Abstract
A general method has been developed for the preparation of microspheres of nanoporous pigments, their formulation into chemically responsive pigment inks, and the printing of these inks as calorimetric sensor arrays. Using an ultrasonic-spray aerosol–gel synthesis from chemically responsive dyes and common silica precursors, 16 different nanoporous pigment microspheres have been prepared and characterized. New calorimetric sensor arrays have been created by printing inks of these chemically responsive pigments as primary sensor elements; these arrays have been successfully tested for the detection, identification, and quantitation of toxic aliphatic amines. Among 11 structurally similar amines, complete identification of each analyte without confusion was achieved using hierarchical cluster analysis (HCA). Furthermore, visual identification of ammonia gas was easily made at the IDLH (immediately dangerous to life or health), PEL (permissible exposure limits), and 0.1 PEL concentrations with high reproducibility.
Introduction
For all chemical sensor technology, the sensor surface is obviously critical to the interactions between the sensor and the analyte that are responsible for the sensor response. Especially with the development of sensor arrays for electronic nose applications (which has emerged as a very powerful approach for the selective identification of chemically diverse analytes1–5), control of analyte–surface interactions is central to improvements in both sensitivity and selectivity. Sensor arrays do not have highly specific receptors for specific analytes; instead, the array uses cross-responsive sensor elements to mimic the mammalian gustatory and olfactory systems by producing a unique composite response for each analyte.1–4 Prior electronic nose technologies have generally employed physical adsorption onto metal oxide surfaces or absorption into conductive polymers as the primary analyte–sensor interaction,2 but such interactions are weak (leading to low sensitivity) and nonselective (leading to poor discrimination). In contrast, we have previously reported on the development of a rather different, but quite simple, optoelectronic approach using a calorimetric sensor array of chemically responsive dyes (i.e., soluble colorants whose colors are affected by a wide range of analyte–dye interactions, including Brønsted and Lewis acid–base, Bipolar, and π–π interactions).4–6
To make more robust calorimetric arrays, one would prefer a solid-state pigment rather than a soluble dye. Nonpermeable pigments, however, cannot function as sensors: only the surface of a pigment particle will be accessible to analytes. The formation of porous pigments or immobilized permeable polymers from soluble dyes has well-developed precedents for bulk films or monoliths, both in sol–gel matrices7–11 and in plasticized polymer films.12 The creation of a printable formulation of nanoporous pigments and the use of such nanoporous pigment inks to form a sensor array, however, have not been previously reported.
We have explored a new methodology for the preparation of chemically responsive inks based on nanoporous silica pigments made by an aerosol–gel method. This synthetic method utilizes an ultrasonic spray to generate an aerosol from homogeneous precursor solutions, which is then heated in a gas stream to provide a continuous flow production of dye–silica microspheres. The precursors in the sprayed homogeneous solutions are confined in each micron-sized aerosol droplet, which act as individual microreactors, providing a facile and quite general method of preparing microcomposite materials.13–17 We report here the application of ultrasonic-spray aerosol–gel synthesis for the preparation of micron-sized spheres of dye-encapsulated nanoporous pigments, demonstrate the use of these inks to print calorimetric sensor arrays, and show their application to the selective identification and detection of amines.
Experimental Section
Nanoporous Pigments Preparation and Characterization
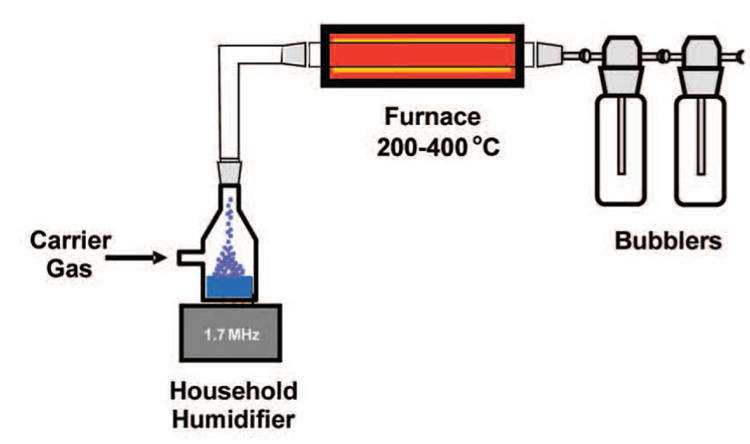
All commercial chemicals were used as received unless otherwise specified. Tetramethoxysilane (TMOS), methyltrimethoxysilane (MTMS), and the organic dyes used in this study were purchased from Sigma-Aldrich. A pictorial representation of a laboratory-scale aerosol–gel setup is shown in Figure 1. In a typical preparation, a precursor solution was made from 2 g of TMOS, 2 g of MTMS,11 g of ethanol, 28 g of water, and 1 g of 0.1 M aqueous hydrochloric acid and combined with 40 mg of dye; the molar ratio of MTMS to TMOS was 1.1. The precursor solution was introduced into an atomization cell and nebulized by a 1.7 MHz household ultrasonic humidifier (Sunbeam model 696), as described elsewhere.17 The resulting aerosol was carried through a tube furnace set at a temperature between 200 and 400 °C in an Ar flow at 1.0 SLPM (standard liters per minute). The product was collected in several bubblers containing deionized water and then isolated by centrifugation. After several washings with ethanol and deionized water, the micron-sized powder was dried at room temperature.
