scholar]
- 25.Fagerstrom KO, Pomerleau O, Giordani B, Stelson F. Nicotine may relieve symptoms of Parkinson’s disease. Psychopharmacology (Berl) 1994;116:117–9. doi: 10.1007/BF02244882. [DOI] [PubMed] [Google Scholar]
- 26.Kelton MC, Kahn HJ, Conrath CL, Newhouse PA. The effects of nicotine on Parkinson’s disease. Brain Cogn. 2000;43:274–82. [PubMed] [Google Scholar]
- 27.Newman MB, Arendash GW, Shytle RD, Bickford PC, Tighe T, Sanberg PR. Nicotine’s oxidative and antioxidant properties in CNS. Life Sci. 2002;71:2807–20. doi: 10.1016/s0024-3205(02)02135-5. [DOI] [PubMed] [Google Scholar]
- 28.Uversky VN, Yamin G, Souillac PO, Goers J, Glaser CB, Fink AL. Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS Lett. 2002;517:239–244. doi: 10.1016/s0014-5793(02)02638-8. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen L, Khurana R, Coats A, Frokjaer S, Brange J, Vyas S, Uversky VN, Fink AL. Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry. 2001;40:6036–46. doi: 10.1021/bi002555c. [DOI] [PubMed] [Google Scholar]
- 30.Groenning M, Olsen L, van de Weert M, Flink JM, Frokjaer S, Jorgensen FS. Study on the binding of Thioflavin T to beta-sheet-rich and non-beta-sheet cavities. J Struct Biol. 2007;158:358–69. doi: 10.1016/j.jsb.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Boraud T, Bezard E, Bioulac B, Gross CE. Dopamine agonist-induced dyskinesias are correlated to both firing pattern and frequency alterations of pallidal neurones in the MPTP-treated monkey. Brain. 2001;124:546–57. doi: 10.1093/brain/124.3.546. [DOI] [PubMed] [Google Scholar]
- 32.Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson’s disease. Prog Neurobiol. 2007;81:29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Ono K, Hirohata M, Yamada M. Anti-fibrillogenic and fibril-destabilizing activity of nicotine in vitro: implications for the prevention and therapeutics of Lewy body diseases. Exp Neurol. 2007;205:414–24. doi: 10.1016/j.expneurol.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Urbanc B, Cruz L, Le R, Sanders J, Ashe KH, Duff K, Stanley HE, Irizarry MC, Hyman BT. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002;99:13990–5. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–8. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 37.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 38.Lansbury PT., Jr Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci U S A. 1999;96:3342–3344. doi: 10.1073/pnas.96.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg MS, Lansbury PT., Jr Is there a cause-and-effect relationship between alpha-synuclein fibrillization and Parkinson’s disease? Nat Cell Biol. 2000;2:E115–119. doi: 10.1038/35017124. [DOI] [PubMed] [Google Scholar]
- 40.Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–84. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–8. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 42.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–11. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 43.Rochet JC, Outeiro TF, Conway KA, Ding TT, Volles MJ, Lashuel HA, Bieganski RM, Lindquist SL, Lansbury PT. Interactions among alpha-synuclein, dopamine, and biomembranes: some clues for understanding neurodegeneration in Parkinson’s disease. J Mol Neurosci. 2004;23:23–34. doi: 10.1385/jmn:23:1-2:023. [DOI] [PubMed] [Google Scholar]
- 44.Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–9. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 45.Lashuel HA, Lansbury PT., Jr Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Q Rev Biophys. 2006;39:167–201. doi: 10.1017/S0033583506004422. [DOI] [PubMed] [Google Scholar]
- 46.Hong D-P, Fink AL, Uversky VN. Structural characteristics of the alpha-synuclein oligomers stabilized by the flavonoid baicalein. J Mol Biol. 2008 doi: 10.1016/j.jmb.2008.08.039. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]