Figure 1.
Ultrasonic spray rig for aerosol–gel synthesis of chemoresponsive pigments.
For characterization of nanoporous pigments, scanning electron microscopy (SEM) was carried out with a Hitachi S-4700, and transmission electron microscopy (TEM) was performed using a JEOL 2010F with an acceleration voltage of 200 kV. Diffuse reflectance ultraviolet–visible (UV–vis) spectra of the products were obtained using a Hitachi 3300 double monochromator UV–vis spectrophotometer.
Array Preparation and Sensing Experimental Procedure
Prior to the printing, the nanoporous silica-dye pigments were dispersed in 7:3 mixtures of deionized water and 2-methoxyethanol. A calorimetric sensor array was then prepared by spotting suspensions of 16 different pigments onto standard chromatography paper (Whatman Chrl) using a 4 ×4 array of free-floating slotted dip-pins (V & P Scientific, Inc., San Diego, CA). For the detection of aliphatic amines, sensing experiments were performed using a static cells5 In the ammonia sensing experiments, a certified premixed gas tank from S. J. Smith, Co. (Urbana, IL) was used. Ammonia was mixed with dry and wet nitrogen gas with a manifold of MKS digital mass flow controllers to achieve the desired concentrations and relative humidity. In order to confirm the reproducibility of the array responses, triplicate runs were performed. Difference maps were obtained from the scanned images (cf. Supporting Information, Figure S2, for an example) by digitally subtracting the before image from the after image and averaging the center half of each spot’s red, green, and blue (RGB) values using a customized software package, ChemEye (ChemSensing, Inc., Champaign, IL; http://www.chemsensing.com); Adobe PhotoShop may also be used for such analyses. This results in a 48-dimensional vector with a potential range from −255 to +255 (i.e., 16 changes in red, green, and blue values) that quantitatively represents the calorimetric response of the array.4 Chemometric analysis (specifically, principal component analysis (PCA) and hierarchical cluster analysis (HCA)18) on the difference vectors was carried out using the Multi-Variate Statistical Package (MVSP, v. 3.13i, Kovach Computing).
Results and Discussion
For sensing array applications involving chemoresponsive pigments based on initially soluble dyes, there are four requirements: (1) all colorant centers must be accessible to analytes; (2) the dye must be immobilized in a porous or permeable host material to prevent leaching or blooming of the colorant; (3) the immobilized dye must be solvated in the host matrix to prevent dye crystallization (because solid dye particles are not generally permeable and therefore unresponsive to analytes); and (4) the pigment must be in a printable form. Among various host materials available, ORMOSILs (organically modified silicates) offer additional advantages of high stability, controllable pore size, and ease of modification (e.g., controllable hydrophobicity).7–11 These features contribute to enhanced analyte adsorption (i.e., effective “preconcentration” of analyte), leading to increased sensitivity.
We have focused on aliphatic amines as our test analyte to demonstrate the effectiveness of our approach. Aliphatic amines are common pollutants found in industrial wastewater effluents and agricultural runoff due to their wide use in numerous industrial applications.19 Consequently, the development of selective and rapid sensing technology for aliphatic amines has been an area of great interest. Furthermore, amines are common bacterial metabolic byproducts and can be used as indicators to estimate food freshness.20 Hence, a practical and robust detection method for amines is of great value in environmental monitoring as well as in food quality control. Several different methods have been previously reported for amine sensing, using various single colorimetric sensors, including the use of metalloporphyrins,4,5,21 molecular imprinting,22 functionalized mesoporous silica,23 and chromogenic reagents.24 The ability to distinguish one closely related amine from another, however, requires the use of a sensor array.4,5
A total of 16 different nanoporous pigment microspheres were prepared via the ultrasonic-spray aerosol–gel method, formulated into chemoresponsive inks, and dip-pen printed to prepare colorimetric sensor arrays. We used two common silicon alkoxides, TMOS and MTMS, as the matrix-forming precursors to manipulate the porosity of the resulting pigments. When the pigments were prepared using only TMOS, the pigments were not porous and did not show any color change either in response to acidic or basic gases or to acidic or basic aqueous solutions. The pigments obtained using the mixture of TMOS and MTMS, however, were highly porous and exhibited very rapid (subsecond) color changes in response to changes in pH. This indicates that the nanoporous structure resulting from the use of MTMS plays an important role in the diffusion of an analyte into the pigments. As the concentration of MTMS increased, the response time became increasingly more rapid. If MTMS was used alone, however, a significant amount of dye could be leached by aqueous solutions. In our study, a nanoporous pigment prepared with a 1.1 molar ratio of MTMS to TMOS was found to be optimal in terms of response time, consistency, and permanence of dye encapsulation.
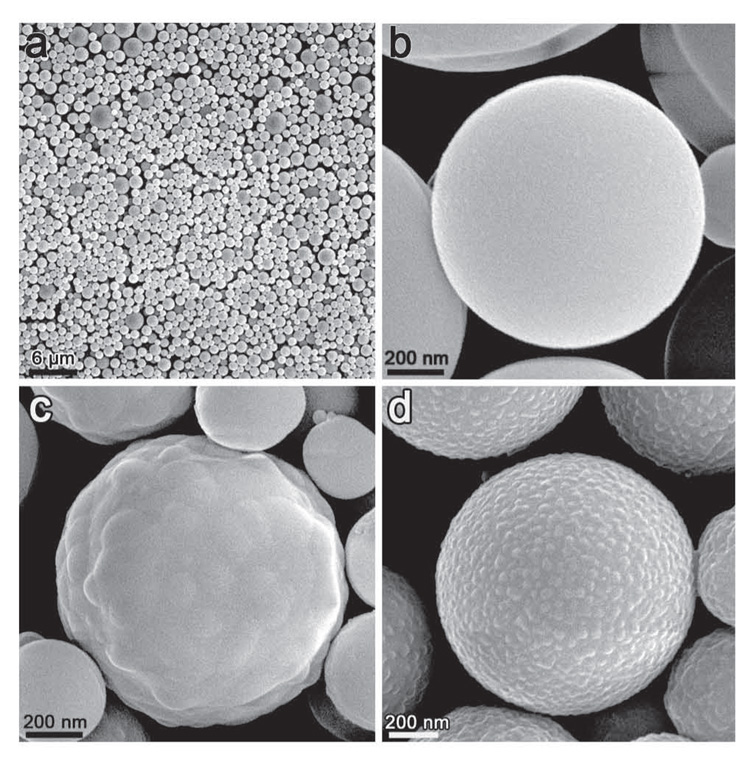
We also observed that the furnace temperature had a significant effect on the properties of the pigments microspheres. Representative SEM images of nanoporous pigment microspheres incorporated with bromocresol green (BCG-SiO2) and prepared at 200, 300, and 400 °C are shown in Figure 2. At 200 °C (Figures 2a and 2b), these nanoporous pigment microspheres are smooth-surfaced with diameters ranging from 0.4 to 1.5 µm and are extremely responsive to exposure to a pH 10 aqueous solution (response time < 1 s). As the furnace temperature was increased to 300 °C (Figure 2c), however, small bumps appeared on the surface of the microspheres, the porosity was diminished, and response time becomes much worse (5–10 min). When the furnace temperature was set to 400 °C (Figure 2d), the formation of these bumps was substantial, the porosity was lost, and the pigment no longer responds to changes in pH even after several hours. We attribute the slow response time observed in pigments obtained at higher temperatures to the collapse of surface pores, which prevents facile diffusion of the analytes to the colorant centers within the silica matrix.
Figure 2.
SEM micrographs of nanoporous silica microspheres that have BCG entrapped within them (BCG–SiO2) synthesized at (a and b) 200 °C, (c) 300 °C, and (d) 400 °C.
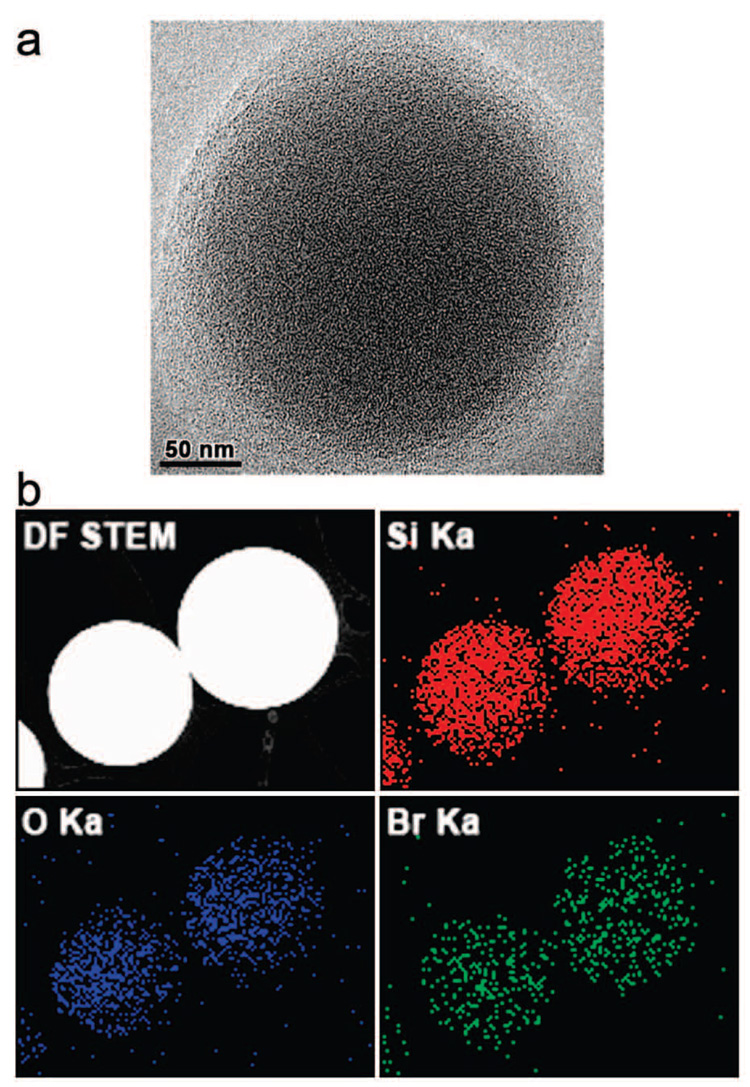
The TEM image (Figure 3a) of a pigment microsphere synthesized at 200 °C shows the nanoporous structure of the silica sphere that results from the amorphous organosilica network formed by the use of MTMS. The incorporation of pH dye molecules into the silica matrix was examined by energy-dispersive X-ray spectroscopy (EDS) elemental mapping analysis conducted in scanning transmission electron microscopy (STEM). The X-ray emission from the Br Kα shell, which originates from the bromine in BCG molecules, was observed along with Si Kα and O Kα signals, showing a uniform distribution of BCG molecules throughout the silica microspheres (Figure 3b).
Figure 3.
(a) TEM image and (b) STEM-EDS elemental mapping analysis of BCG–SiO2, showing a uniform distribution of BCG molecules throughout the silica microspheres.
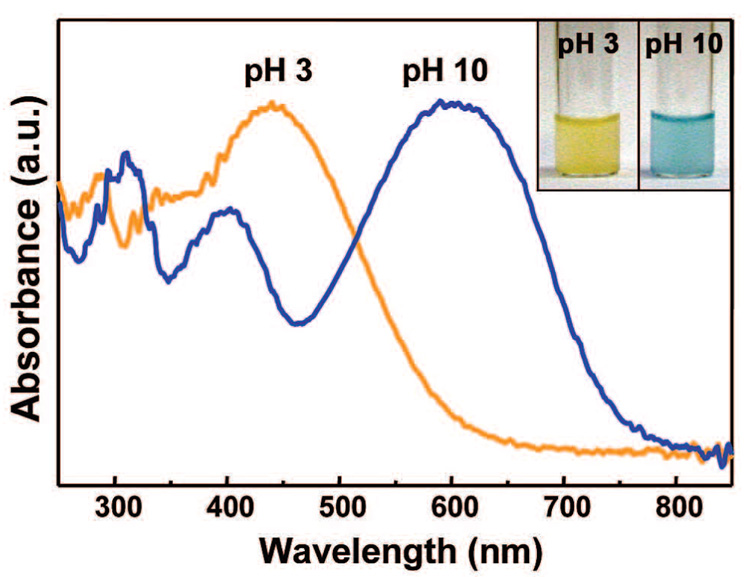
Diffuse reflectance UV–vis spectra of BCG–SiO2 microspheres are shown in Figure 4 as a function of pH. It is well-known that in an acidic solution, BCG ionizes to give a yellow monoanionic form, but further deprotonation at higher pH confers the blue dianionic form to BCG. As shown in Figure 4, BCG–SiO2 washed with a acidic solution exhibits strong absorption in the range of 400–600 nm, whereas BCG–SiO2 washed with a basic solution absorbs visible light mostly in the range of 500–800 nm. The same optical responses were observed in other BCG–SiO2 synthesized at higher temperatures (i.e.,≥300 °C), but the response time was much slower.
Figure 4.
Diffuse reflectance UV–vis spectra of BCG–SiO2 washed with acidic and basic solutions; inset: photograph of BCG–SiO2 suspended in different pH buffer solutions.
The reversible characteristics of the chemical response of the nanoporous pigments led us to exploit them in sensing applications. To demonstrate the utility of these nanoporous pigments in calorimetric sensor arrays, 16 different nanoporous pigment microspheres (incorporating 14 different pH dyes and 2 solvatochromic dyes, Table 1) were prepared using the same procedures as for BCG–SiO2 (i.e., furnace temperature at 200 °C) and resulting in very similar microspheres. For fabrication of the calorimetric sensor arrays, chemoresponsive inks were made from the nanoporous pigment microspheres by dispersal in an aqueous solution of 2-methoxyethanol, printed on standard chromatography paper using slotted dip-pins, and dried under vacuum.
Table 1.
List of the Colorants in the Nanoporous Sol–Gel Pigments of the Colorimetric Sensor Arraya
| bromo-cresol | methyl | chloro-phenol | bromo-phenol |
| green | red | red | red |
| bromo-cresol | alizarin | bromo-thymol | bromo-xylenol |
| purple | blue | blue | |
| nitrazine yellow | phenol red | brilliant yellow | cresol red |
| m-cresol purple | thymol blue | Reichardt’s dye | Reichardt’s dye#3 |
common names of the indicators are given. Reichardt’s dye(4-(2,4,6-triphenyl-1-pyridinio)phenolate)and Reichardt’s dye #3 (2,6-dichloro-4-(2,4,6-triohenyl-1-pyridinio)phenolate)are standard solvatochromic indicators.
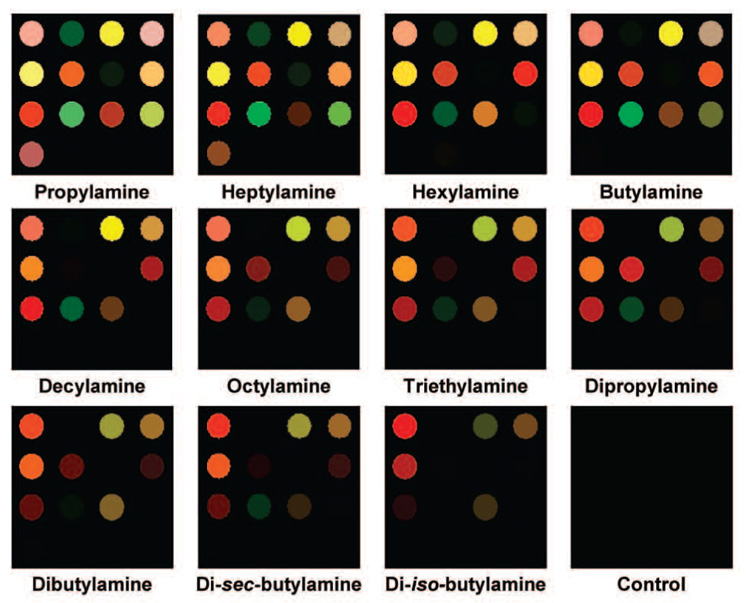
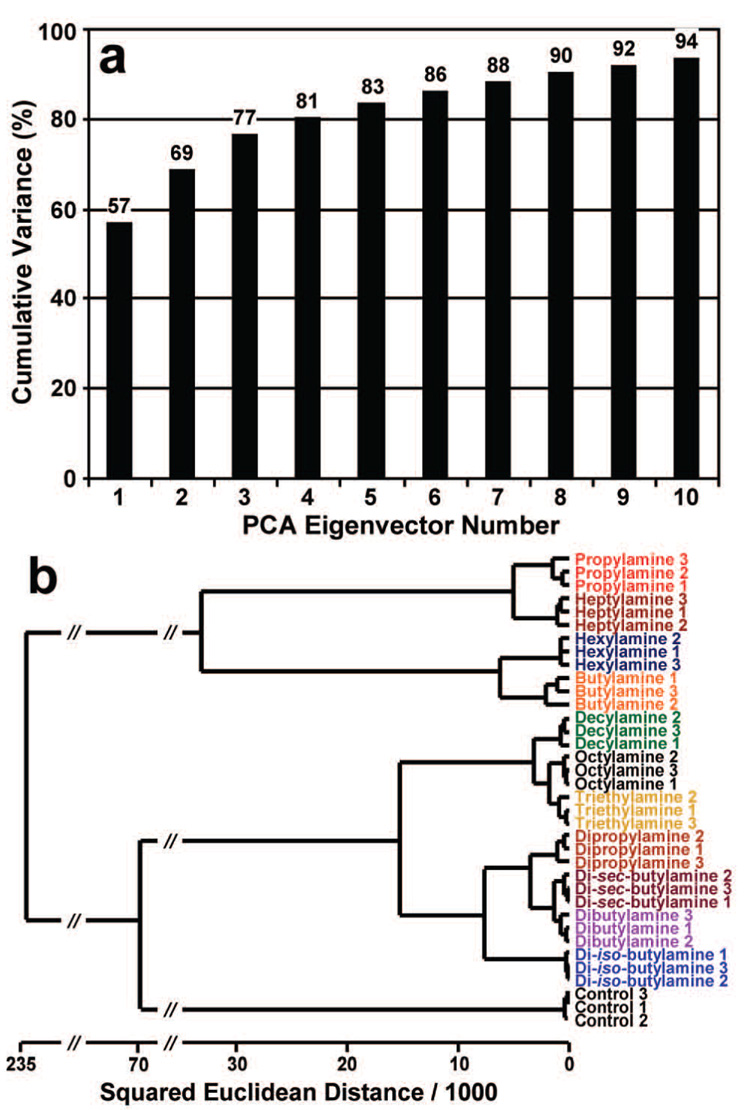
The resulting sensor arrays were used for the analysis of vapors of 11 structurally similar aliphatic amines; average color change profiles were acquired for each amine, as shown graphically in Figure 5. As is obvious even to the eye, the calorimetric sensor array shows a unique color change for each amine. The color change profiles are actually digital data (16 changes in red, green, and blue values ranging from −255 to +255) and were compiled into a library of 48-dimensional vectors. For statistical analyses (Figure 6), PCA and HCA were used to analyze this digital database (Supporting Information, Table S1). PCA provides a quantitative evaluation of the analytical dispersion of a technique based on its number of independent dimensions of variance.18 Prior electronic nose technologies have very few independent dimensions: 95–99% of the total variance among analytes is typically achieved with their first two dimensions. In comparison, there is a very high level of dimensionality (i.e., dispersion) with the pigment-based calorimetric sensor arrays. When PCA is applied even to this family of intimately related analytes, there are still eight dimensions required to capture 90% of the total variance, as shown in Figure 6a, which exemplifies the extraordinary discrimination ability of the colorimetric sensor arrays. To examine the multivariate distances between the analyte responses in this 48-dimensional RGB color space, HCA was performed using the minimum variance (“Ward’s”) method. A response dendrogram was generated as shown in Figure 6b. Remarkably, all of the aliphatic amines were accurately classified and identified against one another without error among the 36 trials.
Figure 5.
color change profiles of 11 structurally similar aliphatic amines after equilibration. For purposes of display, the color range of these difference maps are expanded from 6 to 8 bits per color (i.e., RGB range of 4–67 expanded to 0–255). The full digital data are provided in Supporting Information, Table S1.
Figure 6.
(a) PCA and (b) HCA for 11 structurally similar aliphatic amines using Ward’s method. No misclassifications were observed among the 36 trials. All experiments were run in triplicate. After the amine name, the trial number is given.
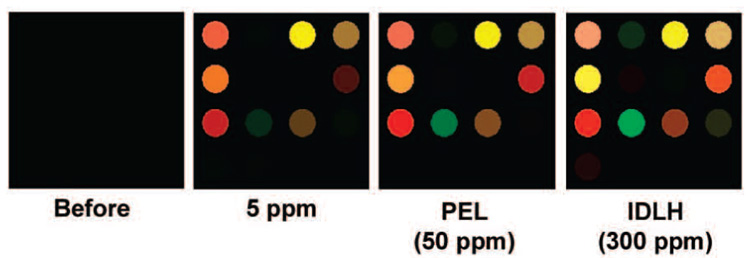
The nanoporous pigment-based array was further evaluated for its sensitivity for the detection of ammonia at various concentrations: 5 ppm, 50 ppm (the permissible exposure limit, PEL), and 300 ppm (the immediately dangerous to life or health concentration, IDLH) at 33% relative humidity (the digital data of the array response is provided in Supporting Information, Table S2). Ammonia vapor is of particular interest because even low concentrations of the gas can lead to serious toxicological consequences.25 In this respect, the development of a simple and sensitive sensor array for gas-phase detection of ammonia is a potentially valuable tool in environmental monitoring. The difference maps of the arrays (shown in Figure 7 after 2 min of NH3 exposure) are, as expected, unique to each concentration of the analyte. Distinct and highly reproducible patterns were obtained at all NH3 concentrations, even at one-tenth of the PEL, with no confusion as shown by HCA (Supporting Information, Figure S2), thus demonstrating an easy method to quantify ammonia concentrations at low detection limits. From the SIN ratio observed at 5 ppm, we estimate that our limit of detection (defined as 5*S/N) is well below 100 ppb (i.e.,<0.2% of the PEL).
Figure 7.
Difference maps of pigment-based arrays after 2 min NH3 exposure. For purposes of display, the color range of these difference maps are expanded from 6 to 8 bits per color (i.e.. RGB range of 4–67 expanded to 0–255). The full digital data and an HCA are provided in the Supporting Information, Table S2 and Figure S2.
Conclusions
The preparation of nanoporous pigment microspheres and their formulation into a chemoresponsive inks has been demonstrated for the first time. The chemically responsive microspheres were prepared via an aerosol–gel synthesis and incorporated into a colorimetric sensor array using dip-pen printing. The use of TMOS and MTMS as silica precursors with the incorporation of pH and solvatochromic dyes provides robust silica microspheres having a nanoporous structure, which not only facilitates the diffusion of analytes for rapid color changes, but also effectively stabilizes the colorants and prevents leaching of dyes from the silica microspheres upon contact with liquids. Colorimetric sensor arrays prepared using these nanoporous pigments are able to discriminate among 11 structurally similar aliphatic amines as well as ammonia gas at various concentrations with detection limits well below the PEL.
Supplementary Material
Color difference databases of 11 structurally similar aliphatic amines and controls and of NH3 at different concentrations; scanned images of the colorimetric sensor array before and after exposure to ammonia at the IDLH; and HCA of the colorimetric array response to ammonia.
Acknowledgment
We gratefully acknowledge Benjamin A. Suslick for his assistance in the sensing experiments. This work was supported by the DOE (DE-FG02-07ER46418), NIH (U-01-E516011), and the Center for Microanalysis of Materials and the Frederick Seitz Materials Research Laboratory at the University of Illinois, which is partially supported by the U.S. Department of Energy under Grants DE-FG02-07ER46453 and DE-FG02-07ER46471. K.S.S. discloses that he has a financial interest in ChemSensing, Inc.
References
- 1.(a) Gardner JW, Bartlett PN. Electronic Noses: Principles and Applications. New York: Oxford University Press; 1999. [Google Scholar]; (b) Pearce TC, Schiffman SS, Nagle HT, Gardner JW. Handbook of Machine Olfaction: Electronic Nose Technology. New York: Wiley VCH; 2003. [Google Scholar]
- 2.(a) Albert KJ, Lewis NS, Schauer CL, Sotzing GA, Stitzel SE, vaid TP, Walt DR. Chem. Rev. 2000;100:2595–2626. doi: 10.1021/cr980102w. [DOI] [PubMed] [Google Scholar]; (b) Lewis NS. Acc. Chem. Res. 2004;37:663–672. doi: 10.1021/ar030120m. [DOI] [PubMed] [Google Scholar]; (c) Anslyn EV. J. Org. Chem. 2007;72:687–699. doi: 10.1021/jo0617971. [DOI] [PubMed] [Google Scholar]; (d) Röck F, Barsan N, Weimar U. Chem. Rev. 2008;108:705–725. doi: 10.1021/cr068121q. [DOI] [PubMed] [Google Scholar]; (e) Hierlemann A, Gutierrez-Osuna R. Chem. Rev. 2008;108:563–613. doi: 10.1021/cr068116m. [DOI] [PubMed] [Google Scholar]
- 3.(a) Anand V, Kataria M, Kukkar V, Saharan V, Choudhury PK. Drug Discovery Today. 2007;12:257–265. doi: 10.1016/j.drudis.2007.01.010. [DOI] [PubMed] [Google Scholar]; (b) Toko K. Biomimetic Sensor Technology. Cambridge, U.K: Cambridge University Press; 2000. [Google Scholar]
- 4.(a) Suslick KS, Bailey DP, Ingison CK, Janzen M, Kosal MA, McNamara WB, III, Rakow NA, Sen A, Weaver JJ, Wilson JB, Zhang C, Nakagaki S. Quim. Nova. 2007;30:677–681. [Google Scholar]; (b) Suslick KS. MRS Bull. 2004;29:720–725. doi: 10.1557/mrs2004.209. [DOI] [PubMed] [Google Scholar]; (c) Suslick KS, Rakow NA, Sen A. Tetrahedron. 2004;60:11133–11138. [Google Scholar]; (d) Rakow NA, Suslick KS. Nature. 2000;406:710–713. doi: 10.1038/35021028. [DOI] [PubMed] [Google Scholar]
- 5.(a) Rakow NA, Sen A, Janzen MC, Ponder JB, Suslick KS. Angew. Chem., Int. Ed. 2005;44:4528–4532. doi: 10.1002/anie.200500939. [DOI] [PubMed] [Google Scholar]; (b) Janzen MC, Ponder JB, Bailey DP, Ingison CK, Suslick KS. Anal. Chem. 2006;78:3591–3600. doi: 10.1021/ac052111s. [DOI] [PubMed] [Google Scholar]
- 6.(a) Zhang C, Suslick KS. J. Am. Chem. Soc. 2005;127:11548–11549. doi: 10.1021/ja052606z. [DOI] [PubMed] [Google Scholar]; (b) Zhang C, Bailey DP, Suslick KS. J. Agric. Food Chem. 2006;54:4925–4931. doi: 10.1021/jf060110a. [DOI] [PubMed] [Google Scholar]; (c) Zhang C, Suslick KS. J. Agric. Food Chem. 2007;55:237–242. doi: 10.1021/jf0624695. [DOI] [PubMed] [Google Scholar]
- 7.(a) Podbielska H, Ulatowska-Jarza A, Muller G, Eichler HJ. In: Optical Chemical Sensors. Baldini F, Chester AN, Homola J, Martellucci Ss, editors. Erice, Italy: Springer; 2006. pp. 353–385. [Google Scholar]; (b) Jeronimo PCA, Araujo AN, Montenegro M. Talanta. 2007;72:13–27. doi: 10.1016/j.talanta.2006.09.029. [DOI] [PubMed] [Google Scholar]; (c) Dunbar RA, Jordan JD, Bright FV. Anal. Chem. 1996;68:604–610. [Google Scholar]
- 8.(a) Rottman C, Grader G, De Hazan Y, Melchior S, Avnir D. J. Am. Chem. Soc. 1999;121:8533–8543. [Google Scholar]; (b) Kowada Y, Ozeki T, Minami T. J. Sol-Gel Sci. Technol. 2005;33:175–185. [Google Scholar]; (c) Makote R, Collinson MM. Anal. Chim. Acta. 1999;394:195–200. [Google Scholar]
- 9.Lev O, Tsionsky M, Rabinovich L, Glezer V, Sampath S, Pankratov I, Gun J. Anal. Chem. 1995;67:22A–30A. [Google Scholar]; (b) Avnir D, Coradin T, Lev O, Livage J. J. Mater. Chem. 2006;16:1013–1030. [Google Scholar]; (c) Rottman C, Ottolenghi M, Zusman R, Lev O, Smith M, Gong G, Kagan ML, Avnir D. Mater. Lett. 1992;13:293–298. [Google Scholar]; (d) Rottman C, Turniansky A, Avnir D. J. Sol-Gel Sci. Technol. 1998;13:17–25. [Google Scholar]; (e) Zusman R, Rottman C, Ottolenghi M, Avnir D. J. Non-Cryst. Solids. 1990;122:107–109. [Google Scholar]
- 10.Carrington NA, Xue Z-L. Acc. Chem. Res. 2007;40:343–350. doi: 10.1021/ar600017w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Zaggout FR. J. Dispers. Sci. Technol. 2005;26:757–761. [Google Scholar]; (b) Zaggout FR. Mater. Lett. 2006;60:1026–1030. [Google Scholar]; (c) Zaggout FR, El-Ashgar NM, Zourab SM, El-Nahhal IM, Motaweh H. Mater. Lett. 2005;59:2928–2931. [Google Scholar]; (d) Zaggout FR, El-Nahhal IM, Qaraman AE-FA, Al Dahoudi N. Mater. Lett. 2006;60:3463–3467. [Google Scholar]; (e) Zaggout FR, El-Nahhal IM, Zourab SM, El-Ashgar NM, El-Dawahedy N, Motaweh H. J. Dispers. Sci. Technol. 2005;26:629–633. [Google Scholar]
- 12.(a) McMurray HN, Albadran J. MRS Bull. 1999;24:55–59. [Google Scholar]; (b) Swindlehurst BR, Narayanaswamy R. In: Optical Sensors: Industrial, Environmental and Diagnostic Applications. Narayanaswamy R, Wolfbeis 0S, editors. Erice, Italy: Springer; 2004. pp. 281–308. [Google Scholar]
- 13.(a) Lu Y, Fan H, Stump A, Ward TL, Rieker T, Brinker CJ. Nature. 1999;398:223–226. [Google Scholar]; (b) Jiang X, Brinker CJ. J. Am. Chem. Soc. 2006;128:4512–4513. doi: 10.1021/ja058260+. [DOI] [PubMed] [Google Scholar]
- 14.Xia B, Lenggoro W, Okuyama K. Adv. Mater. 2001;13:1579–1582. [Google Scholar]
- 15.Tartaj P, González-Carreño T, Serna CJ. Adv. Mater. 2001;13:1620–1624. [Google Scholar]
- 16.Zheng T, Zhan J, Pang J, Tan GS, He J, McPherson GL, Lu Y, John VT. Adv. Mater. 2006;18:2735–2738. [Google Scholar]
- 17.(a) Skrabalak SE, Suslick KS. J. Am. Chem. Soc. 2005;127:9990–9991. doi: 10.1021/ja051654g. [DOI] [PubMed] [Google Scholar]; (b) Suh WH, Suslick KS. J. Am. Chem. Soc. 2005;127:12007–12010. doi: 10.1021/ja050693p. [DOI] [PubMed] [Google Scholar]; (c) Didenko YT, Suslick KS. J. Am. Chem. Soc. 2005;127:12196–12197. doi: 10.1021/ja054124t. [DOI] [PubMed] [Google Scholar]; (d) Suh WH, Jang AR, Suh Y-H, Suslick KS. Adv. Mater. 2006;18:1832–1837. [Google Scholar]; (e) Skrabalak SE, Suslick KS. J. Am. Chem. Soc. 2006;128:12642–12643. doi: 10.1021/ja064899h. [DOI] [PubMed] [Google Scholar]; (f) Bang JH, Han K, Skrabalak SE, Kim H, Suslick KS. J. Phys. Chem. C. 2007;111:10959–10964. [Google Scholar]; (g) Bang JH, Helmich RJ, Suslick KS. Adv. Mater. 2008;20:2599–2603. [Google Scholar]
- 18.(a) Hasswell S. Practical Guide To Chemometrics. New York: Dekker; 1992. [Google Scholar]; (b) Scott SM, James D, Ali Z. Microchim. Acta. 2007;156:183–207. [Google Scholar]; (c) Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 6th ed. Upper Saddle River, NJ: Prentice Hall; 2007. [Google Scholar]; (d) Hair JF, Black B, Babin B, Anderson RE, Tatham RL. Multivariate Data Analysis. 6th ed. Upper Saddle River, NJ: Prentice Hall; 2005. [Google Scholar]
- 19.Gong W-L, Sears KJ, Alleman JE, Blatchley ER., III Environ. Toxicol. Chem. 2004;23:239–244. doi: 10.1897/03-109. [DOI] [PubMed] [Google Scholar]
- 20.(a) Pacquit A, Frisby J, Diamond D, Lau KT, Farrell A, Quilty B, Diamond D. Food Chem. 2007;102:466–470. [Google Scholar]; (b) Pacquit A, Lau KT, McLaughlin H, Frisby J, Quilty B, Diamond D. Talanta. 2006;69:515–520. doi: 10.1016/j.talanta.2005.10.046. [DOI] [PubMed] [Google Scholar]; (c) Veciana-Nogués MT, Mariné-Font A, Vidal-Carou MC. J. Agric. Food Chem. 1997;45:2036–2041. [Google Scholar]; (d) Maynor MS, Nelson TL, O’Sullivan C, Lavigne JJ. Org. Lett. 2007;9:3217–3220. doi: 10.1021/ol071065a. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland IO. Pure Appl. Chem. 1989;61:1547–1554. [Google Scholar]
- 22.(a) Holthoff EL, Bright FV. Acc. Chem. Res. 2007;40:756–767. doi: 10.1021/ar700087t. [DOI] [PubMed] [Google Scholar]; (b) Greene NT, Shimizu KD. J. Am. Chem. Soc. 2005;127:5695–5700. doi: 10.1021/ja0468022. [DOI] [PubMed] [Google Scholar]; (c) Mertz E, Zimmerman SC. J. Am. Chem. Soc. 2003;125:3424–3425. doi: 10.1021/ja0294515. [DOI] [PubMed] [Google Scholar]
- 23.Comes M, Marcos MD, Martínez-Máñez R, Sancenón F, Soto J, Villaescusa LA, Amorós P, Beltrán D. Adv. Mater. 2004;16:1783–1786. [Google Scholar]
- 24.Mohr GJ. Chem. Eur. J. 2004;10:1082–1090. doi: 10.1002/chem.200305524. [DOI] [PubMed] [Google Scholar]
- 25.(a) Schenker S, McCandless DW, Brophy E, Lewis MS. J. Clin. Invest. 1967;46:838–848. doi: 10.1172/JCI105583. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Burns TR, Mace ML, Greenberg SD, Jachimczyk JA. Am J. Forensic Med. Pathol. 1985;6:204–210. doi: 10.1097/00000433-198509000-00006. [DOI] [PubMed] [Google Scholar]; (c) Sigurdarson ST, O’Shaughnessy PT, Watt JA, Kline JN. Am. J. Ind. Med. 2004;46:345–348. doi: 10.1002/ajim.20055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Color difference databases of 11 structurally similar aliphatic amines and controls and of NH3 at different concentrations; scanned images of the colorimetric sensor array before and after exposure to ammonia at the IDLH; and HCA of the colorimetric array response to ammonia.